Strains for Use a Potential Probiotics for Laying Hens
Samantha Roberta Machado Oliveira1,2, Karen Costa1, Ana Paula Trovatti Uetanabaro2, Elisabeth Neumann1, Leonardo Jose Camargos Lara3, Marcelo Resende de Souza3 and Jacques Robert Nicoli1*
1Departamento de Microbiologia, Instituto de Ciencias Biologicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil
2Departamento de Ciencias Biologicas, Universidade Estadual de Santa Cruz, Ilheus, BA, Brazil
3Escola de Veterinaria, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil
*Address for Correspondence: Jacques Robert Nicoli, Departamento de Microbiologia, Instituto de Ciencias Biologicas, Universidade Federal de Minas Gerais, Belo Horizonte, Avenida Antonio Carlos, 6627, 31270-901, MG, Brazil, Tel: +553-134-092-757; ORCiD: 0000-0003-2390-2608; E-mail: [email protected]
Submitted: 31 August 2020; Approved: 09 September 2020; Published: 10 September 2020
Citation this article: Machado Oliveira SR, Costa K, Trovatti Uetanabaro AP, Neumann E, Nicoli JR, et al. In Vitro Selection and In Vivo Trial of LactoBacillus Strains for Use a Potential Probiotics for Laying Hens. Int J Vet Sci Technol. 2020;4(1): 033-042.
Copyright: © 2020 Machado Oliveira SR, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Probiotics; in-vitro screening; in-vivo trial; LactoBacillus; Laying hens
Download Fulltext PDF
Probiotic microorganisms gained the attention of breeders and veterinarians as an alternative following the ban on the use of antibiotics as growth stimulators in animals. Lactobacilli genus pertains to the dominant intestinal microbiota of poultry, and is a beneficial component of the gut microbiome, having a great impact on the health status of these animals. In the present study, three LactoBacillus (L. crispatus MRS1, L. reuteri MRS3 and L. reuteri MRS5) were submitted to In Vitro assays to evaluate their safety (antibiotic susceptibility, hemolytic and gelatinase activities), functional (resistance to acidic pH and bile salts), beneficial (antagonism, exopolysaccharide production and co-aggregation of pathogens) and technological (resistance to lyophilization and storage, reactivation time, growth parameters) characteristics to be used as probiotics for laying hens. L. crispatus MRS3 was selected in this first step and used in an In Vivo trial which showed that its incorporation in diet improved the internal quality of the eggs of laying hens. Concluding, L. crispatus MRS3 presented promising probiotic properties without harmful characteristics to be used in diet for laying hens.
Introduction
The poultry industry is one of the fastest growing segments of the livestock sector in the world. At the same time, due to high production efficiency, the dietary and health needs of poultry require particular care. Enteric disorders are one of the most important problems in the poultry industry, with necrotic enteritis, salmonellosis and colibacillosis regarded as the major bacterial diseases occurring in chicken.
Salmonella Enteritidis and Salmonella Typhimurium are major serovars accountable for foodborne illness, causing 74% of human zoonosis cases [1]. Pathogen resistance caused by wide application of various antibiotics in both the medical and veterinary fields has become a serious worldwide problem. Probiotic microorganisms gained the attention of breeders and veterinarians as an alternative following the ban issued in 2006 by the European Commission on the use of antibiotics as growth promotors in animals [2].
Bacteria of the Lactobacilli genus pertain to the dominant intestinal microbiota of birds and they have been isolated from the gastrointestinal tract of chickens, geese, ducks and pigeons. The most commonly identified species are L. salivarius, L. johnsonii, L. crispatus, L. reuteri and L. agilis [3]. Lactobacilli, as beneficial components of the gut microbiome, have a great impact on the health status of poultry, maintaining the microbial balance of the mucous membranes and providing protection against enteropathogenic infection. Based on to their health-promoting properties, Lactobacilli are used to produce probiotic preparations for humans and animals. The use of selected LactoBacillus strains as feed additives for poultry can produce similar effects to those of antibiotic growth promoters, as demonstrated by increases in weight and feed efficiency, as well as by higher resistance to pathogenic bacteria such as Salmonella sp., Escherichia coli and Campylobacter sp. However, various other bacterial genera (LactoBacillus, Bifidobacterium, Bacillus, Enterococcus), and even bacteriophages, have been studied or commercialized for probiotic use in poultry production [4-9].
Before field trials to evaluate the efficacy of a promising probiotic, preliminary In Vitro screening is required to ensure the safety, functional, beneficial and biotechnological aspects of the tested strains. Probiotic microorganism should be free of undesirable traits, such as transmissible antibiotic resistance (to avoid spreading of resistance determinants in intestinal pathogenic or opportunistic bacteria), and hemolytic and gelatinase activities. A next important criterion is its survival under gastrointestinal conditions to meet the classical definition of probiotic [10]. Such microorganism when orally administered must survive during the passage through the gastrointestinal tract to their site of function to be effective. The main challenge to be overcome during this transport are the survival in acidic pH of the pro-ventriculus and gizzard and to bile salts in the small intestine [11]. The demonstration of antagonistic activity towards pathogenic species in-vitro may be considered a desirable beneficial attribute of probiotic bacteria, as well as the ability to trap pathogenic microorganism by co-aggregation and/or to stimulate the immune system or inhibit pathogen adhesion by production of Exopolysaccharides (EPS). Finally, a potential probiotic must present technological characteristics which allow an economical and practical viability, such as good growth yield, resistance to lyophilization and storage and fast reactivation ability when administered on lyophilized form. Once selected as a candidate for probiotic use, a microorganism must be submitted to field trials to verify that the properties observed In Vitro are confirmed In Vivo.
