Pathomorphological Changes in the Spleen of Turkey Broilers Challenged with aflatoxin B1 Alone or Co-Administered with Mycotox NG?
Nelly Grozeva1, Ivan Valchev2, Rumen Binev2*, Lazarin Lazarov2, Tsanko Hristov2 and Dian Kanakov2
1Department of General and Clinical Pathology
2Department of Internal Non-Infectious Diseases, Faculty of Veterinary Medicine, Trakia University, 6000 Stara Zagora, Bulgaria
*Address for Correspondence: Rumen Binev, Department of Internal Non-Infectious Diseases, Faculty of Veterinary Medicine, Trakia University, 6000 Stara Zagora, Bulgaria, E-Mail: [email protected]
Submitted: 06 July 2017; Approved: 20 July 2017; Published: 24 July 2017
Citation this article: Grozeva N, Valchev I, Binev R, Lazarov L, Hristov T, et al. Pathomorphological Changes in the Spleen of Turkey Broilers Challenged with aflatoxin B1 Alone or Co-Administered with Mycotox NG. Int J Vet Sci Technol. 2017;1(1): 001-006.
Copyright: © 2017 Binev R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Aflatoxin В1; Turkey broilers; Spleen; Mycotox Ng
Download Fulltext PDF
The aim of the present study was to evaluate the toxic effects of aflatoxin B1 (AFB1) on spleen morphology after its independent application or in combination with a mycosorbent (Mycotox NB) in turkey broilers. Experiments was carried out with 60 female turkey broilers 7-day age (meat TM strain) and divided into one control and five treatment groups (n = 10). Groups were as followed: Group I - control (fed standard feed according to the species and age of birds); Group II - feed by supplement with 0.5 g/ kg Mycotox NG, Group III - feed by supplementation with - 0.2 mg/ kg aflatoxin B1, Group IV -feed supplementation with - 0.4 mg/ kg aflatoxin B1, Group V - fed by supplementation with - with 0.2 mg/ kg aflatoxin B1 and 0.5 g/ kg Mycotox NG and Group VI - fed by supplementation with - 0.4 mg/ kg aflatoxin B1 and 0.5 g/ kg Mycotox NG. The duration of the experiments was 42 days. Specimens were collected from the spleen of birds for histological examination after euthanasia by cervical dislocation, fixed in 10% neutral formalin, dehydrated in alcohol series and embedded into paraffin. The 3 μm cross sections were cut on a microtome (Leica RM 2235) and stained with haematoxylin-eosin. Pathomorphological changes affected mainly the lymphatic tissue of germinative centres, whose number and size were reduced in birds from group IV and most cells exhibited degenerative (necrobiotic to necrotic) processes. In birds of group III, degenerative changes were less intensive (granular and vacuolar dystrophy) compared to the other groups. In birds off groups V and VI, the supplementation of toxin binder reduced partly the severity of morphological changes as congestion among lymphatic follicles in red and white pulp, slight granular and vacuolar dystrophy.
Introduction
Aflatoxins are groups of closely related biologically active substances with minor differences in their chemical structure [1]. For the first time, aflatoxins were isolated more than 40 years ago in an outbreak of Turkey X disease Cullen and Newberne, [2] and rainbow trout cancer Blount, [3] after feeding diets comprising peanuts and cotton meal. These mycotoxins are secondary toxic metabolites produced by the genus Aspergillus (Aspergillus flavus and Aspergillus parasiticus) - coumarin derivatives containing a dihydrofurofuran moiety.
Аflatoxins are fluorescent compounds: under ultraviolet rays, aflatoxin B1 (AFB1) and aflatoxin B2 (AFB2) have a blue fluorescence while aflatoxins G1 (AFG1) and G2 (AFG2): yellow-green [4]. Аflatoxin В1 (AFB1) is the commonest metabolite in plant substrates and at the same time, the most toxic one. It is frequently encountered in cereal crops and peanut meal Gowda, et al. [5] and is the most potent liver carcinogen [6]. The toxicity of aflatoxin В2 (AFB2), G1 (AFG1) and G2 (AFG2) is 10%, 20% and 50% of that of AFB1, respectively [7]. Аflatoxins are a group of almost 20 Aspergillus metabolites with similar chemical structure responsible for decay of plant substrates [8].
Аflatoxins are resistant to food processing and therefore could be found both in animal feeds and foodstuffs for direct consumption or processed foods, posing a risk for human health [9].
