Effect of Dietary Acidolysis-Oxidized Konjac Glucomannan (A-OKGM) on Schizothorax Prenanti Immunity and Expression of Immune-Related Genes
Ming Rui Chen, Ying Long Wu*, Mei He, Zhen Zhen Lv, Jia Qi Zhao, Qiu Ping Yan and Li Mei Feng
College of food science, Sichuan Agricultural University, Yaan, 625014 Sichuan, PR China
*Address for Correspondence: Yinglong Wu, College of Food Science, Sichuan Agricultural University, No. 46, XinKang Road, Yaan, 625014 Sichuan, PR, Tel: +86 (0) 835 2882111; E-mail: [email protected]/[email protected]
Submitted: 02 September 2017; Approved: 30 October 2017; Published: 31 October 2017
Citation this article: Chen MR, Wu YL, He M, Lv ZZ, Zhao JQ, et al. Effect of Dietary Acidolysis-Oxidized Konjac Glucomannan (A-OKGM) on Schizothorax Prenanti Immunity and Expression of Immune-Related Genes. Int J Vet Sci Technol. 2017;1(2): 040-047.
Copyright: © 2017 Wu YL, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Schizothorax Prenanti; Immunity; Acidolysis-Oxidized Konjac Glucomannan; Interleukin-1β; Interleukin-6; Tumor Necrosis Factor α
Download Fulltext PDF
The present study was conducted to investigate the effects of dietary Acidolysis-Oxidized Konjac Glucomannan (A-OKGM) (0, 0.4, 0.8 and 1.6 %) supplementation on the immunity and immune related genes expression of Schizothorax prenanti. The C - Reactive Protein (CRP) level was significantly higher than that in control group, regardless of the inclusion level. Similarly, the IgM level was significantly increased by all A-OKGM diets. The lysozyme activity in fish fed with 1.6% A-OKGM was markedly different from other groups. As for immune organ index, the 1.6% A-OKGM diet group significantly increased the spleen index. The liver index in fish fed with 0.8% A-OKGM diet was significantly higher than that of control group. The 0.4% and 1.6% A-OKGM diet groups significantly up-regulated TNFα gene expression in anterior gut. While the 0.8% and 1.6% A-OKGM diet groups also showed a significantly enhanced TNFα mRNA expression in mid and distal gut. The IL-1β gene expression in fish fed with 0.4% and 1.6% A-OKGM diet was significantly enhanced in anterior gut. The 0.8% and 1.6% A-OKGM diets significantly up-regualted the expression of IL-1β in distal gut. In a same pattern, the IL-6 mRNA level in 0.4% and 1.6% diet groups was significantly higher in anterior gut. The 0.8% and 1.6% A-OKGM diet groups significantly induced the IL-6 gene expression in distal gut. Present study suggested that A-OKGM can act as immunostimulant to improve the immune indexes and up-regulate the immune-related gene expressions.
Introduction
Schizothorax prenanti, also known as ‘ya fish’ (Cypriniformes, Cyprinidae, Schizothoracinae), is an endemic cold fish in southwest part of China. It mainly reproduces in upper reaches of Yangtze River and Hanjiang River. S. prenanti is considered to be a good candidate for freshwater culture in China due to its delicious meat and high market value. However, S. prenanti is susceptible to different kinds of diseases, such as motile aeromonad septicaemia [1], which can affect the quality of fish. Recently, infected fish has been reported to be a problem in some fish farms. Hence, finding a solution to improve the intrinsic immunity of fish is urgent.
The intestine, being a multifunctional organ central to both nutrient uptake, pathogen recognition, has not received as much attention as the well documented primary and secondary immune tissues such as spleen, head kidney and liver during disease response and immune stimulation studies [2]. However, the research to the intestinal immunity of fish is of special interest for the fish farming industry for a number of reasons. Firstly, farmed fish kept at high breeding densities are susceptible to intestinal infections, with the gut being an important entry point for pathogens [3]. Secondly, gut microbiota which is a potential factor to modulate fish pathogens are likely to respond to dietary manipulations [4-6]. Thirdly, farmed fish are typically fed commercial pelleted feeds, which opens up gate for manipulating intestine health through the incorporation of various feed additives into the feed. Hence, a comprehensive understanding of the diet-gut interactions could aid in the development of new strategies to prevent and treat their multiple infectious diseases.
Konjac Glucomannan (KGM) which existed in the tuber of Amorphophallus konjac is a kind of prebiotics. Up to date, many authors have studied the effects of prebiotics on immunity of fish [7,8,9]. However, high molecular weight (500-2000 kDa) and viscosity of KGM impede the its applications in aquafeeds industry. So we produced Oxidized Konjac Glucomannan (OKGM). It is an oxidative degradation product of KGM with its higher purity and lower molecular weight. Nonetheless, the molecular weight of OKGM is still high. Therefore, Hydrochloric Acid (HCl) and hydroperoxide (H2O2) were used as degradation reagents to obtain acidolysis-oxidized konjac glucomannan (A-OKGM) whose molecular weight is lower than that of OKGM. Besides, previous study showed that the molecular weight of A-OKGM is 9.8 kDa, and the functional groups of A-OKGM are as same as those of KGM [10].
