Effect of Inclusion Levels of Arachis glabrata in the Diets on the Ingestion and In Vivo Digestibility of Panicum maximum in Guinea Pigs (Cavia porcellus)
Emile Miegoue1*, Fernand Tendonkeng1, Nathalie Mweugang Ngouopo2, Jules Lemoufouet1, Martin Vidal Tatang1, Paulette Ntsafack1, Mouchili Mama1 and Etienne Tedonkeng Pamo1
1Department of Animal Science, Nasarawa State University, Keffi, Nigeria
2University of Ngaoundere, Faculty of Sciences, Department of Animal Science
*Address for Correspondence: Emile Miegoue, University of Dschang, Faculty of Agronomy and Agricultural Sciences, Department of Animal Production Animal Nutrition and production Research Unit B.P. 222 Dschang, Cameroon, Tel: +237-697-121-2 97 / 674 -218-1 74; E-mail: migoumile@yahoo.fr; emile.miegoue@univ-dschang.org
Submitted: 09 November 2018; Approved: 28 November 2018; Published: 01 December 2018
Citation this article: Miegoue E, Tendonkeng F, Ngouopo NM, Lemoufouet J, Tatang MV, et al. Effect of inclusion levels of arachis glabrata in the diets on the ingestion and in vivo digestibility of panicum maximum in guinea pigs (cavia porcellus). Int J Vet Sci Technol. 2018;2(2): 026-032.
Copyright: © 2018 Miegoue E, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Panicum maximum; Arachis glabrata; Guinea pigs; Ingestion; In vivo digestibility
Download Fulltext PDF
This study, which evaluated the effect of graded levels of Arachis glabrata on the ingesting and in vivo digestibility of Panicum maximum and on the caecal rate of bacterial flora, was conducted at the Research and Application Farm of the University of Dschang between July and August 2016. For this trial, 40 adult guinea pigs of local breed including 20 animals per sex, aged about 04 months and weighing between 288 and 374 g, were purchased from breeders in the city of Dschang and its surroundings. Guinea pigs were first acclimatized for 2 weeks in the farm's dressing boxes before being randomly distributed in the individual digestibility cages. After 10 days of adaptation, animals were distributed in 04 batches (T0, T1, T2 and T3) of 10 animals (5 males and 5 females) each then subjected to 04 iso-nitrogenous rations (16% of proteins) containing graded levels of A. glabrata. Animals in each batch received 250g of Panicum maximum associated with 60g of compound food containing 0, 10, 15 or 20% of A. glabrata respectively for lots T0, T1, T2 and T3. During the 07day of digestibility test, each animal's feces were collected in labeled envelopes and weighed every morning before a re-distribution of diet. P Maximum and nutrients ingestion (160.3 g DM / day / animal) in males and regardless of sex was significantly (P < 0.05) higher with diet containing A. glabrata compared to control diet. In contrast, ingestion of concentrate containing legume was significantly (P < 0.05) lower than that of the control diet, regardless of sex. However, females had significantly (P < 0.05) better ingested DM and OM of the control diet than males whose ingestion of these nutrients with other diets was better (P < 0.05). In addition, intake of CP and CF from all rations did not significantly (P > 0.05) varied between the two sexes. Nutrient digestion was not significantly influenced (P > 0.05) by sex and as inclusion level of A glabrata in the diet. The level of bacteria in the caecal flora after the digestibility test was not modified (P > 0.05) by the inclusion level of A glabrata in the diet. Nevertheless, the diet containing 20% of A. glabrata was the most favorable for the development of Lactobacilli at the expense of Enterobacteria.
Introduction
Food security is a real challenge in most African countries where people suffer from protein malnutrition [1]. Emerging challenges such as climate change, environmental sustainability and rapidly evolving technologies are transforming food systems and raising questions about how to sustainably meet the food needs of a growing world population [2]. Average animal protein consumption in Africa is less than one quarter of that in the Americas, Europe and Oceania, and represents only 17% of the recommended level of consumption for all proteins [3]. The same author reported that livestock and livestock products can make an essential contribution to the economic and food security of low-income households and their nutrition. According to Noumbissi et al. [4], setting up short-cycle farms like caviaculture proves to be one of the durable solutions, given the major interest of the guinea pig living in its prolificacy, its high growth rate and its lean meat rich in protein.