All bird species are sensitive to aflatoxin toxicity and although they do not receive relatively high concentrations with the feed to end lethally, low levels could be also deleterious after continuous intake. Growing birds, especially ducklings and turkey poultry are extremely sensitive to the toxic effects of aflatoxins. The total aflatoxin content in the feeds for poultry should not exceed 20 μg/ kg feed. Nevertheless, levels lower than 20 μg/ kg feed also induce lower resistance to diseases and stress [10]. The toxic effects of aflatoxins in domestic fowl are studied in detail with regard to their carcinogenic, teratogenic, mutagenic and growth inhibiting effects [11,12]. The occurring haematological, biochemical (decreased serum total protein, albumin, inorganic phosphorus, uric acid, total cholesterol and the values of haematocrit, red blood cell counts, mean corpuscular volume, haemoglobin, thrombocyte counts, percentage of monocyte counts; increased values of white blood cell and heterophil counts) Oguz, et al. [13], immunological (depression in anti-Brucella abortus antibodies) Qureshi, et al. [14] and morphological (hydropic degeneration in liver; significantly reduced in size bursa of Fabricus and depletion of lymphoid cells from the follicles with necrosis and hyperplasia of mucosal epithelium, intraepithelial cysts oedema in the intraepithelial areas and heterophil infiltration; reduction in the size thymus and depletion of lymphocytes from the cortical and medullary areas; slight lymphoid depletion of periarteriolar lymphoid tussies in spleen; pale kidney with degeneration and/or necrosis of tubular epithelium [15].
The morphological changes in the spleen are characterized with lymphocytic degeneration, fatty dystrophy and haemorrhagic foci [16], congestive events in the red pulp, reduction of lymphocytes, vacuolar dystrophy [17], depletion of lymphoid cells, reticular cell hyperplasia, lymphocytolysis and increased germinal cell counts.
A number of strategies for detoxication of mycotoxin-contaminated feeds are proposed: physical separation, heat inactivation, irradiation, microbial degradation, treatment with chemicals. These methods of detoxication of aflatoxin-contaminated feeds are expensive [18]. The utilization of inert mycosorbents as hydrated calcium sodium aluminosilicate (HSCAS) [19], zeolites [20], bentonites [21], active charcoal [22] or bioproducts - live yeast cultures [23] are among the most extensively studied. Mycosorbents reduce the bioavailability of mycotoxins in blood and prevent their absorption in the intestines. Regardless of that, some of them reduce also the bioavailability of amino acids and/or minerals [24].
The aim of the present investigation was to evaluate the toxic effects of aflatoxin B1 (AFB1) on spleen morphology after its independent application or in combination with a mycosorbent (Mycotox NB).
Material and methods
Experimental design
The experiment was performed with 60 7-day-old female turkey broilers (from the meat TM strain) randomly divided into six groups (n = 10).
All birds were fed standard feed according to the species and age, produced by a feed factory. The experimental design comprised: Group І - control; Group II - experimental, supplemented with 0.5 g/ kg Mycotox NG (micronised yeasts, montmorillonite, thymol); Group ІІI - experimental, whose feed contained 0.2 mg/ kg aflatoxin В1; Group IV - experimental, whose feed contained 0.4 mg/ kg aflatoxin В1; Group V - experimental, supplemented with 0.2 mg/ kg aflatoxin В1 and 0.5 g/ kg Mycotox NG; Group VI - experimental, supplemented with 0.4 mg/ kg aflatoxin В1 and 0.5 g/ kg Mycotox NG.
Aflatoxin В1 used in this experiment was produced by Aspergillus flavus, 99% purity (Sigma-Aldrich, (Germany). All birds were housed under optimum microclimatic conditions as per [25].
After the trial, turkey poults from the control and experimental groups were euthanised via cervical dislocation according to [26]. Spleen specimens for histological study were fixed in 10% neutral formalin, dehydrated in ascending alcohol series and embedded in paraffin. Paraffin blocks were cut on a microtome Leica RM 2235 (Leica Biosystems Nussloch GmbH, Germany), with cross section thickness of 3 µm. They were stained with haematoxylin-eosin using routine histological techniques.
The experiment was approved by the Bulgarian Food Safety Agency - permit No 19218/06.11.2014. Statistical analysis was done with Statistica 6.0 (Windows) software, StatSoft, Inc. (USA, 1993) and ANOVA test. Data are presented as mean ± standard deviation (SD). The level of statistically significance was р < 0.05.
Results
The relative weights of the spleen (g/100 g live weight) аre presented in table 1. They were statistically significantly lower in birds from Groups III and IV (by 28.79% and 31.82%, respectively) compared to controls (p < 0.001). The addition of toxin binder to the feed of Groups V and VI prevented the reduction of relative spleen weights. There was no statistically significant difference between relative spleen weights of groups V and VI vs the control group (р > 0.05).