In this study, we investigated the effects of A-OKGM on the expression of immune related genes in anterior intestine, mid intestine and distal intestine. Besides, the serum also was collected to analyze IgM level, C - Reactive Protein (CRP) level and the lysozyme activity. These results have profound implication for preventing the infectious diseases in S. prenanti.
Materials and Methods
Preparation Of A-OKGM
50 g KGM (KGM was purchased from PaiTe konjac biological technology co. ltd. The purity is 95%) was dissolved in 250 ml 40% ethanol solution, stirring 5 min at 180 r/min under 40°C, used 10% HCl to set pH to 4.3, then added 8.75 ml H2O2 (30%) into the liquid 3 times in 30 min at 10 min intervals, 4 h later, added Na2SO3 (1 mol/L) to terminate the reaction, added 10% NaOH to adjust pH to 7, filtrated with vacuum filter, washed OKGM three times with 50%, 70% and 90% ethanol, filtrated 3 times individually. The moisture contents were subsequently dried at 90°C in the drum wind drying oven, grinded to granule can be through 120 mesh sieve, and then we got the OKGM. 50 g OKGM (obtained from the above step) was suspended in 350 ml absolute alcohol and 100 ml concentrated hydrochloric (HCl) acid (36% v/v), the suspension was magnetically stirred for 2 h at room temperature, then washed with 70% ethanol until the washings became neutral. The samples were then left in a fume hood before being subjected to vacuum-drying at 30°C for 16 h, then we got the A-OKGM.
Diets composition
The A-OKGM was tested at three supplemented levels of 4.0, 8.0, 16.0 g kg-1. The diet without A-OKGM was used as control, Diets meet all nutritional requirements for S. prenanti and were manufactured through the formulation (Table 1).
Feeding trials
200 fish were transferred from a local farm (Yuquantown, Tianquan, Yaan, Sichuan, China) to the laboratory of Functional food of Sichuan Agriculture University where they were allowed to adapt to the environment for three weeks in the indoor 12 fiberglass tanks (50 cm × 70 cm × 40 cm), and thereafter 120 healthy and robust fish were selected for experimental use and they were divided into 4 groups randomly, every group (30 fish) was reared in 3 fiberglass tanks (50 cm × 70 cm × 40 cm) at the temperature of 15-25, under natural photoperiod (12L:12D). The tanks were aerated to maintain the dissolved oxygen levels at saturation, and water in each tank was replenished 100% daily. Each group was fed with experimental diet (control and diets added 4.0, 8.0, 16.0 g kg-1 A-OKGM) for two months.
Sampling
At the end of the experiment, 24 h after the last feeding, fish were killed by a blow to the head. Blood samples (10 fish/each group) were collected from the caudal vein before the dissection of fish. The blood samples were centrifuged at 3000 g for 10 mins at 4°C after clotted, and the supernatants were collected as serum which were used to analyze IgM level, C-Reactive Protein (CRP) level and lysozyme activity. For RNA extraction, the anterior intestine, mid intestine (section between the distal end of the anterior intestine and the increase in diameter indicating the start of the distal intestine) and distal intestine (from the distal end of the mid intestine to the anus) of three fish from each replicate tank were dissected and pulverized with mortars under liquid nitrogen and stored at -80°C for later use. For each group, 4 fish were used for determination of visceral index.
Biochemical analysis
The fish C – Reactive Protein (CRP) and IgM level was determined through ELISA Kits which purchased from Shanghai Yaji biotechnology Institute (Shanghai, China). Lysozyme activity was determined via the commercial kit (Nanjing, Jiangsu, China) according to manufacturer’s instruction. The visceral index was calculated by the following equations:
Spleen index = weight of spleen/body weight.
Head kidney index = weight of head kidney/body weight.
Gut index = weight of gut/body weight.
Mesonephros index = weight of mesonephros/body weight.
Liver index= weight of liver/body weight.
Total RNA Extraction and Cdna Synthesis
The total RNA was extracted from anterior, mid and distal intestine samples using RNA isolater reagent (VazymE, Nanjing, China) and treated with RNase-free H2O to the final volume of 20 µL and the integrity of RNA was tested by electrophoresis in 1.0% agarose gel. Moreover, the quantity of isolated RNA were later determined by measuring the absorbance at 260 and 280 nm. After that, 6 µl of the total RNA was used to synthesize the first-strand cDNA by HiScript®1st Strand cDNA Synthesis Kit (VazymE, Nanjing, China) according to the manufacturer’s instruction, and the cDNA was stored at -80°C for later use.