Therefore, in the present study, three LactoBacillus strains were submitted to a selective process In Vitro to be used as probiotic for laying hens based on safety, functional, beneficial and biotechnological criteria. Once this first step was completed, the selected bacterium was administered to the laying hen ration to assess its ability to improve qualitative and quantitative parameters in egg production.
Material and Methods
TBacteria
The three LactoBacillus strains (L. crispatus MRS1, L. reuteri MRS3 and L. reuteri MRS5) used in the present study have been isolated from the cecum of healthy Lohmann White laying hens (Gallus gallus domesticus) and identified by MALDI-TOF mass spectrometry. These Lactobacilli were grown in de Man, Rogosa and Sharp broth (MRS, Acumedia, Lansing, USA) by incubation at 37°C for 24 hours.
The pathogenic reference strains Escherichia coli ATCC 25723, Staphylococcus aureus ATCC 29213, Listeria monocytogenes ATCC 15313, Salmonella enterica subsp. enterica serovar Typhimurium ATCC 14028 were used as indicator strains for the antagonistic assays, as well as the following bacterial strains isolated from cecum of laying hens and identified at species level by MALDI-TOF mass spectrometry: Klebsiella oxytoca MAC, Klebsiella varicola MAC29, Escherichia fergusonii MAC16, Citrobacter freundii MAC17, Enterococcus faecalis MAC20 and Enterobacter aburiae MAC27. All these bacteria were grown in Brain Heart Infusion broth (BHI, Difco, Sparks, USA) by incubation for 24 hours at 37°C.
In Vitro selection
Antibiotic susceptibility: The LactoBacillus strains were screened for susceptibility to 12 clinically and veterinary relevant antibiotics, namely tetracycline 30 μg, ampicillin 10 μg, gentamicin 10 μg, amicacin 30 μg, erythromycin 15 μg, ceftriaxone 30 μg, chloramphenicol 30 μg, oxacillin 1 μg, penicillin 10 μg, amoxicillin 10 μg, vancomycin 30 μg and nalixic acid 30 μg. The susceptibility to antimicrobials was determined by the disc diffusion method. The Lactobacilli were grown in MRS broth, and after the incubation period they were inoculated on MRS agar and incubated at 37°C for 48 hours. Then, using colonies from the culture in solid medium, a suspension of each sample was prepared and its turbidity was adjusted to a 0.5 value with sterile saline solution according to the McFarland scale equivalent to 108 cells. Then, aliquots of 100 μl of the adjusted suspensions were spread onto MRS agar with the aid of a sterile swab. Immediately after the antimicrobial impregnated disks were placed onto the surface of the plates which were incubated at 37°C for 24 hours. The inhibition zones were measured with a digital caliper (Mitutoyo, Digimatic Caliper, Sao Paulo, Brazil). For each antimicrobial tested, the Lactobacilli were classified as sensitive, moderately sensitive or resistant according to the cut-off points suggested by Charteris [12].
Hemolytic activity: Hemolytic activity was determined after inoculation of the bacteria onto blood agar plates (5% defibrinated sheep blood). The results were analyzed after incubation at 37°C for 48 hours, under aerobiose conditions. The hemolytic activity was detected by the formation of clear zones around the colony (positive for β-hemolysis) or absence of zone (negative).
Gelatinase activity: Test tubes containing MRS broth supplemented with 12% gelatin were inoculated with three colonies of each LactoBacillus, puncturing the medium with the aid of a platinum needle loop. These tubes were incubated at 37°C for 72 hours. During this period, tubes were removed and refrigerated for 30 minutes. After this time, it was checked whether the medium solidified completely (gelatinase negative) or remained liquid (gelatinase positive).
Resistance to gastrointestinal conditions: After incubation for 24 hours at 37°C, the bacteria were recovered by centrifugation for 5 minutes at 5,000 rpm, washed twice with phosphate buffered saline (PBS, pH 7.5) and incubated in simulated gastric juice solution containing pepsin (3 g/l), NaCl (5 g/l) and pH adjusted to 2.0 and 3.0 with 1M HCl. Sample plating was performed after serial decimal dilutions at times 0, 1.5 and 3 hours after inoculation. The number of Colony Forming Units (CFU) was determined after incubation at 37°C on MRS agar.
For bile salts resistance, Lactobacilli were cultured in MRS broth after inoculation and incubation for 24 hours at 37°C. Then, they were washed and resuspended in sterile PBS and samples subjected to serial decimal dilutions. These dilutions were then inoculated onto MRS agar plates supplemented with increasing concentrations of bile salts (Oxgall, Difco 0.15%, 0.3%, 0.6% and 1.0%), and incubated for 48 hours at 37°C. After incubation, the resistance was evaluated as the remaining number of CFU/ml.