Pathomorphological studies
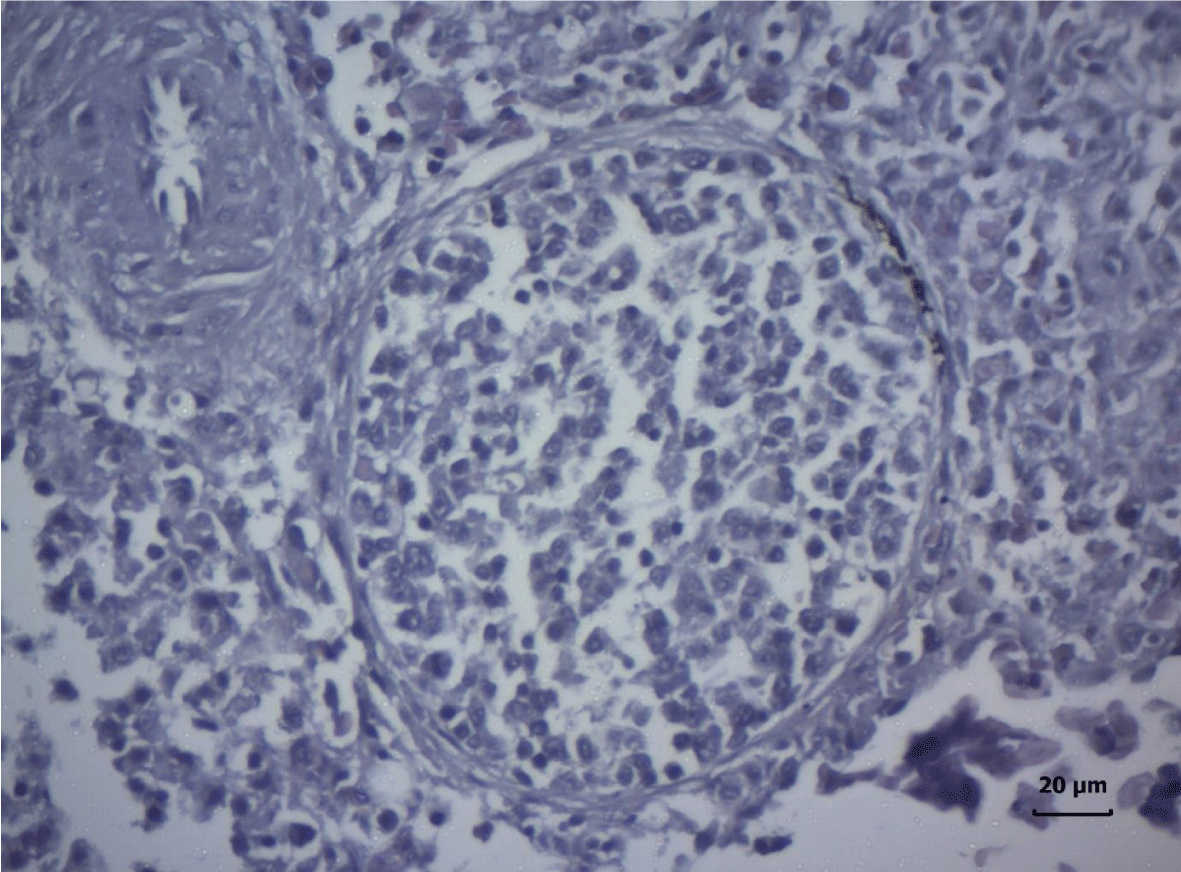
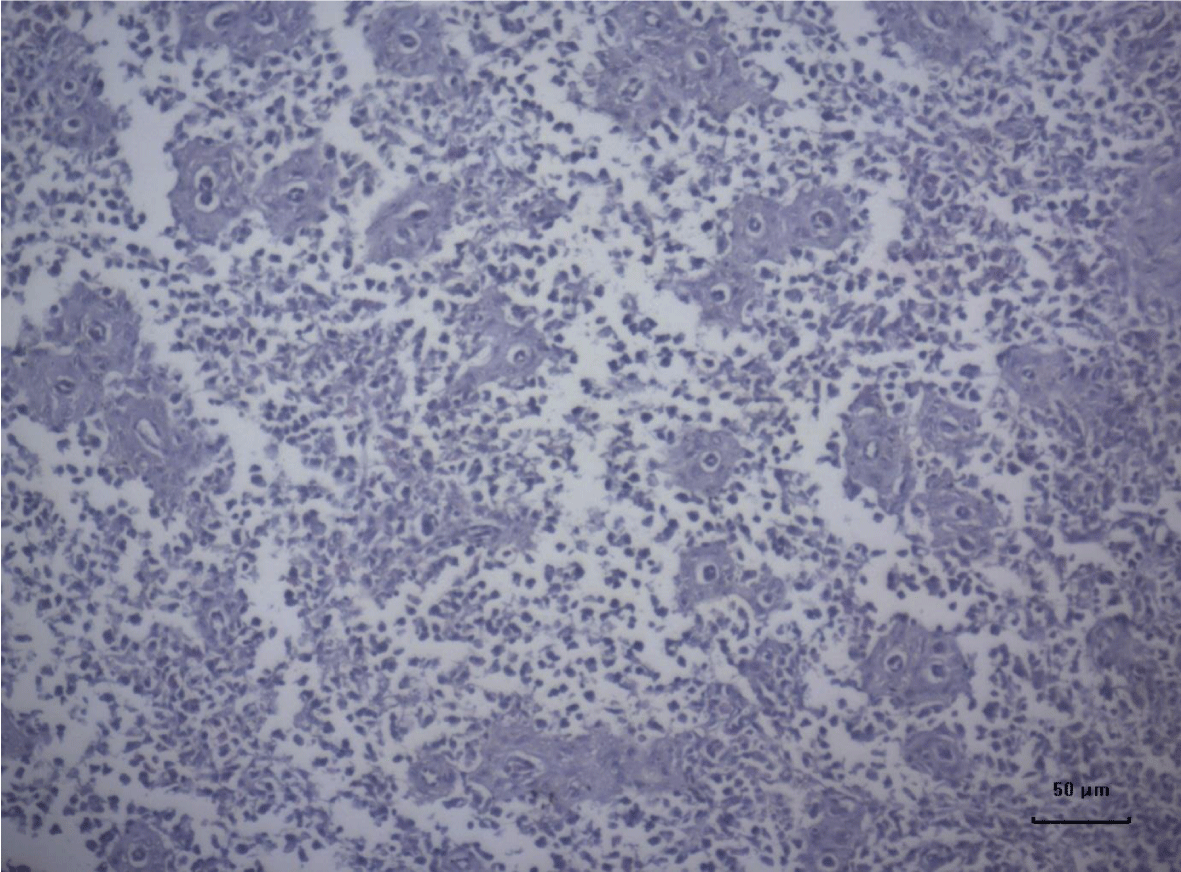
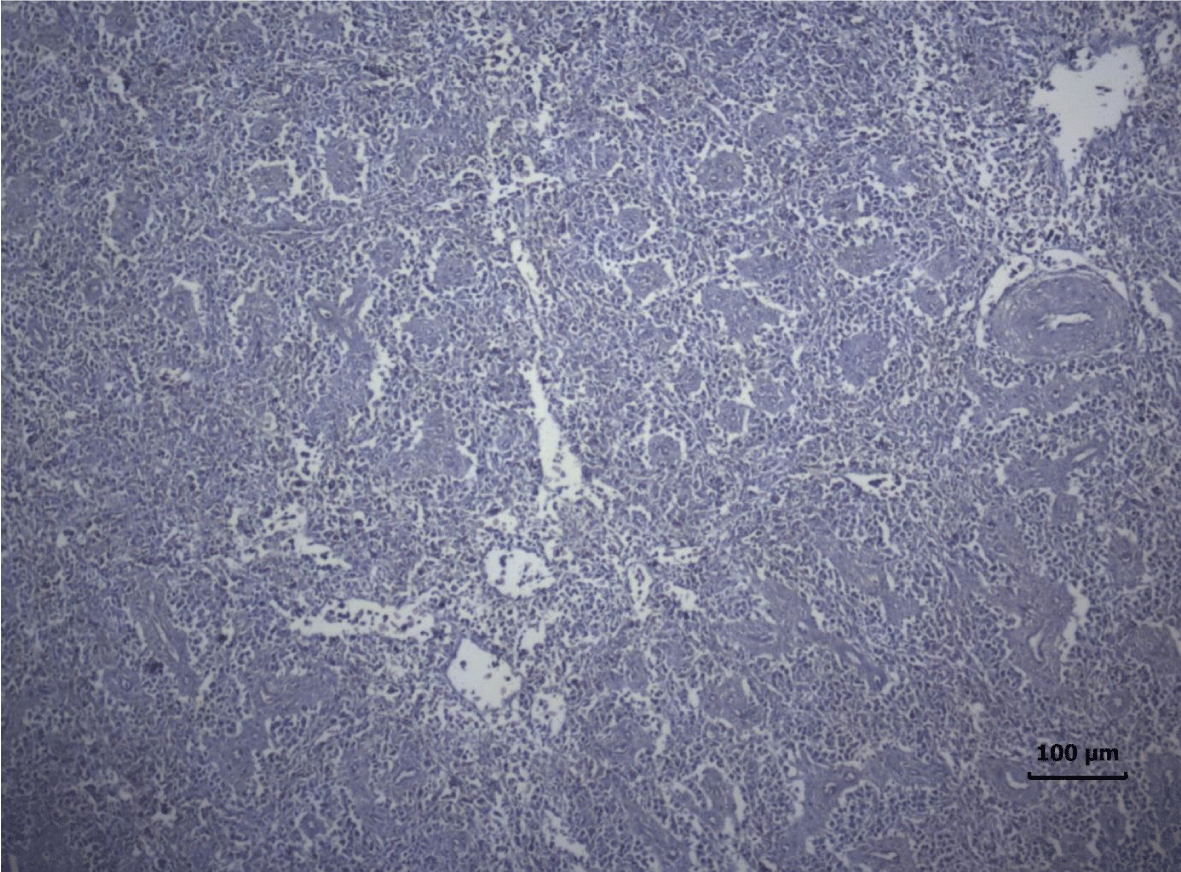
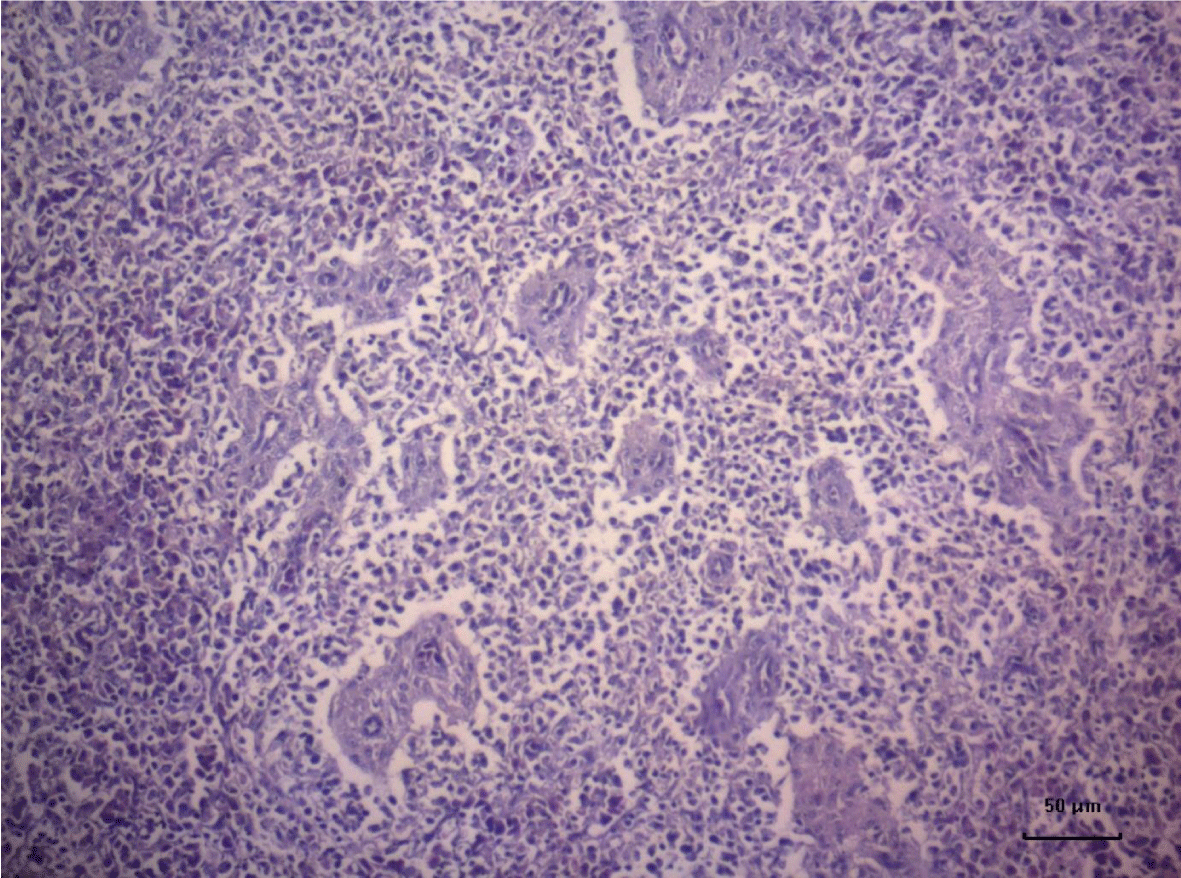
The most important pathomorphological changes in the spleen affected predominantly the lymph tissue