RT-qPCR
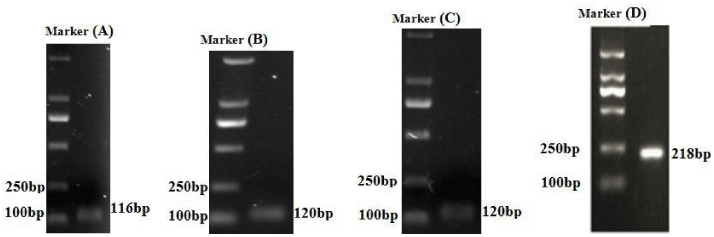
The CFX96 real-time PCR (Bio-Rad) and SYBR Green (VazymE, Nanjing, China) were performed to analyze the expression of immune related genes, and all the genes were designed from the existed sequences of Carassius auratus (KF767100.1), Cyprinus carpio (KF844251.1), Danio rerio (AB183467.1). (Table 2) listed all the primers used in our study. The primer of β-actin was designed as housekeeping gene from S. prenanti β-actin sequence (JQ013000). The RT-PCR was performed with final volume of 10 µl, each reaction contained 5 µl of AceQ qPCR SYBR Green Master Mix (VazymE, Nanjing, China), 0.25 µl of each primer, 1 µl of cDNA and 3.5 µl RNase-free H2O. The amplification of each gene consisted of 95°C for 3 min, followed by 39 cycles of 95°C for 10 s and Annealing temperature for 30 s. After each run, we generated the melting curves which were used to ensure the specificity of primers by increasing the temperature from 65°C for 5 s to 95°C, at 0.5°C/s. All of the reaction were in triplicate. Gene expression results were analyzed using the 2-ΔΔCt method. Moreover, the products of RT-PCR were tested by electrophoresis in 2.0% agarose gel (Figure 1).
Statistical Analysis
All data is presented as the mean ± S.M. Data was subjected to one-way ANOVA and Duncan’s multiple range tests using the software SPSS 16.0 statistical software package (SPSS Inc., Chicago, IL, USA). Differences were considered significant at P < 0.05.
Results
Visceral index
As seen from table 3, spleen index in fish fed with 1.6% A-OKGM was significantly different (P < 0.05) from other groups. Similarly, the liver index in fish fed with 0.8% A-OKGM was markedly higher (P < 0.05) than that in control group. There was no significant difference (P > 0.05) in head kidney index, gut index and Mesonephros index among all groups.
Immune parameters
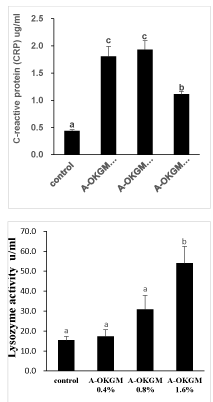
As is shown in figure 2, the CRP and IgM level in fish fed with A-OKGM diets was significantly higher (P < 0.05) than that in control group, regardless of inclusion level. Lysozyme activity in fish fed with 1.6% A-OKGM diet was significantly increased (P < 0.05). However, there is no significantly difference among other groups.
S. Prenanti Tnfα Mrna Expression
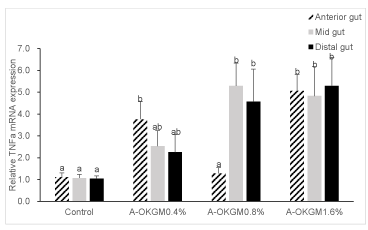
As is shown in figure 3. In the anterior intestine, the TNFα expression in fish fed with 0.4 and 1.6% A-OKGM diets was significantly increased (P < 0.05). In the mid intestine, the TNFα mRNA level in fish fed with 0.8% and 1.6% A-OKGM diets was significantly higher (P < 0.05) than that in control group. In the distal intestine, the TNFα expression was significantly promoted (P < 0.05) by both 0.8% and 1.6% A-OKGM diets.
S. prenanti IL-1β mRNA expression
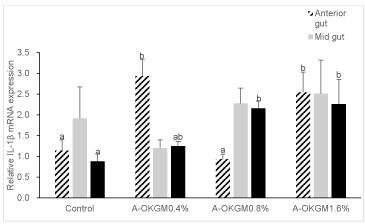
As is shown in figure 4. In the anterior intestine, the expression of IL-1β in fish fed with 0.4% and 1.6% A-OKGM diets was significantly increased (P < 0.05) when compared with other groups. However, the IL-1β expression in mid intestine didn’t reach significant level (P > 0.05). In the distal intestine, the 0.8% and 1.6% A-OKGM diets significantly promoted (P < 0.05) the expression of IL-1β.
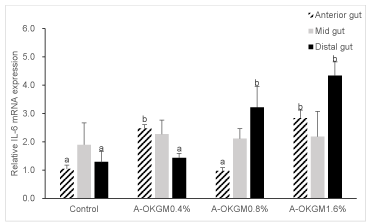
S. prenanti IL-6 mRNA expression
As seen from figure 5. In the anterior intestine, the IL-6 expression in fish fed with 0.4% and 1.6% A-OKGM diets was significantly higher (P < 0.05) than that in control group. However, in the mid intestine, there was no significantly difference (P > 0.05) in IL-6 mRNA level between each group after the feeding trial. In the distal intestine, the expression of IL-6 in fish fed with 0.8% and 1.6% A-OKGM diets was significantly higher (P < 0.05) when compared with control group.