However, food is the main limiting factor for the expression of the production potential of animals in tropical environments [5]. Indeed, the high price of compound food used as a protein supplement, in addition to raising the cost of production, does not always make it possible to obtain good productivity [6]. However, to minimize feed costs for farmed guinea pigs, suggested maximizing feed intake and diet concentrates of vitamin and vitamin supplements (CMV) [7].
In this logic, several studies have been conducted on the use of Arachis glabrata in the guinea pig diet. Indeed, the work of Miegoue et al. [8] and Miegoue et al. [9] confirmed the good use of this legume in ingesting and in vivo digestibility of Pennisetum purpureum or Panicum maximum in guinea pigs. These authors have reported that an adequate association of local forages (grasses and legumes) can constitute an undeniable food base for the growth of domestic guinea pigs with regard to their strictly herbivorous diet. However, supplementation makes it possible to compensate for deficits in nutritional principles that the simple increase in the level of forage intake cannot compensate [10].
In addition, the digestibility of the wall of plant matter is highly dependent on the action of cecal microorganisms [11], which can digest fibrous foods in the same way as polygastrics [7].
However, very little work has been done on an assessment of the level of inclusion of Arachis glabrata on grass digestibility. In order to contribute to a better use of Arachis glabrata in the guinea pig diet, the present work consisted in evaluating the effect of different levels of Arachis glabrata in the diet on the ingestion and digestibility of Panicum maximum and bacterial flora of cecum in guinea pigs.
Materials and Methods
The study took place between August and September 2016 at the Research and Application Farm (RAF) of University of Dschang. The locality is located between 5° 25' and 5° 30' North Latitude, 10° 0' and 10° 05' East Longitude and at an altitude of about 1420 m west of Cameroon. The climate of the region is equatorial of Cameroonian type, with an average annual temperature of 20°C. The months of July and August are the coldest. The average annual rainfall varies between 1500 and 2000 mm, with a relative humidity ranging between 40% (in the dry season) and 97% (during the heavy rains). The dry season alternates with the rainy season [12].
Housing and animal sample
The walls and floor of the breeding lodges as well as the breeding equipment (feeders, drinking troughs, and digestibility cages) were previously cleaned and disinfected with a solution of sodium hypochlorite and potassium permanganate. The realization of the crawl space for two weeks preceded the introduction of guinea pigs in the breeding unit reserved for this purpose, Forty (40) local adult guinea pigs including 20 females and 20 males of mean age 04 months and mean weight 331 ± 42.86 g were used for this test. These animals were purchased from stockbreeders in the town of Dschang and surrounding areas. The guinea pigs were acclimatized on the farm for two (02) weeks in breeding lodges, males isolated from females. Guinea pigs were raised on the ground on untreated dry wood chip litter and renewed every 2 days to prevent the accumulation of feces and urine. Each lodge was equipped with a lighting device and 2 concrete drinkers in which the drinking water enriched with vitamin C at a rate of 250 mg tablet for 1.5 liters, was available ad libitum and renewed daily. It is after this period of acclimatization that animals were introduced into individual cages of digestibility to be subjected to different tests.
Plant sample
The grass (Panicum maximum) was harvested before flowering in the forage plot of the farm the day before and pre-washed before being served the next day to the animals. The leaves of the legume (Arachis glabrata) were harvested before flowering, dried and then crushed and incorporated into the feed. A 100 g sample of each plant dried at 60°C in an oven to a constant weight was milled and stored in plastic bags for chemical composition evaluation [13] as is shown in table 2.
Conduct of the test
Formulation of different diet: The combination of the ingredients, the proportions of which are presented in table 1, made it possible to formulate four (04) iso-nitrogen diets (16% CP).
Evaluation of the ingestion: The present digestibility test was preceded by a 10-day period of animal adaptation to the digestibility cage and experimental rations. During this period, the amount of feed was adjusted to the estimated daily feed consumption of 60 g and the animals, randomly distributed in individual cages, continued to receive vitamin C in the drinking water renewed daily.
During the trial, each treatment was repeated over ten (10) guinea pigs, five (05) of each sex.
For evaluation of ingestion, 250 grams of Panicum maximum associated with 60 grams of compound food (CA0, CA10, CA15 or CA20) were served once a day every day between 8 and 9 hours per animal and refusals were collected and weighed before any new distribution.
