Comparative Activity of Metabolic Enzymes of Acaricides in F1 Larvae of Rhipicephalus (Boophilus) Microplus (Acar. Ixodidae) and Susceptible Strains IVRI-I: Correlation with Massive Selection of Resistant Ticks on Farms in Benin, West Africa
Soumanou Salifou1*, Houenagnon MA. Houngnimassoun1, Aboubakar Sidick2, Osse Razaki2, Minassou J Ahouandjinou2, Sabbas Attindehou1, Martin C Akogbeto2 and Sahidou Salifou1
1Laboratory of Applied Biology Research, University of Abomey-Calavi, Benin
2Entomological Research Center of Cotonou (CREC), Benin
*Address for Correspondence: Soumanou Salifou, Laboratory of Applied Biology Research, University of Abomey-Calavi, 01 BP 2009 Cotonou, Benin, Tel: +229 -975-06173; E-mail: [email protected]
Submitted: 30 September 2020; Approved: 13 October 2020; Published: 15 October 2020
Citation this article: Salifou S, Houngnimassoun HM, Sidick A, Razaki O, Ahouandjinou MJ, et al. Comparative Activity of Metabolic Enzymes of Acaricides in F1 Larvae of Rhipicephalus (Boophilus) Microplus (Acar. Ixodidae) and Susceptible Strains IVRI-I: Correlation with Massive Selection of Resistant Ticks on Farms in Benin, West Africa. Int J Vet Sci Technol. 2020;4(2): 047-052.
Copyright: © 2020 Salifou S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Enzymes; Metabolic resistance; Acaricides; Rhipicephalus (Boophilus) Microplus
Download Fulltext PDF
The role of enzymes in the metabolic processes of acaricides is well known for their crucial involvement in the resistance mechanisms of several arthropods, including ticks and other mites. The present study therefore aimed to evaluate the level of expression of esterases, monooxygenases and glutathione-S-transferases in F1 populations of Rhipicephalus (Boophilus) Microplus not exposed to an acaricide molecule. The results obtained indicate a significant difference (p < 0.0001) between the level of expression of these enzymes in R. (B.) Microplus populations and the reference susceptible strain (IVRI-I ). This may be positively correlated with resistance of R. (B.) Microplus to common acaricides. These results thus lift a corner of veil on a massive selection of individuals resistant to conventional molecules used in the fight against ticks in Benin. However, this population is not yet multiresistant because the level of expression of these enzymes compared to that of the multiresistant IVRI-V strain, remains significantly (p < 0.0001) lower. Therefore, it is urgent to adopt strategies for the use of acaricides in the control of these ticks.
Introduction
Tick resistance to acaricides is a global phenomenon and almost all tropical and sub-tropical countries face the problem of resistance to one or more acaricide formulations [1]. It renders available products and vector control strategies ineffective, resulting in increased prevalence of pathogens and the diseases they transmit [2-4]. Mareover it requires an increase in the quantities of products used and the development of new molecules or formulations thus increasing the cost.
Over the last two decades, tick research has shown numerous cases of acaricide resistance in R. (B.) Microplus [5-11] and the role of overexpression of metabolic enzymes such as Esterases (ESTs), Utathione-S-Transferases (GSTs) and cytochrome P450 is reported to be involved in the metabolic detoxification of acaricides [12, 13].
However, most of his work in the laboratory quantifies the enzymatic activity of field strains after bioassays such as AIT (Adult Immersion Test), LIT (Larval Immersion Test) and LPT (Larval Packet Test) with conventional acaricides. But, we all know that any organism that suffers an aggression of any kind is called up on to defend itself by reacting. Therefore does not account for the real level of expression of its enzymes in unexposed populations after years of massive and abusive use of synthetic molecules in the field. The present study therefore proposes to quantify the enzyme profile in an F1 population of R. (B.) Microplus not exposed to synthetic acaricides. The objective is to evaluate the level of expression of detoxification enzymes in populations of R. (B.) Microplus of Kpinnou.