Discussion
KGM consists of β-D-glucose and β-D-mannose monomers mainly connected by β1, 4-glycosidic bonds, with some side chains connected through β1, 3-glycosidic bonds [11]. KGM is a kind of Polysaccharides with prebiotic property. It has been well established that host exhibited better growth performance and higher expression of immune regulatory genes after administrated with prebiotics [12,13]. Recently, KGM has been applied in various kinds of field due to its structure, such as film preparation in drug and food industries [14,15]. However, high viscosity and molecular weight of KGM impede its application in aquaculture field. Hence it is necessarily to have KGM with lower viscosity and molecular weight. Our previous study has showed that the S. Prenanti exhibited a greater immunity and growth performance after fed with the diet supplemented with OKGM [16]. Nevertheless, the viscosity and molecular weight of OKGM was still too high, and we further degraded OKGM with Hydrochloric acid to obtain A-OKGM. [17] Showed that dietary pulverized KGM suppressed allergic rhinitis-like symptoms in mice upon immunization and nasal sensitization with ovalbumin. Moreover, [18]. Reported that acid-hydrolyzed KGM exerted a greater prebiotic effect than does KGM in BALB/c mice. In our present study, A-OKGM, as a potential prebiotic, have a positive effects on the serum immune parameters and immune genes expression in the gut of S. Prenanti.
Fish have both a well-developed innate and an adaptive immune system [19-21]. The innate immune system consists of specialized cells such as granulocytes, macrophages, cytotoxic cells and dendritic cells, and a number of proteins that play a pivotal role in protecting fish against pathogenic infection. Lysozyme is an paramount parameter in innate immune system, which exists both in vertebrate and invertebrate. [22,23] demonstrated that serum lysozyme activity in 2 g kg-1 MOS diet group of rainbow trout was significantly increased. The serum lysozyme activity of Carassius auratus gibelio was significantly higher in the treatment group of 480 mg kg-1 MOS than the control group [24]. Moreover, Labeo rohita fed with 100-500 mg kg-1 β-glucan for 56 days also showed significantly higher serum lysozyme activity compared to control fish [25,26]. Demonstrated that lysozyme activity was significantly higher than other groups when red sea bream was fed with the diet (0.1% β-glucan in combination with 0.025% HK-LP). Due to the fact that the molecular structure of A-OKGM similar to the MOS, β-glucan. In our present study, the serum lysozyme activity in fish fed with 1.6% A-OKGM diet was significantly increased when compared with control group.
C - Reactive Protein (CRP), which belongs to the pentraxin family, is commonly associated with the acute phase response [27,28] which is the first line of host defense against infection, injury or trauma. This reaction aim at eliminating infective organisms and preventing further tissue damage, but also at restoring the host’s normal functions [29]. As CRP is a kind of acute phase proteins, it function in a variety of defense related activities such as limiting the dispersal of infectious agents, inactivation of proteases, killing of microbes, repair of tissue damage and other potential pathogens, and restoration of the healthy state [30]. The concentration of CRP is quite high in the normal serum of many fish species, which increases up to 20-fold following high temperature shock or administration of inflammatory agent [31-33]. Up to date, the inflammatory agents are mainly pathogens [34-36]. Limit paper study the effects of prebiotics, such as A-OKGM, on the level of CRP. In the carp, the oral administration of β-glucan for 25 days significantly increased serum CRP levels [37]. In our present study, the serum CRP level in S. Prenanti fed with A-OKGM diets was significantly higher than that in control group, regardless of the inclusion level. Besides, the IgM content was also significantly increased, regardless of inclusion level [16]. Demonstrated that the content of IgM in S. prenanti fed with 2000 mg/kg OKGM was significantly higher than other groups. The major serum immunoglobulin in teleost fish is IgM which is an important molecule mediating humoral immune responses [38]. As for the immune organs, the spleen and liver index was significantly increased after fish fed with 1.6% A-OKGM and 0.8% A-OKGM, respectively. Taken together, present results indicated that diet supplemented with A-OKGM could in some degree enhance immunity of S. Prenanti.
To our best knowledge, our research is the first to explore the effects of A-OKGM on the immune related genes expression in the gut of S. Prenanti. Our present results indicated that the treatment diet stimulated the immune cell cytokines secretion in the gut. It is interesting that the expression of TNF a in the mid gut and distal gut was increased steadily in a dosage-dependent manner (0.4% to 1.6%). Moreover, IL-6 mRNA level in distal gut was also increased in dosage-dependent manner. However, the same result didn’t be observed in the expreession of IL-1β.