of germinal centers. Turkey poults from the experimental group III supplemented with 0.2 mg/ kg AFB1, exhibited mild degenerative changes comprising karyopyknosis, karyorrhexis and karyolysis of nuclei and rarefaction of lymphoid cells (Figure 1). Generalized hyperplasia of tunica intima of splenic arterioles could be detected (Figure 2).
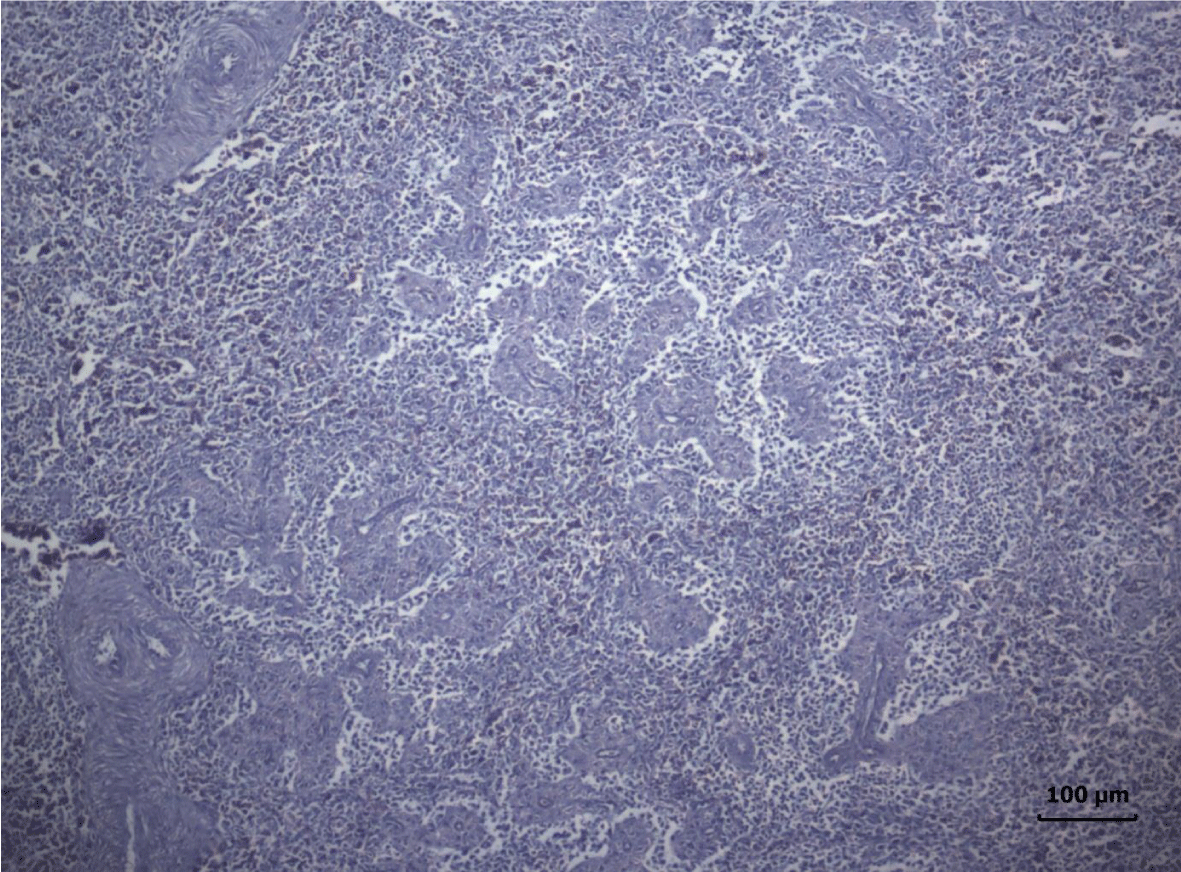
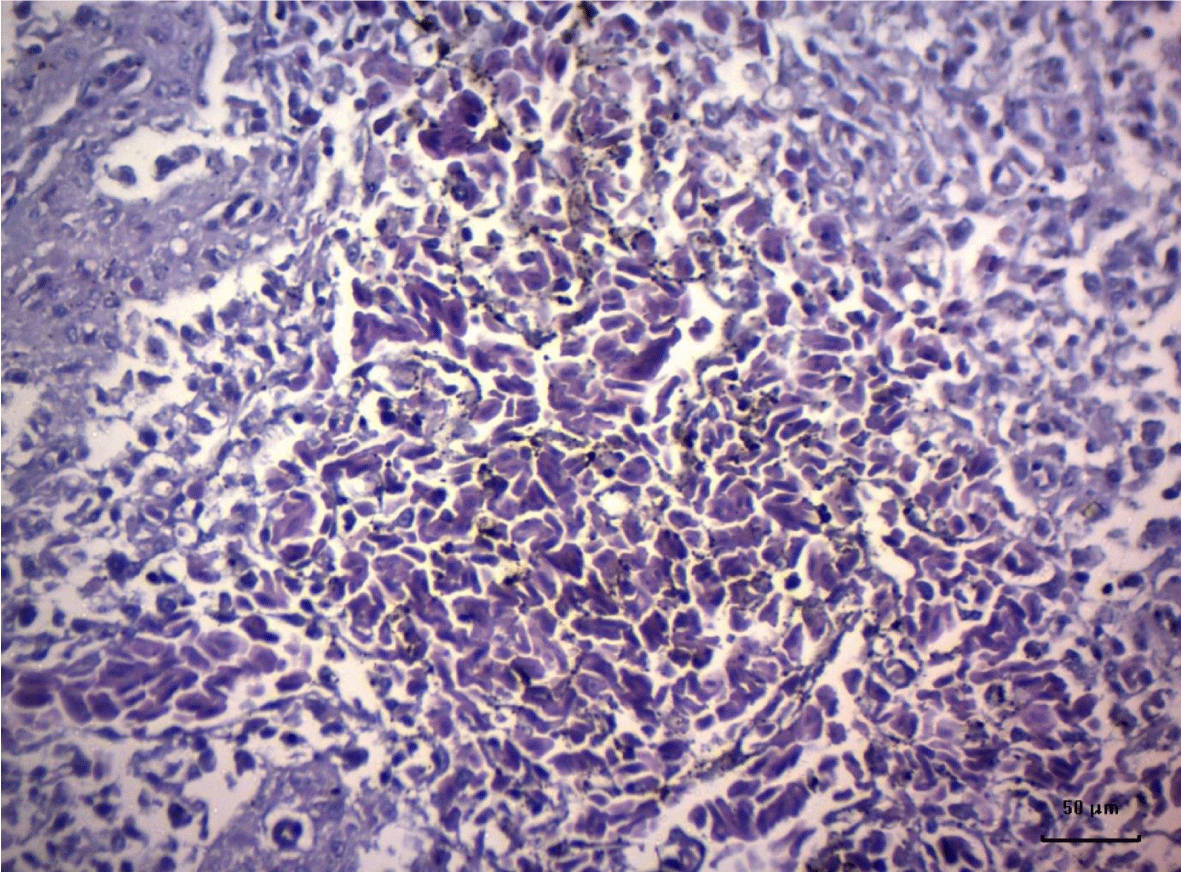
In turkey poults from Group ІV -, which received 0.4 mg/ kg AFB1 in their feed, the degenerative changes in the spleen were rather more extensive. Lymph follicle rarefaction was enhanced, with necrotic foci in some areas (Figure 3). Occasionally, hemorrhagic foci could be seen in the splenic pulp. Red pulp hemosiderosis was observed as well (Figure 4). Along with these changes, sclerosis of the central artery was present (Figure 5).