Materials and Methods
Obtaining the F1 larvae
A population of ticks engorged females ( ̴ 200 ticks) was collected from Girolando cattle from the Kpinnou Breeding Farm (FEK) in the commune of Athieme (Mono County, 6°35'58.78"North and 1°44'26. 84"East). The animals have not been treated recently with a conventional acaricide formulation, the last treatment being more than 30 days ago.
The collected ticks were kept alive in an isothermal box, which guaranteed good aeration and relative humidity until the National Laboratory of Veterinary Parasitology (LNPV).
Morphological identification and egg-laying of female Rhipicephalus (Boophilus) Microplus
The morphological identification of the species Rhipicephalus (Boophilus) Microplus was carried out at the National Laboratories of Veterinary Parasitology using OPTIKA binocular magnifiers at 10X magnification, according to the dichotomous identification key of Walker, et al. [14]. The Boophilus subgenus was identified on the basis of the hexagonal-based capitulum, presence of distinct eyes, Coxa I with small paired spurs, Coxa II to IV without spurs, presence of small palm segments.
The ticks were then laid at laboratory room temperature (28°C ± 2) until oviposition. After oviposition, which usually started 72 h after, and lasted about 16 days, the eggs were carefully separated from the females and weighed at a rate of 0.1g/package in standard FAO [15] larval packages using the precision balance (Explorer® Pro; max = 210 g; d = 0.1) and then incubated still at laboratory room temperature (28°C ± 2) until hatching, which took place after 21- 23 days.
At hatching, F1 larvae take about 15 days to complete their chitinization. Once chitinization is complete, the larvae with negative geotropism move up to the upper edge of the packs where they gather in clusters (swarms). This behaviour is similar to that observed in nature where they climb to the end of the grass stems to wait for the passage of a possible host to take the blood meal. It is therefore warming larvae, i.e. larvae capable of taking the blood meal that have been manipulated for this study.
Reference tick strains
The acaricide-sensitive reference strain IVRI-I (Reg. No. NBAII/IVRI/BM/1/1998) and the multiresistant strain IVRI-V (registration pending) of R. (B.) Microplus maintained in the
Entomology Laboratory, Parasitology Division, Indian Veterinary Research Institute, and used for standardization and validation of biochemical assays were used as reference for resistance level characterization.
Biochemical assays of detoxification enzymatic activities
We have worked on 3 of the most frequent enzyme families known for their considerable role in acaricide resistance, namely cytochrome P450 monooxygenases (Cyt P450), Glutathion-S-Transferases (GST) and esterases α and β [16- 20].
The activities of Cyt P450, Esterase and GST are expressed as the amount of product formed per mg of protein from enzyme extracts. Protein quantities are determined with bovine serum albumin as reference.
Preservation and preparation of ticks
The presence of enzyme activities appropriate for acaricide resistance is often performed using microplate assays .As larvae must not be destroyed before use, the F1 obtained were stored at -80°C. The standardized protocol for characterizing acaricide resistance to field ticks by Fular, et al. [21] (in press) was used to assess enzyme activity.
Pools of 40 R. (B.) Microplus larvae with an average of 15 days were formed in Eppendorf tubes. A total of 40 samples were collected.
Since enzymes degrade quickly at room temperature, all the work was done on ice. The ticks were crushed in 200 ml of distilled water using sterilized plungers and an Argos brand pellet. The crushed material was centrifuged at 12,000 rpm for 2 min in a centrifuge of the type MIKRO® 200R V2.01, and the supernatant was recovered in sequence from wells of a microplate with a micropipette. A volume of 2 x 20 µl was distributed in 2 wells of a microplate for oxidases. The other 4 microplates were filled with 10 ml in 2 replicates for the amount of proteins and the evaluation of GST activities and then esterases.
Measurement of Glutathione S-Transferase (GST) activity
The enzymatic activity of GST was determined by spectrophometer monitoring the conjugation of 1-chloro-2,4-dinitrobenzene (CDNB, Sigma) with glutathione (microfluorometric method). 10 µl of tick meal in two replicas was placed in each Nunc plate well and 200 µl of a solution of 0.06 g glutathione in reduced form (GSH) and 0.013 g
CDNB (1-chloro-2,4-dinitrobenzene) previously and completely dissolved in 1 ml methanol was added.