Interleukin (IL)-1β is an important inflammatory mediator and belongs to the interleukin-1 family of cytokines. IL-1β is produced by variety of cells, including monocytes, macrophages, T and B lymphocytes [39]. It plays a key role in the inflammatory process, enhancing cell-mediated immunity by inducing the growth and proliferation of lymphocytes, and by stimulating immune and inflammatory response effector cells [40]. In mammals, IL-1β mRNA expression of pigs fed Distillers Dried Grains With Solubles (DDGS) was significantly higher in ileum tissue than that of pigs fed other diets. DDGS, which was obtained from ethanol plants, contains plenty of β-glucan and MOS [41]. In fish, the diets supplemented with β-glucan can stimulate the carp macrophages and promote IL-1β expression [42]. In Gadus morhua, the spleen cell of cod incubated with β-glucan showed a significant up regulation of IL-1β at 24 h post-incubation when compared to control cells [43,44] demonstrated that IL-1β mRNA level was significantly up-regulated in gilthead seabream fed with β-glucan for 4 weeks when compare with other groups. In our present study, 0.4% and 1.6% A-OKGM diets significantly enhanced the IL-1β expression in anterior gut. Besides, S. Prenanti fed with 0.8% and 1.6% A-OKGM diets showed significantly higher expression of IL-1β in distal gut.
Interleukin-6 (IL-6) is a multifunctional cytokine with a wide range of biological activities associated with regulation of immune response and inflammation in many cell types, including macrophages and lymphocytes [45,46]. IL-6 is induced by response to viral or bacterial infection and pro-inflammatory cytokines, such as IL-1, Tumor Necrosis Factor (TNF)-a, or Platelet Derived Growth Factor (PDGF) [47-49]. Similar to IL-1β, IL-6 can act as a pro-inflammatory cytokine in innate immune response. To stimulate inflammatory activity, IL-6 promotes the production of acute phase response proteins, including the C reaction proteins and the related serum amyloid A proteins [50]. Our present results indicate that A-OKGM can up-regulate the IL-6 expression. In detail, IL-6 mRNA level in 0.4% and 1.6% A-OKGM diet groups was significantly promoted in anterior gut. The 0.8% and 1.6% A-OKGM diets significantly enhanced IL-6 expression in distal gut. In carp, the β-glucan enriched bath stimulates the wound healing process by significantly up-regulated IL-6 expression after three days post-wounding [51]. At present, many authors only focused on the IL-6 expression changes in response to bacterial challenge. Limited papers studied on the effects of prebiotics, such as A-OKGM, on the IL-6 expression [52]. Demonstrated that the IL-6 expression of rainbow trout was significantly induced after infected with Yersinia ruckeri. In Oncorhynchus mykiss larvae, the IL-6 was up-regulated and the expression slowly increased until end of the experiment after infected with Ichthyophthirius multifiliis [53] Moreover, [54] reported that the mRNA levels of IL-6 in spleens, intestine tissue and livers (Figure 6A, B, C) of blunt snout bream all increased significantly (P < 0.05), with maximum values attained at 6 h, 3 h, 6 h (10, 6, 18-fold, respectively) after Aeromonas hydrophila injection.
Tumor Necrosis Factor α (TNF a), is a major mediator of pro-inflammatory and antimicrobial defense mechanisms, able to eliminate various pathogens by inducing a variety of cellular responses such as phagocytosis and chemotaxis, and is considered an excellent biomarker and health indicator for both mammals and fish [55-57]. Many studies have reported that polysaccharides induce the expression of TNFa mRNA. In mammals, the TNFa expression in rat fed with Grifola frondosa polysaccharide was markedly increased [58]. In fish, [59] reported that administration of Astragalus polysaccharides significantly up-regulated TNFα mRNA level in carp. Similarly, Immunogen® (mainly β-Glucan and MOS) significantly enhanced the TNFα gene expression in rainbow trout [60]. In our present study, in the anterior gut, 0.4% and 1.6% A-OKGM diet groups significantly enhanced TNFα gene expression. Besides, diets supplemented with 0.8% and 1.6% A-OKGM significantly promoted expression of TNFα in mid and distal gut.
In short, feeding A-OKGM supplemented diet to S. Prenanti induced the immune response in the gut. Although the mechanisms of the action of A-OKGM on the gut immune system are unclear, we suspect that dietary A-OKGM is closely related to the production of immune cytokines. It is clearly showed that A-OKGM promoted IL-1β, IL-6 and TNFα genes expression. The Cytokines (such as IL-1β, IL-6 and TNF α) are indispensable for macrophage, neutrophil, and lymphocyte recruitment to the infected tissues and their activation as pathogen eliminators (61). Meanwhile, cytokines can also induce acute phase proteins, including Mannose-Binding Lectin (MBL) and C - Reactive Protein (CRP) [62]. The increased content of CRP in serum was also observed in our present study.
In conclusion, A-OKGM modified from KGM can act as Immunostimulant to enhance the immunity of S. Prenanti by promoting serum immune parameters and immune related gene expressions.