Co-aggregation: Lactobacilli were activated in MRS broth for 24 hours at 37ºC and the indicator bacteria under the same conditions, but in BHI broth. Then, indicator and LactoBacillus strains were centrifuged at 5,000 rpm for 5 minutes, washed twice and resuspended in PBS. After this process, volumes containing 2 ml of the indicator plus 2 ml of LactoBacillus were transferred to tubes and shaken for 2 minutes. As controls, tubes containing only the indicator or the LactoBacillus were used were. OD600nm reading was performed at time zero and 5 hours after rest. Gram staining of the best results was performed. The following formula was used to calculate the co-aggregation percentage: ((Ax + Ay) - A (x + y)) / (Ax + Ay) / 2 * 100, in which Ax and Ay correspond to the OD600 nm of the control tubes and (x + y) corresponds to the OD600nm of the tube containing the mixture [13].
Antagonism assay: The detection of the production of diffusive inhibitory substances was carried out by the agar double-layer diffusion method. Initially, a micro-drop of 5 μl of each culture of LactoBacillus previously grown in MRS broth for 24 hours at 37°C was spot inoculated in the center of plates containing MRS agar. These plates were incubated at 37°C for 48 hours. To stop microbial multiplication, the culture was exposed to chloroform vapor for 30 minutes and then, the plates were then opened for the same time for evaporation of the residual solvent. Following, a 3.5 ml overlay of semi-solid BHI agar (0.75%) inoculated with 200 μl of the indicator bacteria culture was spread over the MRS agar. The plates were again incubated at 37°C for 24 hours and, when present, the inhibition zones were measured with a digital caliper (Mitutoyo).
EPS production: To detect EPS production, Lactobacilli were grown in MRS broth in aerobiose during 24 hours at 37°C. After incubation, bacteria were inoculated in streaks onto MRS agar plates containing 2% glucose (MRS), 8% sucrose (sMRS) or 5% lactose (1MRS) which were incubated for 48 hours at 37°C. EPS production was detected by the viscous appearance of the inoculated colonies. The strains were classified qualitatively in producer (EPS +) and non-producer (EPS -) [14].
Growth curves: To determine the growth kinetics of the LactoBacillus strains, the cell quantification was estimated by both OD600 nm and CFU determinations. Isolated colonies of the bacteria on MRS agar were used to inoculate an Erlenmeyer flask containing 150 ml of MRS broth. After inoculation, samples were taken every three hours to determine OD600 nm as well as CFU counts by serial decimal dilutions in sterile saline solution followed by plating on MRS agar and incubation during 24 hours at 37°C. During the growth period and at the same sampling times, the pH of the medium was determined to evaluate acid production.
Resistance to lyophilization and storage: The freeze-drying technique was performed according to the methodology described by Bolla et al. [15] with modifications. Each LactoBacillus was grown in 200 ml of MRS broth for 24 hours at 37°C. After incubation, culture was aliquoted into Falcon tubes, centrifuged at 5,000 rpm for 5 minutes and washed twice with sterile saline solution. Then, pellets were resuspended in 10% (w/v) skim milk (Difco) as cryoprotectant or saline solution as the control. Bacterial cell biomass was resuspended in the cryoprotective or control suspension to make up an initial minimum concentration of 109 CFU/ml. From each bacterial suspension in skim milk or saline, glass vials with a volume of 1.0 ml were prepared in liquid nitrogen and dehydrated in a lyophilizer for 24 hours at a temperature of -98°C and a pressure of 10 mm Hg. After lyophilization process, vials were sealed with a rubber stopper and parafilm. The samples were divided and stored under refrigeration at 5°C or in BOD incubator at 25°C considered as equivalent to room temperature.
The viable cell count of each lyophilized culture was performed before (T0) and after lyophilization (T1), as well as at different storage times (15, 45, 60 and 90 days) for both temperatures. At these different times, lyophilized cultures were submitted to serial decimal dilutions in sterile saline solution followed by plating onto MRS agar. The number of viable cells was determined after 48 hours of incubation at 37°C. The number of UFC/ml at T0 was considered to be 100%. The percentage of viable cells was calculated as: log10 CFU per gram at T1, T15, T45, T60 or T90/log10 CFU per gram at T0 x100.
Reactivation kinetics: The reactivation capacity of the lyophilized bacteria was determined as described by Martins et al. [16]. MRS broth were 2% inoculated with lyophilized cultures T0, T1, T15, T45, T60 and T90 after storage at 5°C or 25°C. Aliquots of 200 μl of inoculated media were distributed into wells of 96-well microplates which were incubated in an ELISA reader (Microplate SpectroMax 340) at 37°C. Growth of cultures was monitored by OD600nm reading every 30 min during 24 hours.
In Vivo trial
Animals and diet: The trial was conducted at the Experimental Farm Prof. Helio Barbosa of the Veterinary School of the Federal University of Minas Gerais (UFMG), located in the municipality of Igarape MG, during the first semester of 2019. Four hundred and thirty two 60-week-old Hisex® laying hens were used and housed in experimental cages measuring 45 x 50 cm. In these cages equipped with gutter feeders and cup-type drinkers, four birds were housed per cage (375 cm2/bird). The light program adopted was 14 h of light/day (natural and artificial). The duration of the trial was of 10 weeks and two weeks of adaptation. Water and feed were provided ad libitum. The experimental plots consisted of six cages, which had their feeders separated by wooden dividers, preventing birds from one experimental unit from having access to the feed of the other unit. The diet was formulated to meet the nutritional requirements of the layers according to age and production phase. The nutritional level calculations were made according to the chemical composition and the energetic values of the ingredients. The LactoBacillus was added to the diet in its lyophilized form to obtain 106 CFU per gram. Diet composition and its calculated nutritional values were shown in table 1.