Assessment of digestibility
The digestibility test was preceded by a 10-day period of animal adaptation to the digestibility cage and experimental diet. To evaluate the digestibility that lasted seven (07) days, feces were collected and weighed daily in the morning before any further distribution of the diet and a sample of one hundred (100) grams of feces was then collected and dried at 60°C in an oven to constant weight. Subsequently, the dried feces were crushed using a home-made mill and stored in plastic bags for evaluation of their Dry Matter (DM), Organic Matter (OM), Crude Protein (CP) and Crude Cellulose (CF) according to the method described by AOAC [13].
Analysis of caecal flora
At the end of the digestibility test, 03 randomly selected guinea pigs of each sex and treatment were sacrificed for evaluation of the composition of the caecal flora (Enterobacteria and Lactobacilli) according to the method described [14].
Statistical analyzes
Data on dietary intake and nutrient digestibility, as well as on the caecal flora were subjected to the 2-factor analysis of the variance (food ration and sex of the animal) according to the General Linear Model (MLG).
When significant differences existed between treatments, the separation of means was done by the Waller Duncan test at the 5% threshold [15]. The statistical analysis software used was SPSS 20.0. The comparison between the sexes was made by Student's "t" test at the 5% threshold.
Results
Chemical composition of fodder and different diets
The chemical composition of these forages revealed that the dry matter, organic matter and crude protein contents of Arachis glabrata were higher than those of Panicum maximum (Table 2). On the other hand, the crude fiber and ash content of the legume was rather low compared to that of the grass.
The table 3 shows dry matter, organic matter, crude protein, digestible energy and ash contents of different diet. It showed very little variation between diets. In contrast, the fat content of the control was higher (8.74% DM) than the mean value of that obtained with diets containing Arachis glabrata (4.58% DM). The crude fiber content of the legume-free diet was lower than the values obtained with other diets.
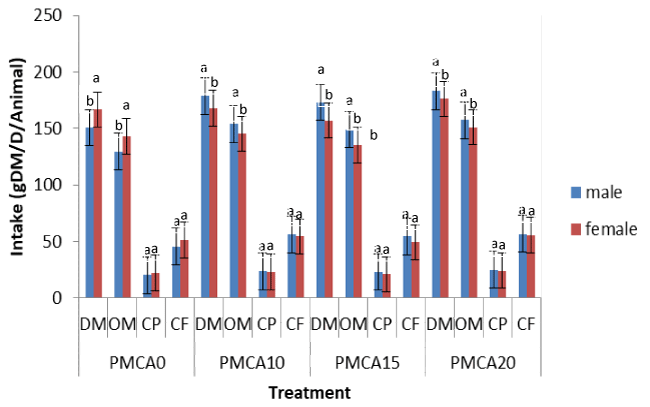
Effect of inclusion level of Arachis glabrata in the diet on ingestion of Panicum maximum in guinea pigs
In male guinea pigs and regardless of sex (Table 4), ingestion of Panicum maximum, compound feed, total Dry Matter (DM), Organic Matter (OM), Crude Protein (CP) and Crude Fiber (CF) did not vary (p > 0.05) among A. glabrata-containing diets but significantly (p < 0.05) higher than that of the control diet. Compared with the legume-free diet, intake of the diet consisting of A. glabrata-containing diets was significantly (p < 0.05) lower than that of the control diet. The highest intake of DM (179.5 g DM/ animal/ day), OM (154.3 g DM/ animal/ day), CP (24.7 g DM/ animal/ day) and CF (56.1 g DM/ animal/ day) was obtained with the diet containing 20% A. glabrata.
Effect of sex on nutrient ingestion in guinea pigs fed with P. maximum associated with diets containing different levels of A. glabrata
It is apparent from figure 1 that sex significantly influenced (p < 0.05) ingestion of DM and OM in favor of females with the legume-free diet, whereas with diet containing A. glabrata, males are those who had better ingested the food. In contrast, CP and CF of all diets were ingested in the same manner (p > 0.05) between males and females.