- Mu XJ, Pridgeon JW, Klesius PH. Comparative transcriptional analysis reveals distinct expression patterns of channel catfish genes after the first infection and re-infection with aeromonas hydrophila. Fish Shellfish Immunol. 2013; 35: 1566-1576. https://goo.gl/QrVoe6
- Martin SAM, Dehler CE, Krol E. Transcriptomic responses in the fish intestine. Dev Comp Immunol. 2016; 64: 103-117. https://goo.gl/tPt7f2
- Salinas I, Parra D. Fish mucosal immunity: intestine. In: Beck, B.H., Peatman, E. (Eds.), Mucosal Health in Aquaculture. Academic Press, London, 2015; 135-171. https://goo.gl/M2o92W
- Merrifield DL, Rodiles A, The fish microbiome and its interactions with mucosal tissues. In: Beck, B.H., Peatman, E. (Eds.), Mucosal Health in Aquaculture. Academic Press, London, 2015; 273-295. https://goo.gl/z7343b
- Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL-6 and related molecules (IL-1 and TNF). FASEB J. 1990; 4: 2860-2867. https://goo.gl/bKh3d7
- Rurangwa E, Sipkema D, Kals J, Ter VM, Forlenza M, Bacanu GM, et al. Impact of a novel protein meal on the gastrointestinal microbiota and the host transcriptome of larval zebrafish Danio rerio. Front Physiol. 2015; 6: 133. https://goo.gl/1CZatB
- Zhou QC, Buentello JA, Iii DMG. Effects of dietary prebiotics on growth performance, immune response and intestinal morphology of red drum (sciaenops ocellatus). Aquaculture. 2010; 309: 253-257. https://goo.gl/dHZGFH
- Munir MB, Hashim R, Chai YH, Marsh TL, Nor SAM. Dietary prebiotics and probiotics influence growth performance, nutrient digestibility and the expression of immune regulatory genes in snakehead (channa striata) fingerlings. Aquaculture. 2016; 460: 59-68. https://goo.gl/gkwz5o
- Dawood MAO, Koshio S, Ishikawa M, Yokoyama S, Basuini MFE, Hossain MS, et al. Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream, pagrus major. Aquac Nutr. 2015; 43: 385-387. https://goo.gl/TgUVBK
- Chen MR, Wang HJ, Yan QP, Zheng QR, Yang M, Lv Z, et al. Effects of dietary oxidized konjac glucomannan sulfates (OKGMS) and acidolysis-oxidized konjac glucomannan (A-OKGM) on the immunity and expression of immune-related genes of schizothorax prenanti. Fish Shellfish Immunol. 2016; 56: 96-105. https://goo.gl/m9CcXt
- Tester RF, Al-Ghazzewi FH. Mannans and health, with a special focus on glucomannans. Food Res Int. 2013; 50: 384-391. https://goo.gl/APgEXQ
- Pourabedin M, Zhao X. Prebiotics and gut microbiota in chickens. FEMS Microbiol Lett. 2015; 362. https://goo.gl/AMWmKb
- Dawood MAO, Koshio S. Recent advances in the role of probiotics and prebiotics in carp aquaculture: A review. Aquaculture. 2016; 454: 243-251. https://goo.gl/5CREKA
- Zhang YQ, Xie BJ, Xin G. Advance in the applications of konjac glucomannan and its derivatives. Carbohyd Polym. 2005; 60: 27-31. https://goo.gl/XrWXKS
- Man X, Li W, Corke H, Yan W, Ni X, Fang Y, Jiang F. Characterization of konjac glucomannan-ethyl cellulose film formation via microscopy. Int J Biol Macromol. 2016; 85: 434-441. https://goo.gl/jWR5dF
- Zhang L, Wu Y, Wang L, Wang H. Effects of Oxidized Konjac glucomannan (OKGM) on growth and immune function of Schizothorax prenanti. Fish Shellfish Immunol. 2013; 35: 1105-1110. https://goo.gl/yKSWhs
- Onishi N, Kawamoto S, Ueda K, Yamanaka Y, Katayama A, Suzuki H, et al. Dietary pulverized konjac glucomannan prevents the development of allergic rhinitis-like symptoms and IgE response in mice. Biosci Biotech Bioch. 2007; 71: 2551-2556. https://goo.gl/4oz716
- Chen HL, Fan YH, Chen ME, Chan Y. Unhydrolyzed and hydrolyzed konjac glucomannans modulated cecal and fecal microflora in Balb/c mice. Nutrition. 2005; 21: 1059-1064. https://goo.gl/ejVQfg
- Magnadottir B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006; 20: 137-151. https://goo.gl/cL2SKP
- Ellis AE. Innate host defense mechanisms of fish against viruses and bacteria. Dev Comp Immunol. 2001; 25: 827-839. https://goo.gl/Cfy2ZR
- Iwama G, Nakanishi T. The fish immune system: organism, pathogen, and environment. Fish Physiology. 1996. https://goo.gl/2BXcyi
- Caballero MJ, Montero D, Torrecillas S, Makol A, Benítez-Santana T, Sweetman J, et al. Reduced gut bacterial translocation in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides (MOS). Fish Shellfish Immunol. 2011; 30: 674-681. https://goo.gl/DCvrLJ
- Staykov Y, Spring P, Denev S, Sweetman J. Effect of a mannan oligosaccharide on the growth performance and immune status of rainbow trout ( Oncorhynchus mykiss ). Aquacult Int. 2007; 15: 153-161. https://goo.gl/YLVxzj
- Liu B, Xu L, Ge X, Xie J, Xu P, Zhou Q, et al. Effects of mannan oligosaccharide on the physiological responses, HSP70 gene expression and disease resistance of Allogynogenetic crucian carp (Carassius auratus gibelio) under Aeromonas hydrophila infection. Fish Shellfish Immunol. 2013; 34: 1395-1403.