Experimental design: The experimental design was completely randomized, consisting of three treatments with six replications of 24 birds each, totaling 432 chickens. For egg quality analysis 24 eggs were used and each egg was considered as a repetition. The three following groups were formed: (CTL) control group received a standard diet; (ANT) received diet with antibiotic (avilamycin); and (PRO) received diet supplemented with probiotic.
Egg production and quality of eggs: At the end of the experimental period six eggs per repetition (96 per treatment) were used to determine specific weight. Initially each egg was weighed in analytical balance and then weighed in water. The specific weight will be obtained by the following formula: (egg weight in air / (egg weight in air - egg weight in water), and results expressed in (g/ml H2O). The same eggs used to determine specific weight were used to determine shell thickness, which was measured by using a Mitutoyo micrometer at three different points of the shell (apical, equatorial and basal region). The result was obtained by averaging the three points, and expressed in millimeter. Six eggs per repetition (96 per treatment) were used to determine the percentages of shells. The eggs were weighed individually, its contents discarded, and the excess of the white removed in water. Then, it was dried at room temperature for further weighing, determining the percentage of bark expressed as percentage. Haugh Unit (H.U.) was calculated using the formula: 100 x log( h−1.7 w.0.37 + 7.6) where, h is albumen height in millimeters, measured by a spherometer, and w is the observed weight of the egg in grams.
Ethics of biological experiments: All experimental procedures were carried out according to the standards set forth by the Brazilian Society of Laboratory Animal Science/Brazilian College for Animal Experimentation. The study was approved by the Ethics Committee in Animal Experimentation (CEUA/UFMG).
Statistical analysis: All experiments were performed in triplicate. Bacterial numbers were represented as the average of log10 CFU ± SEM, or average values of absorbance ± SEM per well of 96-well polystyrene microtiter plates. The t-test was used for statistical analysis. Qualitative data of the eggs were analyzed using the ANOVA procedure and means compared by Tukey test. Values of p <0.05 were considered as statistically significant.
Results
Safety aspects
Hemolysis and gelatinase activities were not detected in the LactoBacillus strains. Table 2 shows the sensitivity of the three Lactobacilli to 12 antimicrobials. The three Lactobacilli were sensitive to most of the antimicrobials except vancomycin, amicacin, nalidixic acid and oxacillin.
Functional aspects
Table 3 shows that all the LactoBacillus strains were similarly quite resistant to the simulated acidic conditions of the gastric environment, as well as to different concentrations of bile salts. However, the two L. reuteri strains showed a slightly better resistance when high bile salts concentrations where tested.
Beneficial aspects
Table 4 shows that the three Lactobacilli were able to produce diffusible inhibitory compounds against most of the indicator bacteria tested in the In Vitro assay, with a similar exception for E. fergusonii 16 and E. aburiae 27.
In the co-aggregation assays, L. reuteri MRS3 showed the best ability to aggregate and deposit with most of the bacterial strain tested (Table 4).
All the three LactoBacillus strains produced EPS onto MRS agar supplemented with sucrose, but not onto MRS added with glucose or lactose.
Biotechnological aspects
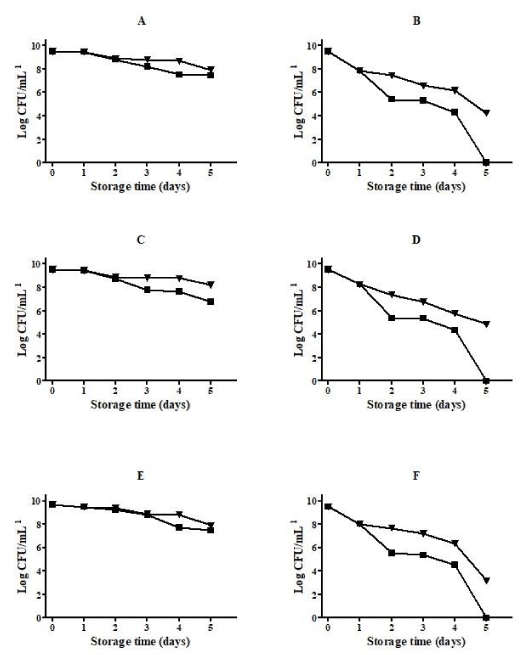
Table 5 shows a high resistance of the three Lactobacilli to the lyophilization process when skim milk was used as cryoprotectant.
On its lyophilized form, using skim milk as cryoprotectant, the three Lactobacilli maintained a similar high viability, in particular when storage was performed at 5°C and during the initial 60 days. As expected, when bacterial cells were lyophilized in saline solution, a high loss in viability was observed during the storage, particularly at 25°C after 90 days of storage (Figure 1).