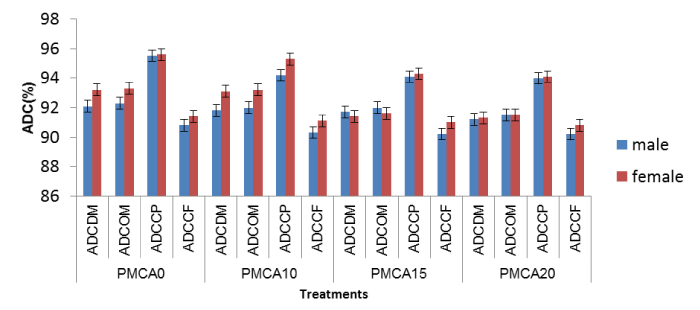
Effect of inclusion level of Arachis glabrata in the diet on Apparent Digestibility Coefficients (ADC) of nutrients in guinea pigs fed with P. maximum
The inclusion level of Arachis glabrata in the diet had no significant effect (p > 0.05) on digestive utilization of nutrients in males or regardless of the sex in guinea pigs fed with P. maximum (Table 5). It was with the control diet that the highest ADC values of DM (92.6 g DM/ animal/ day), OM (92.8 g DM/ animal/ day), CP (95.6 g DM/ animal/ j) and CF (91.1 g DM/ animal/ day) were recorded.
Effect of sex on apparent digestibility coefficients of nutrients in guinea pigs fed P. maximum associated with diets containing different levels of Arachis glabrata
Apparent Digestibility Coefficients (ADC) of nutrients were not significantly influenced (p > 0.05) by sex when guinea pigs were fed with P. maximum regardless of diet (Figure 2).
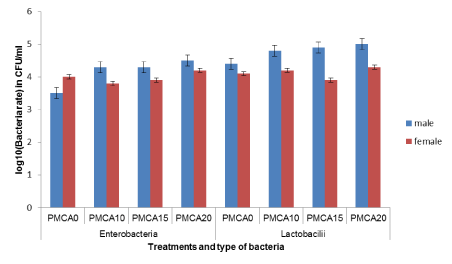
Effect of the level of inclusion of Arachis glabrata in diet on the caecal rate of Enterobacteria or Lactobacillus in the bacterial flora of guinea pigs fed with P. maximum
The rate of Enterobacteria and Lactobacilli in guinea pigs fed P. maximum did not significantly (p > 0.05) change with the inclusion level of A. glabrata in the diet (Table 6). The highest rate of Enterobacteria (4.3 CFU/ ml) and Lactobacilli (4.6 CFU/ ml) was obtained with the 20% A glabrata diet. Enterobacteria levels were comparable (p > 0.05) to those of Lactobacilli in guinea pigs fed with P. maximum regardless of diet (Figure 3). Comparison of the levels of these bacteria between males and females revealed no significant difference (Figure 4).
Discussion
Effect of inclusion level of Arachis glabrata in diet, on ingestion of Panicum maximum in guinea pigs
Compared with the control diet, P. maximum ingestion (160.3 g DM/ animal/ d) was significantly higher with A. glabrata-containing diets. This shows that the protein intake of this legume improved the palatability of guinea pigs. Moreover, several works by kouakou et al. [10]; Miegoue et al. [8] and Miegoue et al. [9] illustrate this observation.
The best intake of these grasses obtained with the ration containing 20% of A. glabrata is explained by the fact that protein supplements of plant origin (more adapted to the herbivore diet of guinea pigs) would promote a sufficient proliferation of intestinal microorganisms involved in digestion in herbivores. This would favor the increase of food fermentation and digestive transit which would thus de-clutter the caecum with the consequent increase in food intake (Kouakou et al. [10], Ramirez-Riviera et al. [16], Dougnon et al. [17].
The low intake of the compound feed in rations containing the legume compared to the control diet could be explained by the substitution phenomenon illustrated [18]. In fact, when a food supplementing self-service feed is distributed separately, a portion of the supplement is substituted for the staple food and occupies part of the digestive tract of the animal which would mean that the presence of A. glabrata in the diet increased the palatability of P. maximum in the animal that has more ingested the grass at the expense of the compound feed. Moreover the inclusion of A. glabrata in the diet would have increased its capacity for congestion and satiety in the guinea pig, thus reducing ingestion of rations containing this legume contrary to the control diet. The level of intake of total Dry Matter (DM) and Organic Matter (OM) from A. glabrata-containing diets higher in male guinea pigs compared females could be explained by the fact that in general in adulthood, males have a high weight compared to that of females. As a result, they tend to ingest more food because food intake is very often correlated with the weight of the animal. Indeed according to many authors, dietary intake in male guinea pigs is higher than that in females Noumbissi et al. [6], Miegoue et al. [8].