The change in optical density resulting from the binding between the thiol group of glutathione and the CDNB (substrate) is measured at 340 nm for 5 minutes at contact (t0) and after 1 minute of reaction (t1), with a microplate spectrophometer. For the control measurements, the same protocol was followed by replacing the extraction solutions containing the enzymes with the extraction solution alone (experimental blank). The results were expressed in nanomoles of products (conjugated form of glutathione) per milligram of protein per minute.
Measurement of oxydase activity
After having put 20 µl of broth in two replicas in each well, we added 80 µl of a solution of 3.3',5, 5' Tetramethyl Benzidine (TMBZ) 0.25M pH 5.0 dissolved in 6 ml methanol. Then 18 ml of 0.25M Sodium Acetate Buffer (NaC2H3O2) pH 5.0 and 25 µl of a 3% hydrogen peroxide solution were added to each well. The plate was then incubated for 30 min at room temperature with a lid and read at 630 nm every 10 min.
The oxidase activity of each tick in nmol P450equivalent units/mg protein was calculated as follows nmol P450 equivalent units per 20 μl of feed / (2x (mg protein in 10 μl feed))
Measurement of non-specific esterase activity (α- and β-naphthol)
The esterase activities were measured with the microfluorometric method by measuring the production of α- and β-naphthol from a substrate of α- and β-Naphthyl Acetate (NA). The reaction is performed in multi-well plates. Thus, 10 µl of the broth in two replicas were put in each plate well to which we added 90 μl of 1% Triton Phosphate Saline Buffer (PBS) pH
After mixing, the plate is left for 10 minutes at room temperature. Then 100 µl of a solution consisting of 600 μl 0.3 M alpha-Naphthyl acetate (or beta-Naphthyl acetate), 2.5 ml 1% Triton PBS buffer pH 6.5 and 7 ml water is added. The plate will be incubated again for 30 min at room temperature. The reaction is stopped by adding 100μl of a solution consisting of 0.010 g Fast Garnett Salt (FGBC) dissolved in10ml distilled water. After mixing, the plate is incubated for 10 minutes at room temperature with a lid. It will then be read at 550 nm as an endpoint. Absorbances of the reaction medium were recorded at 527 nm for α-naphthyl acetate and at 505 nm for β-naphthyl acetate using a microplate reader. The tick esterase activity in μmol of alpha-Naphthol produced/min/mg protein was determined as follows (alpha-Naphthol in μmol produced by 10 μl of grinding / amount of protein (in mg) in 10 μl of grinding) /30
Statistical analysis
Comparative measurements of mean enzyme activities were performed by a one-factor analysis of variance (ANOVA) using Graph Pad Prism 5 software, between susceptible a IVRI-I strains and R. (B.) Microplus from the Kpinnou farm on the one hand, and between R. (B.) Microplus ticks and multi-resistant IVRI-V strains other hand. The nonparametric Mann-Whitney U test was used to indicate a significant increase in the different means.
Results
Esterase activity
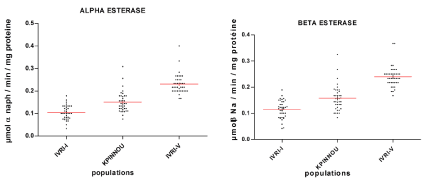
The average specific activity of α and β esterases of R. (B.) Microplus populations of the Kpinnou farm not exposed to acaricides is estimated to be 0.160 ± 0.045 μmol α- naphthol formed/min/mg protein and 0.166 ± 0.048 μmol β-naphthol formed/min/mg protein, respectively, versus 0.099 ± 0.031 μmol α-naphthol formed/min/mg protein (α-esterase) and 0.110 ± 0.034 (β esterase) μmol β-naphthol formed/min/mg protein for the sensitive reference strain (IVRI-I) (Table 1).
αThis observed difference is very significant (p < 0.0001) and the α and β esterases of R. (B.) Microplus populations of Kpinnou are 1.61 and 1. 50 times higher respectively than those of the susceptible IVRI-I strain (Figure 1). On the other hand, these esterase activities recorded for the field population are significantly lower (p < 0.0001) compared to those of the multiresistant.