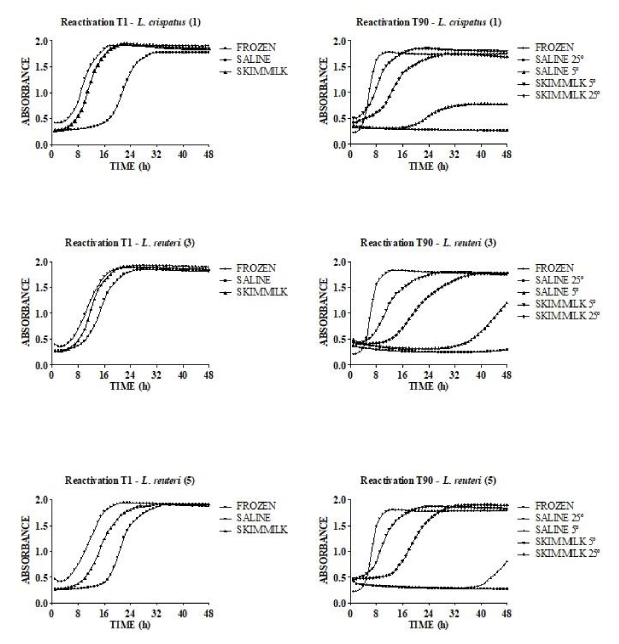
When the reactivation kinetic of the three Lactobacilli on their frozen or lyophilized forms was evaluated, a similar behavior was observed for the three Lactobacilli. During the storage, a decrease in reactivation speed was observed only for bacterial cells lyophilized in saline solution (Figure 2).
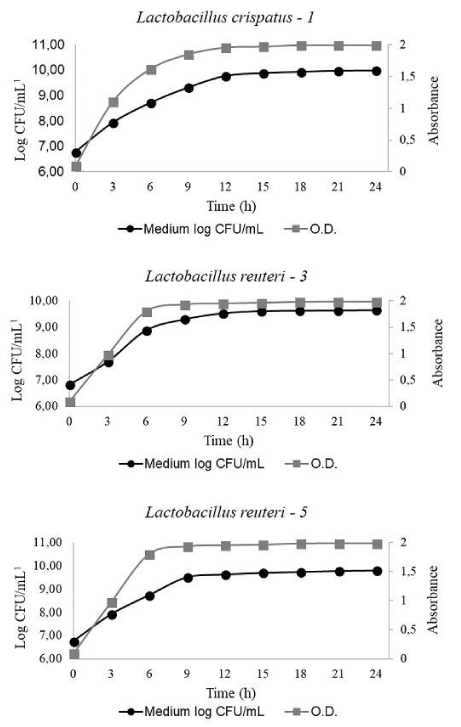
Figure 3 shows similar growth rates for the three LactoBacillus strains, with a stationary phase reached between 9 and 12 hours. During the growth, the culture pH evolved in a similar way for the three Lactobacilli from 5.5 to 4.2.
In Vivo trial
The results of egg weight, specific weight, shell percentage and shell thickness were not influenced by the treatments (p >0.05). Results of Haugh Units were influenced by treatments (p <0.05). Birds fed the PRO diet had better results when compared to the other two treatments (Table 6).
Discussion
Probiotics have been used in poultry for decades and have become common in the pet bird industry [17]. Benefits attributed to probiotic use in birds are disease prevention, promotion of growth, and reduction of excreta noxious gas emission [18]. With regards to disease prevention, probiotics can competitively exclude gut pathogen adhesion, modulate gastrointestinal immune responses, and produce metabolites that inhibit or kill pathogenic bacteria. For promotion of growth, probiotics can counteract dysbiosis, maintaining and replenishing normal microbiota balance, leading to normal nutrient absorption, especially after antibiotic therapy. Probiotics may also be able to modulate excreta noxious gas emission.
The search for new probiotic strains is driven by the growing demand for reducing the antimicrobials use in food-production animals. The initial selection criteria for a new probiotic strain include a series of In Vitro experiments which are used to evaluate safety (antibiotic susceptibility, hemolytic and gelatinase activities), functional (resistance to acidic pH and bile, hydrophobicity, auto-aggregation), beneficial (antagonism, EPS production and co-aggregation of pathogens) and technological (resistance to lyophilization and storage, reactivation time, growth parameters) characteristics of candidates to probiotic use. The present study was designed to select in a first stage and In Vitro a potentially probiotic LactoBacillus that was tested posteriorly in a field trial.
Safety aspects
The determination of hemolysin and gelatinase are pathogenic factors which have been widely used in determining the potential pathogenicity of microorganisms [19]. These extracellular compounds can cause host cell damage and degeneration and would facilitate in invading the host and establishing infection. These activities were not detected in the Lactobacilli tested.
Studies on antibiotic resistance of microorganisms used as probiotic agents are an area of growing concern [20]. In Europe, for example, the absence of acquired or transferable resistance factors must be established for a candidate probiotic in order to achieve Qualified Presumption of Safety (QPS) status recommended by the European Food Safety Authority [21]. It is believed that low antibiotic levels used for food-producing animals can promote the emergence of acquired antibiotic resistance in bacteria present in the intestinal microbiota. Then, the antibiotic-resistant bacteria can transfer the resistance factor to other pathogenic bacteria through the exchange of genetic material [22]. Despite their safety status, many Lactobacilli have been reported as being antibiotic resistant, and resistance to vancomycin is one of the best-characterized intrinsic mechanisms [23]. Most of the LactoBacillus is intrinsically resistant to gentamicin, kanamycin, streptomycin, neomycin, ciprofloxacin and trimethoprim, and sensitive to penicillin, chloramphenicol, tetracycline, and erythromycin [24]. The three Lactobacilli tested in the present study presented such pattern of susceptibility, except for its sensitivity to gentamicin.