Effect of inclusion level of Arachis glabrata in the diet on the digestibility of Panicum maximum in guinea pigs
The lack of a significant difference between the Apparent Digestibility Coefficients (ADC) of nutrients from different diets in guinea pigs fed P. maximum could be explained by the fact that the diets used in this trial were iso nitrogenated and of little variable chemical composition. Indeed, according [18], diet is the factor that has the strongest influence on digestibility [19]. Also reported that one of the environmental factors that seems most likely to modify the bacterial colonization process of the gastrointestinal tract is the food ingested by the host. Digestibility will therefore be affected by the quality and physical state of the ration, that is to say the form in which the food is presented to the animal as much as its chemical composition. These factors condition the action of the microbial flora and digestive juices. The rations served to guinea pigs were in powder form, which favored both ingestion and digestive utilization of the nutrients.
Effect of inclusion level of Arachis glabrata in rations on the caecal rate of Enterobacteria or Lactobacillus bacterial flora in guinea pigs fed P. maximum
The level of inclusion of Arachis glabrata in diet had no significant effect on the variation of bacterial flora in the enteric bacterial and lactobacilli species in guinea pigs. This shows a certain balance between this bacterial population. Indeed, According Losson [20] the balance of this flora is unstable, easily altered by abrupt food change or the administration of a narrow-spectrum antibiotic Gram (+), which can lead to enteropathies. The absence of variation of the flora would therefore mean that the inclusion of Arachis glabrata in the experimental rations did not alter the composition of the flora and consequently the digestive use of the nutrients. Similar results were obtained by Miegoue et al. [8] who had shown that the inclusion of legumes in the guinea pig diet did not significantly affect the caecal flora. Diets thus composed were therefore adapted to guinea pig feeding. Compared with the rate of Enterobacteria (Gram -), Lactobacilli (Gram +) were the most numerous, whatever the diet used. This result reflects the quality of the experimental foods. In fact, according to Andreu and Lormeau most of the caecal microbial population is predominantly composed of Gram (+) and anaerobic organisms, Gram (-) being present in a smaller quantity [21-23].
Conclusion
Evaluation of the effect of different dietary supplementation levels with Arachis glabrata on the in vivo digestibility of Panicum maximum in guinea pigs showed that:
- The ration containing 20% of Arachis glabrata significantly improved the intake of Panicum maximum. Males had better ingested DM and OM of diet containing legumes than females.
- Inclusion levels of Arachis glabrata in the diet did not improve digestive utilization of nutrients ingested by guinea pigs.
- Cecal flora was not significantly affected by the inclusion level of Arachis glabrata when guinea pigs were fed with P. maximum.
Thus, Arachis glabrata can be used up to 20% in cavies diet without any in food intake or utilization.
- Niba AT, Meutchieye F, Fon D, Laisin AG, Taboh H, Njakoi H, et al. Current situation of cavy production in Cameroon: Challenges and opportunities. Livestock Research for Rural. 2012 ; 24. https://goo.gl/iVk5Tw
- FAO. FAO rolle in nutrition. 2017.
- FAO. Livestock farming and food security. 2011.
- Noumbissi M, Tendonkeng F, Zougou T, Miegoue E, Lemoufouet J, Boukila B, et al. Effect of the complementation with Tithonia diversifolia on the evolution of post-partum weight and pre-weaning growth of cavies (Cavia porcellus L.). Livestock Research for Rural Development. 2013; 25. https://goo.gl/tRa4Nj
- Tendonkeng F, Boukila B, Pamo TE, Mboko AV, Zogang FB. et Matumuini NEF. 2011. Direct and residuel Effects of differents level of Nitrogen fertilisation on the chemical composition of Brachiaria ruziziensis at the flowering stage in the west Cameroon. International Journal of Biological and Chemical Sciences.2011; 5: 570-585. https://goo.gl/dPHYCL
- Noumbissi MNB, Tendonkeng F, Zougou TG. et Pamo TE. Effect of different supplementation level of Tithonia diversifolia Hemsl. Leaves on the ingestion and the in vivo digestiblity of Penissetum purpureum K. Schum. On cavy (Cavia porcellus L). Tropicultura. 2014 ; 3: 138-146.
- Bindelle J. et Picron P. The cavy : a small herbivorous animal easy to feed in small plots. Troupeaux et Cultures des Tropiques : Special rodents breeding, Kinshasa. RDC. CAVTK. 2013; 1-10.