IVRI-V strain (0.236 ± 0. 043 μmol α-naphthol formed/min/mg protein for α-esterase and 0.244 ± 0. 040 μmol β-naphthol formed/min/mg protein for β-esterase).
Cyt P450 monooxygenase activity
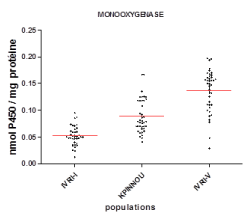
Biochemical tests showed that the oxidase activity of the sensitive IVRI-I strain (0.052 ± 0.018 nmol P450/min/mg protein) was significantly different (p < 0.0001) from that recorded in R. (B.) Microplus Kpinnou populations (0.095 ± 0. 031 nmol P450/min/mg protein). However, this activity remains lower than that of the multi-resistant IVRI-V strain (0.121 ± 0.039 P450/min/mg protein) (Figure 2).
Glutathione-S-transferase (GST) activity
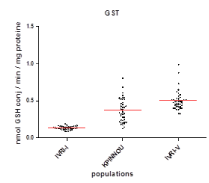
The results in figure 3 show that the mean GST activity ofR. (B.) Microplus populations of Kpinnou was 3.14 times (0.411 ± 0.160 nmol GSH conj,/min/mg protein) higher (p < 0.0001) than that of the sensitive reference strain IVRI-I (0.131 ± 0.023 nmol GSH conj,/min/mg protein). This overproduction of GST recorded in R. (B.) Microplus populations of Kpinnou are, on the other hand, significantly lower (p < 0.0003) than those of the resistant IVRI-V strain (0.522 ± 0.129 nmol GSHconj,/min/mg protein)as well as in esterases and oxydases.
Discussion
Among the two major mechanisms involved in the development of resistance, the role of enzyme over expression on acaricide metabolism has been established in a number of arthropod species [22-26].
In this study, a significant difference (p < 0.001) in enzyme activity (esterases, GST and cytochrome P450monooxygenase ) was observed between the reference susceptible tick strain (IVRI-I ) and Microplus R. (B. ) populations not exposed to acaricide. This highlights the whole issue of massive selection of individuals resistant to acaricides commonly used in breeding. Indeed, esterase elevation is a well-known mechanism for detoxification of organophosphates and, to a lesser extent, for carbamate resistance in insects and mites [27]. Its involvement in the detoxification of pyrethroids has also been demonstrated in Tetranychus urticae mites [28]. These enzymes hydrolyze ester bonds, cleaving xenobiotics and generating acid and alcohol as metabolites.
Although in arthropods, this class of enzymes is involved in various important physiological activities such as reproductive behaviour [29] and nervous system function [30], the esterase activity obtained from R. (B.) Microplus populations of Kpinnou was higher than that of the reference susceptible strain and cannot be positively correlated with the development of resistance to commercially available acaricide molecules.
Similarly, the high level of GST expression recorded in Kpinnou's R. (B.) Microplus ticks indicates a strong aptitude for the development of resistance. Li, et al. [5] reported that the increase in GST activity may be related to the metabolic detoxification of OPs that conferred resistance to San Roman R. (B.) Microplus populations. He, et al. [31] and Li, et al. [5,32] showed a significant correlation between GST overproduction and resistance of R. (B.)
Microplus to coumaphos, diazinon and amitraz. A 4 to 6-fold increase in GST activity among populations of R. bursa resistant to synthetic pyrethroids compared to a susceptible population was demonstrated by Enayati, et al. [33]. Their conclusion appears to be consistent with the present results where they found a 3.14-fold increase in GST activity of IVRI-I strain. Indeed,
GSTs represent a group of multigenic isozymes classified into five families of genes, namely alpha (α), mu (μ), pi (Π), theta (θ), and sigma (σ), based on their sequence similarity and cross immunoreactivity [34]. They are generally involved in the detoxification of endo- and xenobiotics. GSTs are present in all aerobic eukaryotes as catalysts for the conjugation of the reduced form of Glutathione (GSH) with molecules with an electrophilic center, athioether bond is formed between the sulphur of the GST and the substrate, resulting in the resulting conjugates containing more soluble water and facilitating their excretion from the cells [35].