Functional aspects
Survival of probiotic bacteria during passage through the gastrointestinal tract is an indispensable property to reach alive the intestine and provide potentially beneficial effect. For this reason, screening of potential probiotic is focused on the selection of acid and bile salts tolerant isolates. A study of Lee et al. [25], using real-time pH measurements, showed that pH can fluctuate between pH 0.6 and 3.8 in the gizzard of broilers depending on the type of diet. The study presently done indicated that the tested bacterial strains were resistant to pH 2.0 even after 3 hours of exposure. These results are similar with those obtained from other studies, where LactoBacillus strains were able to retain their viability when exposed to pH values of 2.5 [11,26]. However, it has also been observed that some other strains did not survive In Vitro in gastric juice with pH 2.0 for more than 15 min, but reached the colon in viable state and exerted a beneficial effect in In Vivo experiments. In these last cases, it is important to consider also the buffering capacity of the ingested food which can protect acid-sensitive strain during gastrointestinal transit.
Resistance to bile salts is generally considered as an essential property for probiotic strains to survive the conditions in the small intestine. Thus, it is necessary that efficient probiotic bacteria should be able to grow when exposed to the physiological concentrations of bile which range from 0.10 to 0.30% (w/v). The L. reuteri strains tested in the present study were quite resistant to high bile salts concentrations, and various mechanisms can be responsible for this characteristic. Bile Salt Hydrolase (BSH) genes are particularly abundant in lactic acid producing bacteria, such as Lactobacilli and bifidobacteria. Jones et al. [27] have determined that BSH enzymes are restricted to intestinal microorganisms, and this characteristic may be explained as an adaptive resistance to the antimicrobial nature of bile salts [28]. However, BSH activity can has a negative impact on host fat digestion and energy harvest, and for this reason, the impact of the BSH activity must be evaluated before the use of Lactobacilli or bifidobacteria as probiotics.
Beneficial aspects
The results of the antagonism assays showed that LactoBacillus bacteria originated from laying hens have growth-inhibiting properties against the bacterial poultry pathogens tested. This antagonistic effect depends on the type of pathogen and is probably due to the production of antimicrobial diffusible substances, such as organic acids, hydrogen peroxide, carbon peroxide, diacetyl, or bacteriocins [29].
Exopolysaccharides (EPS) are polymers synthesized in substantial amounts during fermentation by Lactic Acid Bacteria (LAB) and bifidobacteria [30]. In the present study, all the three Lactobacilli were able to produce EPS in medium containing sucrose. The literature has demonstrated that EPS produced by Lactobacilli may be responsible for their immunomodulatory actions, acting as immunostimulatory or immunosuppressive agents. Several carbohydrate recognition receptors located on the intestinal epithelium seems to be involved in the interaction between bacterial EPS and modulation of immunologic response [31]. Additionally, it has been demonstrated that EPS also play a role in the persistence of the producing bacteria in the intestinal tract [32].
Biotechnological aspects
In its metabolically active form, a probiotic maintain its viability during a relative short period (few months) and this is not a problem for probiotic administered as fermented or supplemented forms (yogurt, acidophilic milk) which have a short shelf life. However, for pharmaceutical products or probiotics to be incorporated in a ration, survival over long storage periods (some years) is necessary. In this case, the microorganism must be maintained alive but on a metabolically inactive form and for this lyophilization is one of the most efficient methods to be used [15]. But not all the microorganisms are able to resist to such a drastic process and an evaluation of the resistance to freeze-drying steps must be done. Addition of a cryoprotectant, such as skim milk, generally helps to maintain the cell viability during the lyophilization. In the present study, the three Lactobacilli showed a high resistance to lyophilization when skim milk was used, and maintain high viability levels during storage, even at room temperature.
Another desired biotechnological property for a probiotic in lyophilized form is a high speed of reactivation [16]. After ingestion, the microorganism must return to its metabolically active form as soon as possible to be active even in the upper parts of the digestive tract. In this way, L. reuteri MRS3 showed the best reactivation kinetic, even after a long storage period at room temperature.
Finally, to be economically viable, a probiotic candidate must present high growth yields. In the present study, the three Lactobacilli showed similar efficient growth curves that could allow rapid and high production of viable cells.
In Vivo trial
In the literature, studies using probiotic supplementations containing LactoBacillus spp., Bifidobacterium spp., Bacillus spp. and Enterococcus spp. reported contradictory results in relation to egg mass, production and quality when administered to laying hens. Yoruk et al. [33], Zeweil et al. [34], Panda et al. [35], Guo et al. [36] and Liu et al. [37] demonstrated that the Haugh unit was not influenced by feeding with probiotics, whereas eggshell strength increased significantly. On the other hand, Ma et al. [38] and Zhang et al. [39] demonstrated that egg quality greatly improved when laying hens were fed with probiotic-supplemented diets. The difference in results among these various studies with probiotics may be attributed to the difference in strains, trial period, or environment. In the present study, a clear improvement in internal quality of the egg was observed with the bacterial supplementation in the diet.