- Miegoue E, Tendonkeng F, Lemoufouet J, Mweugang Ngouopo N, Noumbissi M, Fogang MD, et al. intake and digestibility of Pennisetum purpureum associate to one legume (Arachis glabrata, Calliandra calothyrsus ou Desmodium intortum) as protein source on cavy. Livestock Research for Rural Development. 2016a; 28. https://goo.gl/e76n7E
- Miegoue E, Tendonkeng F, Lemoufouet J, Noumbissi M N B, Mweugang N N, Zougou TG,et al. pre-weaning growth of cavies fed on Panicum maximum supplemented with diet containing Arachis glabrata, Calliandra calothyrsus or Desmodium intortum. Int J Biol Chem Sci. 2016b; 10: 313-325.https://goo.gl/PpsTts
- Kouakou N’GDV, Thys E, Assidjo EN. et Grongnet JF. Ingestion and in vivo digestibility of Panicum maximum associed to three complements: Jatropha curcas seed cake, tourteau de coton seed cake (Gossypium hirsutum) and Euphorbia heterophylla on cavy (Cavia porcellus L.). Tropicultura. 2010; 28: 173-177. https://goo.gl/xtUkub
- Michelland R, Combes S, Monteils V, Bayourthe C, Cauquil L, Enjalbert F, et al. compare Analyzis of digestive ecosystems of rumen of caw and rabbits caecum. Prod Anim. 2012 ; 25 : 395-406. https://goo.gl/76n8pf
- Pamo TE, Niba A T, Fonteh FA, Tendonkeng F, Kana JR, Boukila B. et al. Effect of the supplementation with Moringa oleifera or with multinutritionnals blocks on the evolution of post-partum weight and pre-weaning growth of cavies (Cavia porcellus L.). Livestock Research for Rural Development. 2005; 17. https://goo.gl/fJhSp7
- AOAC. (Association of Official Analytical Chemist). 15th edition. Washington DC. Official method of analysis.1990; 10.
- Benson HJ. Microbiological applications. Laboratory manual in general microbiology. Complete version. Eighth edition. Mc. Graw Hill higher Education International edition ISBN 0-07-112169-2. 2002; 478.
- Steele R G, Torrie JH. Principles and procedures of statistics. Mc Graws Hill Book C. 1980; 633.
- Ramirez-Rivera U, Sangines-Garcia JR, Escobedo-Mex JG, Cen-Chuc F, Rivera-Lorca JA, Lara-Lara PE. Effect of diet inclusion of Tithonia diversifolia on feed intake, digestibility and nitrogen balance in tropical sheep. Agroforest System. 2010 ; 80: 295-302. https://goo.gl/pTvkSg
- Dougnon TJ, Aboh BA, Kpodekon TM. Ingestion, digestibility and ponderal growth on crictomies (Cricetomys gambianus) fed on Moringa oleifera and Leucaena leucocephala based concentrate. Livestock Research for Rural Development. 2011; 23: 15-27. https://goo.gl/roFGhW
- Rivière R. Domestic ruminants feeding Manual in tropical milieu. manual and precise of breeding Collection. Ministery of cooperation and development. 1991 ; 529.
- Rainbaud P. et Ducluzeau R. Study of bacterian colonization bactérienne gastro-intestinal tract using experimental model. Rev. Sc. Tech. off. Int. Epiz. 1989; 8: 361-373. https://goo.gl/7wR9ky
- Losson B. The cavy and his principales deseases. herd and Cultures of Tropics : Special rodents breeding, Kinshasa, RDC. Laboratory of Parasitology, Faculty of veterinary Medecine, University of Liège, Bd de Colonster 20 Bât. B43, 4000 Liège, Belgique. Theisis. Ecole Nationale Vétérinaire d’Alfort, Alfort. 2013; 122.
- Andreu de Lapierre E. The cavy. Medecine pratique Dictionary of NCA, Ed. MED’COM. 2001; 18-29.
- Lormeau E. Contribution of the study of Cavia porcellus (Linne, 1758): Atlas of radiographiez and osteology. Exercice Theisis, veterinary Medecine, Toulouse. 2010; 207.
- Pamo T E, Niba A T, Fonteh FA, Tendonkeng F, Kana JR, Boukila B,et al. Effect of the supplementation with Moringa oleifera or with multinutritionnals blocks on the evolution of post-partum weight and pre-weaning growth of cavies (Cavia porcellus L.). Livestock Research for Rural Development. 2005; 17. https://goo.gl/6fqXEE


Sign up for Article Alerts