Also, studies have shown that cytochrome P450 is involved in the resistance of ticks to various acaricides. Metabolic resistance to synthetic pyrethroids due to cytochrome P450 activity has already been observed in the Mexican population of R. (B.) Microplus [9]. Enayati, et al. [33] reported an increase in monooxygenase content up to 4.9- fold in the pyrethroid-resistant R. bursa population of Iran compared to the susceptible strain. In this study, the significant level of monooxygenase expression (p < 0.0001) recorded in R. (B.) Microplus ticks from the Kpinnou farm was 1.83 times higher than that of the reference susceptible strain (IVRI-I). This is a strong indication of the possible role of monooxygenases in detoxifying active acaricides or preventing them from reaching their target site.
Cytochrome P450 is a superfamily of enzymes, also called CYP, present in all organisms. More than 200 CYP enzymes have been described and code for proteins whose structural characteristics are retained even when sequence similarity is relatively low [36]. Cytochrome P450 enzymes play different roles in eucaryotes, including carbon uptake, biosynthesis of hormones and cellular components, and degradation of xenobiotics via an oxidation reaction that takes place through conformational changes.
Moreover, it should be noted that, despite this overproduction of detoxification enzymes in the R. (B.) Microplus populations of the Kpinnou farm, the level of expression of these enzymes is significantly different (p < 0.0001) from that recorded in the multiresistant strain (IVRI-V).
Conclusion
Resistance to acaricides is a widespread phenomenon in bovine tick populations .The role of enzymes such as cytochromes P450. Esterases and GST in the metabolic processes of acaricides has been well documented. This metabolic process actually results from the degradation and increased excretion of xenobiotic molecules, a mechanism that probably evolved from an ancestral ability to degrade food toxins. Therefore, these enzymes are expected to be presentat aslightly moderate level intick populations in livestock. In the present
study, significantly different values (p < 0.0001) ofthese enzymes were recorded in R. (B.) Microplus populations from the Kpinnou farm not exposed to an acaricide molecule compared to the reference susceptible strain (IVRI-I). These results therefore shed some light on the massive selection of individuals resistant to the conventional molecules used to control these ectoparasites. However, further studies are necessary to identify the classes of acaricide molecules to which these ticks are resistant in Benin in order to adopt strategies for the use of acaricides in the fight against ticks.
Acknowledgements
The authors would like to thank all those who contributed to the realization of this study, in particular the managers of Entomological Research Center of Cotonou (CREC), for their collaboration.
- Abbas RZ, Zaman MA, Colwell DD, Gilleard J, Iqbal Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet Parasitol. 2014; 203: 6-20. DOI: 10.1016/j.vetpar.2014.03.006
- Georghiou GP, Lagunes Tejeda A. The occurrence of resistance to pesticides in arthropods. Rome: Food and Agriculture Organization.1991; 318. https://bit.ly/2GNx2TG
- Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Manag Sci. 2007; 63: 628-633. DOI: 10.1002/ps.1406
- Whalon ME, Mota Sanchez D, Hollingworth RM. Globalpesticide resistance in arthropods. Oxford University. 2008; 169.