- Misra CK, Das BK, Mukherjee SC, Pattnaik P. Effect of long term administration of dietary β-glucan on immunity, growth and survival of Labeo rohita fingerlings. Aquaculture. 2006; 255: 82-94. https://goo.gl/vC3Dzh
- Dawood MAO, Koshio S, Ishikawa M, Yokoyama S. Interaction effects of dietary supplementation of heat-killed Lactobacillus plantarum and β-glucan on growth performance, digestibility and immune response of juvenile red sea bream, Pagrus major. Fish Shellfish Immunol. 2015; 45:33-42. https://goo.gl/uLvoiW
- Cartwright JR, Tharia HA, Burns I, Shrive AK, Hoole D, Greenhough TJ. Isolation and characterisation of pentraxin-like serum proteins from the common carp Cyprinus carpio. Dev Comp Immunol. 2004; 28: 113-125. https://goo.gl/VEkT3T
- Pepys MB, Dash AC, Fletcher TC, Richardson N, Munn EA, Feinstein A. Analogues in other mammals and in fish of human plasma proteins, C-reactive protein and amyloid P component. Nature. 1978; 273:168-170. https://goo.gl/jZvwyN
- Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994; 15: 74-80. https://goo.gl/6PNhEL
- Bayne CJ, Gerwick L .The acute phase response and innate immunity of fish. Dev Comp Immunol. 2001; 25: 725-743. https://goo.gl/7owytZ
- Kodama H, Matsuoka Y, Tanaka Y, Liu Y, Iwasaki T, Watarai S. Changes of C-reactive protein levels in rainbow trout (Oncorhynchus mykiss) sera after exposure to anti-ectoparasitic chemicals used in aquaculture. Fish Shellfish Immunol. 2004; 16: 589-597. https://goo.gl/LKNARg
- Szalai AJ, Bly JE, Clem LW. Changes in serum concentrations of channel catfish (Ictalurus punctatus Rafinesque) Phosphorylcholine-Reactive Protein (PRP) in response to inflammatory agents, low temperature-shock and infection by the fungus Saprolegnia sp. Fish Shellfish Immunol. 1994; 4: 323-336. https://goo.gl/eCWYYu
- Winkelhake JL, Chang RJ. Acute phase (C-reactive) protein-like macromolecules from rainbow trout (Salmo gairdneri). Dev Comp Immunol. 1982; 6: 481-489. https://goo.gl/g26Ff9
- Kodama H, Yamada F, Murai T, Nakanishi Y, Mikami T, Izawa H. Activation of trout macrophages and production of CRP after immunization with Vibrio angillarum. Dev Comp Immunol. 1989; 13:123-132. https://goo.gl/vHXA4b
- Pionnier N, Falco A, Miest J, Frost P, Irnazarow I, Shrive A, Hoole D. Dietary β-glucan stimulate complement and C-reactive protein acute phase responses in common carp ( Cyprinus carpio ) during an Aeromonas salmonicida infection. Fish Shellfish Immunol. 2013; 34: 819-831. https://goo.gl/6F1udx
- Li MF, Chen C, Li J, Sun L. The C-reactive protein of tongue sole Cynoglossus semilaevis is an acute phase protein that interacts with bacterial pathogens and stimulates the antibacterial activity of peripheral blood leukocytes. Fish Shellfish Immunol. 2013; 34: 623-631. https://goo.gl/uCUnDg
- Pionnier N, Falco A, Miest JJ, Shrive AK, Hoole D. Feeding common carp Cyprinus carpio with β-glucan supplemented diet stimulates C-reactive protein and complement immune acutephase responses following PAMPs injection. Fish Shellfish Immunol. 2014; 39: 285-295. https://goo.gl/6BuasD
- Hordvik I, Kamil A, Bilal S, Raae A, Fjelldal PG, Koppang EO. Characterization of serum IgM in teleost fish, with emphasis on salmonids. Fish Shellfish Immunol. 2013; 34: 1656.