The degenerative changes in Group V (0.2 mg/ kg AFB1 and 0.5 g/kg Mycotox NG) were of lower intensity compared to those established in turkey poults from Groups III and IV. Morphological changes consisted mainly in congestive events in the red pulp and decreased density of lymphoid cells (Figure 6).
Turkeys from Group VI (0.4 mg/ kg AFB1 + 0.5 g/ kg Mycotox NG) demonstrated mild lymphoid cell depletion with slight thickening of the intima of splenic arteries and occasionally, hemosiderosis (Figure 7).
There were no dystrophic changes in the spleen of control (Group І) and Mycotox NG supplemented (Group II) turkey poults.
Discussion
Mycotoxicoses are diseases induced by feeding mould-contaminated feed ingredients. Aflatoxin is the prevailing and primary metabolite found in animal feeds [27]. Aflatoxins are a serious concern for poultry industry due to their high toxicity and common occurrence in compound poultry feeds [28,29]. In most cases, aflatoxin content in poultry feeds is higher than the maximally allowed amount of 20 μg. kg-1 feed [23].
Birds and fish are highly sensitive to the toxic impact of AFB1, and react to low doses within the range 15-30 ppb [30]. The bioactivation of aflatoxins is done in hepatocytes through microsomal enzyme systems - cytochrome Р450 (CYP450) where they are transformed into reactive aflatoxin-8,9-epoxide (AFBO), which is the primary most toxic metabolite. Тhis reactive compound inhibits protein synthesis, causes liver damage, immunosuppressive effects and decreases productive performance. Domestic turkeys are highly sensitive to the toxic effects of AFB1. In them, liver class alpha glutathione S-transferases (GSTA) are not capable to detoxify AFBO, which is probably the main reason for the high susceptibility of the species [30].
Sehu, et al. [31] reported that aflatoxins reduced the weight of immunocompetent organs (thymus, spleen, bursa of fabricius) in quails fed feed with total aflatoxin content of 2.5 mg/ kg. Compared to control group, the relative weight of the spleen in dietary treatments with AFB1 only was lower. This reduction of immunocompetent organ weights was possibly due to necrosis and decreased density of lymphoid cells [32].
Aflatoxins induce immunosuppression in domestic fowl by altering the morphological structure of immunocompetent organs (thymus, spleen, bursa of Fabricius) [33]. Rats supplemented with 1 mg/kg AFB1 with the feed for 6 weeks exhibited degenerative and haemorrhagic foci in the splenic pulp, degenerated blood cells, megakaryocytes, irregular arrangement of leukocytes in the white pulp, haemosiderin granules, and congestion [34]. In broiler chickens treated with 0.2 mg/ kg AFB1, Sakhare, et al. [35] observed necrotic foci in germinative centers, central artery sclerosis, reduced number of lymphocytes, hyperplasia of tunica intima and destruction of elastic fibres. Decreased density of lymphoid cells in immunocompetent organs (bursa of Fabricius, thymus, spleen, caecal tonsils, Harderian glands) were found out in broiler chickens treated with citrinin and aflatoxin either alone or in combination [36]. Balachandran and Hashem, Mohamed, [37,38] demonstrated lower lymphoid cell density and necrotic changes in the bursa of Fabricius and the spleen, increased number of germinal centres and reticular hyperplasia in broiler chickens receiving 1 mg aflatoxin/ kg feed over 28 days. Observed splenic lesions also supported the immunotoxic and haemotoxic effects of aflatoxins [39,40]. Immunotoxic effects of aflatoxins are well studied in domestic poultry [33,41,42]. In previous studies of ours with broiler chickens Valchev, et al. [43] treated by supplementation of the feed with 0.5 mg/ kg AFB1 or 0.8 mg/ kg AFB1 dystrophic changes in lymphoid cells and reduced lymphoid cell density (lymph tissue reduction) were found out. Rats treated with 1 mg/ kg AFB1 for 6 weeks exhibited lymphocyte degeneration, hemorrhages, degenerated blood cells, megakaryocytes, congestion in blood sinuses, necrotic foci, atrophy of the white pulp and irregular arrangement of leukocytes in the white pulp, [16]. The morphological structural changes demonstrated by this study were similar to changes in immunocompetent organs (thymus and bursa of Fabricius) reported by Kumar and Balachandran, [44] in broiler chickens treated with 1 mg/ kg AFB1 over 28 days. It was demonstrated that the toxic effect of aflatoxins on lymphoid or hematopoetic organs resulted in rarefaction of lymphoid cell density in the white pulp and red pulp hemosiderosis [45].