- Li AY, Davey RB, Miller RJ, George JE. Resistance to coumaphos and diazinon in Boophilus Microplus (Acari: ixodidae) and evidence for the involvement of an oxidative detoxification mechanism. J Med Entomol. 2003; 40: 482-490. DOI: 10.1603/0022-2585-40.4.482
- Rodriguez Vivas RI, Rivas AL, Chowell G, Fragoso SH, Rosario CR., Garcia Z, et al. Spatial distribution of acaricide profiles (Boophilus Microplus strains susceptible or resistant to acaricides) in southeastern Mexico. Vet Parasitol. 2007; 146: 158-169. DOI: 10.1016/j.vetpar.2007.01.016
- Fernandez Salas A, Roger Ivan Rodriguez Vivas, Miguel AAD. Resistance of Rhipicephalus Microplus to amitraz and cypermethrin intropical cattle farms in Veracruz, Mexico. J Parasitol. 2012; 98: 1010-1014. DOI: 10.1645/GE-3074.1
- Reck J, Klafke GM, Webster A, Dall'Agnol B, Scheffer R, Souza UA, et al. First report of fluazuron resistance in Rhipicephalus Microplus: A field tick population resistant to six classes of acaricides. Vet Parasitol. 2014; 201: 128-136. DOI: 10.1016/j.vetpar.2014.01.012
- Klafke G, Webster A, Agnol BD, Pradel E, Silva J, de La Canal LH, et al. Multiple resistance to acaricides in field populations of Rhipicephalus Microplus from Rio Grande do Sul state, Southern Brazil. Ticks Tick Borne Dis. 2017; 8: 73-80. DOI: 10.1016/j.ttbdis.2016.09.019
- Cuore U, Solari MA, Trelles A. Current status of resistance and first diagnostic of multiple resistance Rhipicephalus (Boophilus) Microplus tick simultaneously resistant to five drugs in Uruguay. Veterinaria (Montevideo). 2017; 53, 13-19. https://bit.ly/3iXf7XF
- Bandara KJ, Karunaratne SP. Mechanisms of acaricide resistance in the cattle tick Rhipicephalus (Boophilus) Microplus in Sri Lanka. Pest Biochem Physiol. 2017; 139: 68-72. DOI: 10.1016/j.pestbp.2017.05.002
- Braga IA, Valle D. Aedes aegypti: History of control in Brazil. Epidemiol serv Health. 2007; 16: 113-118. https://bit.ly/373Fx7K
- Russell RJ, Scott C, Jackson CJ, Pandey R, Pandey G, Taylor MC, et al. The evolution of new enzyme function: Lessons from xenobiotic metabolizing bacteria versus insecticide-resistant insects. Evol Appl. 2011; 4: 225-248. DOI: 10.1111/j.1752-4571.2010.00175.x
- Walker AR, Bouattour A, Camicas JL, Estrada Pena A, Horak I, Atif A, et al. Ticks of domestic animals in Africa: A guide to identification of species. Bioscience Reports, Edinburgh. 2003. https://bit.ly/3drALlK
- Ticks and tick-borne disease control. A practical field manual. Vol. 1 FAO. 1984; 247-248. https://bit.ly/2Fqyg6n
- Baffi MA, Cicero DP, De Souza GR, Ceron CR, Bonetti AM. Esterase profile in the postembryonic development of Rhipicephalus Microplus. Pesq Agropec Bras Brasilia. 2007; 42: 1183-1188. https://bit.ly/34UVhqL
- Li Y, Farnsworth CA, Coppin CW, Teese MG, Liu JW, Scott C, et al. Organophosphate and pyrethroidhydrolase activities of mutant esterases from the cotton bollworm Helicoverpa armigera. PLoS ONE. 2013; 8: 77685. DOI: 10.1371/journal.pone.0077685
- Chevillon C, Koffi BB, Barre N, Durand P, Arnathau C, De Meeus T. Direct and indirect inferences on parasite mating and gene transmission patterns: Pangamy in the cattle tick Rhipicephalus (Boophilus) Microplus. Infect. Genet. Evol. 2007; 7: 298-304.