Conclusion
Following the subtractive In Vitro screening strategy, the strain L. crispatus MRS3 was selected based on promising probiotic properties without harmful characteristics. When ingested by laying hens in its lyophilized form added to the diet, this bacterium improved the internal quality of the eggs. In conclusion, L. crispatus MRS3 could be applied as a new probiotic strain in poultry feed supplements.
Acknowledgments
This study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
- The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA Journal. 2015; 13: 3991
- Regulation (EC) No 1831/2003 of the European Parliament and of the council of 22 September 2003 on additives for use in animal nutrition. OJL 268. 2003; 29-43. https://bit.ly/3ic2SXD
- Dec M, Puchalski A, Urban-Chmiel R, Wernicki A. 16S-ARDRA and MALDI-TOF mass spectrometry as tools for identification of LactoBacillus bacteria isolated from poultry. BMC Microbiology. 2016; 16: DOI: 10.1186/s12866-016-0732-5
- Park JW, Jeong JS, Lee SI, Kim IH. Effect of dietary supplementation with a probiotic (Enterococcus faecium) on production performance, excreta microflora, ammonia emission, and nutrient utilization in ISA brown laying hens. Poult Sci. 2016; 95: 2829-2835. DOI: 10.3382/ps/pew241
- Dastar B, Khosravi A, Boldajie F, Ghoorchi T. Effect of calcium with and without probiotic, lactose, or both on organ and body weights, immune response and caecal microbiota in moulted laying hens. J Anim Physiol Anim Nutr. 2016; 100: 243-250. DOI: 10.1111/jpn.12358
- Adhikari PA, Cosby DE, Cox NA, Lee JH, Kim WK. Effect of dietary bacteriophage supplementation on internal organs, fecal excretion, and ileal immune response in laying hens challenged by Salmonella Enteritidis. Poult Sci. 2017; 96: 3264-3271. DOI: 10.3382/ps/pex109
- Tang SGH, Sieo CC, Ramasamy K, Saad WZ, Wong HK, Ho YW. Performance, biochemical and haematological responses, and relative organ weights of laying hens fed diets supplemented with prebiotic, probiotic and symbiotic. BMC Vet Res. 2017; 13: 248. DOI: 10.1186/s12917-017-1160-y
- Adhikari PA, Lee JH, Cosby DE, Cox NA, Kim WK. Effect of probiotics on fecal excretion and immune gene expression in the ileum of laying hens challenged with Salmonella Enteritidis. Poult Sci. 2019; 98: 1235-1242. DOI: 10.3382/ps/pey443
- Yan FF, Murugesan GR, Cheng HW. Effects of probiotic supplementation on performance traits, bone mineralization, cecal microbial composition, cytokines and cortisone in laying hens. Animal. 2018; 13: 33-41. DOI: 10.1017/S175173111800109X
- Guidelines for the Evaluation of Probiotics in Food. Food and Agriculture Organization of the United Nations and World Health Organization (FAO/WHO). Expert Consultation Report. 2002; 1-11. https://bit.ly/2F2CCk9
- Yamazaki M, Ohtsu H, Yakabe Y, Kishima M, Abe H. In vitro screening of Lactobacilli isolated from chicken excreta to control Salmonella Enteritidisand Typhimurium. Br Poult Sci. 2012;53: 183-189. DOI: 10.1080/00071668.2012.678814
- Charteris A. Kelly PM, Morelli L, Collins JK. Antibiotic susceptibility of potentially probiotic LactoBacillus species. J Food Protec. 1998; 61: 1636-1643. DOI: 10.4315/0362-028x-61.12.1636
- Kos B, Suskovic J, Vukovic S, Simpraga M, Frece J, Matosic S. Adhesion and aggregation ability of probiotic strain LactoBacillus acidophilus M92. J Appl Microbiol. 2003; 94: 981-987. DOI: 10.1046/j.1365-2672.2003.01915.x
- Waldherr FW, Doll VM, Meissner D, Vogel RF. Identification and characterization of a glucan-producing enzyme from LactoBacillus hilgardii TMW 1.828 involved in granule formation of water kefir. Food Microbiol. 2010; 27: 672-678. https://bit.ly/32aXePE
- Bolla PA, Serradell MA, Urraza PJ, Antoni GL. Effect of freeze-drying on viability and in-vitro probiotic properties of a mixture of lactic acid bacteria and yeasts isolated from kefir. J Dairy Res. 2011; 78: 15-22. DOI: 10.1017/S0022029910000610
- Martins FS, Veloso LC, Arantes RME, Nicoli JR. Effects of yeast probiotic formulation on viability, revival and protection against infection with Salmonella enterica subsp. enterica serovar Typhimurium in mice. Lett Appl Microbiol. 2009;49: 738-744. DOI: 10.1111/j.1472-765X.2009.02732.x
- Smith JM. A review of avian probiotics. J Avian Med Surg. 2014; 28: 87-94. DOI: 10.1647/2012-031
- Zhang ZF, Kim IH. Effects of probiotic supplementation in different energy and nutrient density diets on performance, egg quality, excreta microflora, excreta noxious gas emission, and serum cholesterol concentrations in laying hens. J Anim Sci. 2013; 91: 4781-4787. DOI: 10.2527/jas.2013-6484