- Oppenheim JJ, Kovacs EJ, Matsushima K, Durum SK. There is more than one interleukin 1. Immunol Today. 1986; 7: 45-56. https://goo.gl/Yh81J6
- Pleguezuelos O, Zou J, Cunningham C, Secombes CJ. Cloning, sequencing, and analysis of expression of a second? gene in rainbow trout (Oncorhynchus mykiss). Immunogenetics. 2000; 51: 1002-1011. https://goo.gl/8o2nq6
- Weber TE, Ziemer CJ, Kerr BJ. Effects of adding fibrous feedstuffs to the diet of young pigs on growth performance, intestinal cytokines, and circulating acute-phase proteins. J Anim Sci. 2008; 86: 871-881. https://goo.gl/pehP9C
- Pietretti D, Vera-Jimenez NI, Hoole D, Wiegertjes GF. Oxidative burst and nitric oxide responses in carp macrophages induced by zymosan, MacroGard ® and selective dectin-1 agonists suggest recognition by multiple pattern recognition receptors. Fish Shellfish Immunol. 2013; 35: 847-857. https://goo.gl/GwP58h
- Caipang CMA, Lazado CC, Brinchmann MF, Kiron V. Transcription of selected immune-related genes in spleen cells of cod, Gadus morhuafollowing incubation with alginic acid and β-glucan. J Exp Mar Biol Ecol. 2012; 416: 202-207. https://goo.gl/j38Twf
- Guzmán-Villanueva LT, Tovar-Ramirez D, Gisbert E, Cordero H, Guardiola FA, Cuesta A, et al. Dietary administration of β-1,3/1,6-glucan and probiotic strain Shewanella putrefaciens, single or combined, on gilthead seabream growth, immune responses and gene expression. Fish Shellfish Immunol. 2014; 39: 34-41. https://goo.gl/XCj5tG
- Naka T, Nishimoto N, Kishimoto T, The paradigm of IL-6: from basic science to medicine. Arthritis Res. 2002; 4: 233-242. https://goo.gl/zSxf3s
- Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL-6 and related molecules (IL-1 and TNF). FASEB J. 1990; 4: 2860-2867. https://goo.gl/bKh3d7
- Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986; 46: 705-716. https://goo.gl/eXxL9s
- Hirano T. Interleukin 6 and its receptor: ten years later. Int Rev Immunol. 1998; 16: 249-284. https://goo.gl/2PFfPY
- Ray A, Tatter SB, Santhanam U, Helfgott DC, May LT, Sehgal PB. Regulation of expression of interleukin-6. Molecular and clinical studies. Ann Ny Acad Sci.1989; 557: 353-362. https://goo.gl/WWkpnH
- Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990; 265: 621-636. https://goo.gl/UMw5Un
- Przybylska-Diaz DA, Schmidt JG, Vera-Jiménez NI, Steinhagen D, Nielsen ME. β-glucan enriched bath directly stimulates the wound healing process in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2013; 35: 998-1006. https://goo.gl/gqSkDQ
- Raida MK, Buchmann K. Development of adaptive immunity in rainbow trout, Oncorhynchus mykiss (Walbaum) surviving an infection with Yersinia ruckeri. Fish Shellfish Immunol. 2008; 25: 533-541. https://goo.gl/pr3SYU
- Heinecke RD, Buchmann K. Inflammatory response of rainbow trout Oncorhynchus mykiss (Walbaum, 1792) larvae against Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2013; 34: 521-528. https://goo.gl/ggHgH3
- Zhang CN, Zhang JL, Liu WB, Wu QJ, Gao XC, Ren HT. Cloning, characterization and mRNA expression of interleukin-6 in blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2016; 54: 639-647. https://goo.gl/RJDASG
- Kevin OK, Michael H, Gill D, Leonid Heifets. Tumor necrosis factor alpha stimulates killing of mycobacterium tuberculosis by human neutrophils. Infect Immun. 2002; 70: 4591-4599. https://goo.gl/DEDa2Y
- Cunha FQ, Assreuy J, Moss DW, Rees D, Leal LM, Moncada S, et al. Differential induction of nitric oxide synthase in various organs of the mouse during endotoxaemia: role of TNF-alpha and IL-1-beta. Immunology. 1994; 81: 211-215. https://goo.gl/hcsb49
- Secombes CJ, Wang T, Hong S, Peddie S, Crampe M, Laing KJ, et al. Cytokines and innate immunity of fish. Dev Comp Immunol. 2001; 25: 713-723. https://goo.gl/b4dK17
- Xu H, Liu JH, Shen ZY, Fei Y, Chen XD. Analysis of chemical composition, structure of Grifola frondosa polysaccharides and its effect on skin TNF-α levels, lgG content, T lymphocytes rate and caspase-3 mRNA. Carbohyd Polym. 2010; 82: 687-691. https://goo.gl/rbU5pA
- Yuan C, Pan X, Yi G, Xia A, Wu G, Tang J, Han X. Effects of Astragalus polysaccharides (APS) on the expression of immune response genes in head kidney, gill and spleen of the common carp, Cyprinus carpio L. Int Immunopharmacol. 2008; 8: 51-58. https://goo.gl/XFg6Ds
- Ahmadi PY, Farahmand H, Miandare HK, Mirvaghefi A, Hoseinifar SH. The effects of dietary Immunogen ® on innate immune response, immune related genes expression and disease resistance of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2014; 37: 209-214. https://goo.gl/aT9eQV
- Svanborg C, Godaly G, Hedlund M. Cytokine responses during mucosal infections: role in disease pathogenesis and host defence. Curr Opin Microbiol. 1999; 2: 99-105. https://goo.gl/tpiyky
- Devries ME, Ran L, Kelvin DJ. On the edge: The physiological and pathophysiological role of chemokines during inflammatory and immunological responses. Semin Immunol. 1999; 11: 95-104.

Sign up for Article Alerts