- I da Silva Vaz Jnr S, Imamura K, Ohashi M, Onuma. Cloning expres-sion and partial characterization of a Haemaphysalis longicornis anda Rhipicephalus appendiculatus glutathione-S-transferase. Insect Mol Biol. 2004; 13: 329-335. DOI: 10.1111/j.0962-1075.2004.00493.x
- Konus M, Koy C, Mikkat S, Kreutzer M, Zimmermann R, Iscan M, et al. Molecular adaptations of Helicoverpa armigera midgut tissue under pyrethroid insecticide stress characterized bydifferential proteome analysis and enzyme activity assays. Comp Biochemt Physiol Pt D Genom Proteom. 2013; 8: 152-162. DOI: 10.1016/j.cbd.2013.04.001
- Fular A, Gupta S, Sharma AK, Kumar S, Upadhaya D, Shakya M, et al. Standardization of tick specific biochemical tools for estimation of esterases, monooxygenases and glutathione S-transferase for characterization of acaricide resistance pesticide biochemistry and physiology. 2020. https://bit.ly/3719ANi
- Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000; 45: 371-391. https://bit.ly/3lNKEx1
- Rosario CR, Almazan C, Miller RJ, Dominguez Garcia DI, Hernandez Ortiz R, de la Fuente J. Genetic basis and impact of tickacaricide resistance. Front Biosci. 2009; 14 : 2657-2665. DOI: 10.2741/3403
- Rosario CR, Guerrero FD, Miller RJ, Rodriguez Vivas RI, Tijerina M, Dominguez Garcia DI, et al. Molecular survey of pyrethroid resis-tance mechanisms in Mexican field populations of Rhipicephalus (Boophilus) Microplus. Parasitol. Res. 2009; 105: 1145-1153. DOI: 10.1007/s00436-009-1539-1
- Bass C, Jones C. Editorial overview: Pests and resistance: Resistance to pesticides in arthropod crop pests and disease vectors: mechanisms, models and tools. Curr Opin Insect Sci. 2018; 27: 4-6. DOI: 10.1016/j.cois.2018.04.009
- Rabbit WAC, Pereira JS, Fonseca ZAAS, Andre WPP, Bessa EM, et al. Resistance of Rhipicephalus (Boophilus) Microplus against cypermethrin and amitraz in dairy cattle in northeast Brazil. Act Vet Bras. 2013; 7: 229-232.
- Karunaratne SHPP. Direct and indirect inferences on parasite mating and gene transmission patterns: Pangamy in the cattle tick Rhipicephalus (Boophilus) Microplus. Infect Genet Evol. 1998; 7: 298-304.
- Sanchez Arroyo H, Koehler PG, Valles SM. Effects of the Synergists Piperonyl Butoxide and S, S,S-Tributyl Phosphorotrithioate on Propoxur Pharmacokinetics in Blattella germanica (Blattodea: Blattellidae). Journal of Economic Entomology. 2001;94: 1209-1216. https://bit.ly/2GPOffc
- Labate J, Bortoli A, Game AY, Cooke PH, Oakeshott JG. The number and distribution of esterase 6 alleles in populations of drosophila melanogaster. Heredity. s1989; 63: 203-208. DOI: 10.1038/hdy.1989.93
- Villatte F, Bachmann TT. How many genes encode cholinesterase in arthropods? Pest Biochem. Physiol. 2002; 73: 122-129. https://bit.ly/3jXazBN
- He H, Chen AC, Davey RB, Ivie GW, George JE. Identification of a point mutation in the Para-type sodium channel gene from a pyrethroids resistant cattle tick. Biochem Biophys Res Commun. 1999; 261: 558-561.
- Li AY, Davey RB, Miller RJ, George JE. Detection and characterization of amitraz resistance in the southern cattle tick, Boophilus Microplus (Acari: Ixodidae). J Med Entomol. 2004; 41: 193-200. DOI: 10.1603/0022-2585-41.2.193
- Enayati AA, Asgarian F, Amouei A, Sharif M, Mortazavi H, Boujhmehrani H, et al. Pyrethroid insecticide resistance in Rhipicephalus bursa (Acari, ixodidae). Pestic Biochem Phys. 2010; 97: 243-248. https://bit.ly/34YR4T9
- Coelho WAC, Pereira JS, Fonseca ZAAS, Andre WPP, Bessa EM, et al. Resistance of Rhipicephalus (Boophilus) Microplus against cypermethrin and amitraz in dairy cattle in northeastern Brazil. Act Vet Bras. 2013 ; 7: 229-232.
- Muller P, Warr E, Stevenson BJ, Pignatelli PM, Morgan JC, Steven A, et al. Field caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet. 2008; 4. DOI: 10.1371/journal.pgen.1000286
- Werck-Reichhart D, Feyereisen R. Cytochromes P450: A success story. Genome Biol. 2001; 1. https://bit.ly/34YFFm2

Sign up for Article Alerts