- Poeta P, Costa D, Klibi N, Rodrigues J, Torres C. Phenotypic and genotypic study of gelatinase and beta-haemolysis activities in faecal enterococci of poultry in Portugal. J Vet Med B Infect Dis Vet Public Health. 2006; 53: 203-208. DOI: 10.1111/j.1439-0450.2006.00941.x
- Campedelli I, Mathur H, Salvetti E, Clarke S, Rea MC, Torriani S, et al. Genus-wide assessment of antibiotic resistance in LactoBacillus spp. Appl Environ Microbiol. 2018; 85: 01738-18. DOI: 10.1128/AEM.01738-18
- Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012; 10: 2740 -2750. https://bit.ly/3k2uoaC
- Hummel AS, Hertel C, Holzapfel WH, Franz C. Antibiotic resistances of starter and probiotic strains of lactic acid bacteria. Appl Environ Microbiol. 2007; 73: 730-739. DOI: 10.1128/AEM.02105-06
- Goldstein E, Tyrrell KL, Citron DM. LactoBacillus species: Taxonomic complexity and controversial susceptibilities. Clin Infect Dis. 2015; 60: 98 -107. DOI: 10.1093/cid/civ072
- Abriouel H, Casado Muñoz MDC, Lavilla Lerma L, Perez Montoro B, Bockelmann W, et al. New insights in antibiotic resistance of LactoBacillus species from fermented foods. Food Res Int. 2015; 78: 465-481. DOI: 10.1016/j.foodres.2015.09.016
- Lee SA, Dunne J, Mottram T, Bedford MR. Effect of diet phase change, dietary Ca and P level and phytase on bird performance and real-time gizzard pH measurements. Br Poultry Sci. 2017; 58: 290-297. DOI: 10.1080/00071668.2017.1293799
- Van Coillie E, Goris J, Cleenwerck I, Grijspeerdt K, Botteldoorn N, Van Immerseel F, et al. Identification of Lactobacilli isolated from the cloaca and vagina of laying hens and characterization for potential use as probiotics to control Salmonella Enteritidis. J Appl Microbiol. 2007; 102: 1095-106. DOI: 10.1111/j.1365-2672.2006.03164.x
- Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. PNAS. 2008; 105: 13580-13585. https://bit.ly/3m3Ot1X
- Begley M, Hill C, Gahan CG. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol. 2006; 72: 1729-1738. DOI: 10.1128/AEM.72.3.1729-1738.2006
- Servin AL. Antagonistic activities of Lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004; 28: 405-440. DOI: 10.1016/j.femsre.2004.01.003
- Ryan PM, Ross RP, Fitzgerald GF, Caplice NM, Stanton C. Sugar-coated: Exopolysaccharide producing lactic acid bacteria for food and human health applications. Funct Food. 2015; 6: 679-693. DOI: 10.1039/c4fo00529e
- Laiño J, Villena J, Kanmani P, Kitazawa H. Immunoregulatory effects triggered by lactic acid bacteria exopolysaccharides: New insights into molecular interactions with host cells. Microorganisms. 2016; 4: 27. DOI: 10.3390/microorganisms4030027
- Castro-Bravo N, Wells JM, Margolles A, Ruas-Madiedo P. Interactions of surface exopolysaccharides from Bifidobacterium and LactoBacillus within the intestinal environment. Front Microbiol. 2018; 9: 2426. DOI: 10.3389/fmicb.2018.02426
- Yoruk M, Gul M, Hayirli A, Macit M. The effects of supplementation of humate and probiotic on egg production and quality parameters during the late laying period in hens. Poult Sci. 2004;83: 84-88. DOI: 10.1093/ps/83.1.84
- Zeweil H, Genedy S, Bassiouni M. Effect of probiotic and medicinal plant supplements on the production and egg quality of laying Japanese quail hens. Proceeding of the 12th European poultry conference. SWANS. 2006; 1-6. https://bit.ly/2GJWA3B
- Panda AK, Rama Rao SS, Raju MV, Sharma SS. Effect of probiotic (LactoBacillus sporogenes) feeding on egg production and quality, yolk cholesterol and humoral immune response of White Leghorn layer breeders. J Sci Food Agri. 2008; 88: 43-47. https://bit.ly/3bFeMXh
- Guo JR, Dong XF, Liu S, Tong JM. Effects of long-term Bacillus subtilis CGMCC 1.921 supplementation on performance, egg quality, and fecal and cecal microbiota of laying hens. Poult Sci. 2017; 96: 1280-1289. DOI: 10.3382/ps/pew389
- Liu X, Peng C, Qu X, Guo S, Chen JF, He C , et al. Effects of Bacillus subtilis C-3102 on production, hatching performance, egg quality, serum antioxidant capacity and immune response of laying breeders. Anim Physiol Anim Nutr. 2019; 103: 182-190. https://bit.ly/3hc9GmJ
- Ma Q, Gao X, Zhou T, Zhao L, Fan Y, Li X, et al. Protective effect of Bacillus subtilis ANSB060 on egg quality, biochemical and histopathological changes in layers exposed to aflatoxin B1. Poult Sci. 2012; 91: 2852-2857. DOI: 10.3382/ps.2012-02474
- Zhang J, Xie Q, Ji J, Yang W, Wu Y, Li C, et al. Different combinations of probiotics improve the production performance, egg quality, and immune response of layer hens. Poult Sci, 2012;91: 2755-2760. DOI: 10.3382/ps.2012-02339

Sign up for Article Alerts