Aflatoxins impair the synthesis of proteins by forming adducts with DNA, RNA and proteins [46], RNA synthesis inhibition, reduced activity of DNA-dependent RNA polymerase and degranulation of granulated endoplasmic reticulum [47] - mechanisms through which the structure of a number of tissues (liver, kidneys, skeletal muscles, heart, pancreas, immunocompetent organs) [48-50]. It is reported that aflatoxins enhance lipid peroxidation (LPO) and induce cellular damage resulting in alteration of normal morphological structure of parenchymal organs [51,52].
The addition of Mycotox NG to the diet of birds from Groups V and VI reduced the deleterious effects of AFB1 on histopathological changes in the spleen of broiler chickens. Less severe histological lesions of immunocompetent organs were found out in broiler chickens after supplemented of aflatoxin-contaminated feed with natural zeolite (clinoptilolite) [53,41], herbo-mineral mycosorbent-Toxiroak [35] or of Mycotox NG [43]. The negative polarity of mycotoxins is attracted by the positive polarity of the toxin binder - a mechanism through which toxin are immobilized and eliminated from the organism of animals [54].
Conclusion
The tested AFВ1 amounts induced dose-dependent morphological alterations in the spleen (degenerative changes in lymph follicle, hyperplasia of tunica intima of splenic arterioles, rarefaction with necrotic foci), the relative weight of the spleen was lower compared to that of control birds. The addition of 0.5 g/ kg mycosorbent (Mycotox NG) to the ratio of Groups V and VI that contained either 0.2 mg/ kg or 0.4 mg/ kg AFB1 partly reduced the deleterious effects of AFB1 on spleen relative weight and the histological changes (congestive events decreased, density of lymphoid cells and thickening of the intima of splenic arteries and occasionally, hemosiderosis) in this organ.
- Grochulski P, Masson L, Borisova S, Pusztai-Carey M, Schwartz JL, Brousseau R, et al. Bacillus thuringiensis Cry1A (a) insecticidal toxin: Crystal structure and channel formation. J Mol Biol. 1995; 254: 447-64. https://goo.gl/zzP7KH
- Cullen JM, PM Newberne. Acute Hepatotoxicity of Aflatoxins. In: Eaton DL, Groopman, JD. (eds). The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance. 1993; 1-26. https://goo.gl/81cE9K
- Blount WP. Turkey “X” disease. Journal of the British Turkey Federation. 1961; 9: 52-4. https://goo.gl/FEGk8m
- Verma RJ. Aflatoxin Cause DNA Damage. International Journal of Human Genetics. 2004; 4: 231-236. https://goo.gl/J2Ype8
- Gowda NKS. Malathi V and Suganthi RU. Screening for aflatoxin and the effect of moisture, duration of storage and form of feed on fungal growth and toxin production in livestock feeds. Animal Nutrition and Feed Technology. 2004; 3: 45-51. https://goo.gl/Co4mXC
- Wilson DM, Payne GA. Factors affecting Aspergillus flavus group infection and aflatoxin contamination of crops. In:Eaten, DL and Groopman, JD (Eds.), The toxicology of aflatoxins. (1st. Edn.), San Diego, Academic Press, Inc., 1994; PP: 123-145.
- Leeson S, Diaz GJ, Summers JD. "Poultry Metabolic Disorders And Mycotixns." University Books, Guelph, Ontario, Canada: 1995; p. 249-298. https://goo.gl/aWvV48
- Cortes G, Carvajal M, Mendez-Ramirez I, Avila-Gonzalez E, Chilpa-Galvan N, Castillo-Urueta P, Flores CM. Identification and qualification of aflatoxins and aflatoxicol from poultry feed and their recovery poultry Litter. Poult Sci. 2010; 89: 993-1001. https://goo.gl/35cV2Y
- Nizamlyoglu F, Oguz H. Occurrence of aflatoxins in layer feed and corn samples in Konya province, Turkey. Food Addit Contam. 2003; 20: 654-8. https://goo.gl/u9JXhM
- Coelho. Molds, mycotoxins and feed preservatives in the feed industry. BSAF Corparation, 100. Cherry Hill Road, Parsippany; NJ07054: 1990. p.159. https://goo.gl/oVkgRn
- Oguz H, Kurtoglu V, Coskun B. Preventive efficacy of clinoptilolite in broiler during chronic aflatoxin (50 and 100 ppb) exposure. Res Vet Sci. 2000; 69: 197-201. https://goo.gl/xGirGH
- Sur E, Celik I. Effects of aflatoxin B1 on the development of chicken thymus and blood lymphocyte alpha-naphthyl acetate esterase activity. Vlaams Diergeneeskundig Tijdschrift. 2005;74: 432-439. https://goo.gl/VuJAad
- Oguz H, Kececi T, Birdane YO, Onder F, Kurtoglu V. Effect of clinoptilolite on serum biochemical and haematological characters of broiler chickens during experimental aflatoxicosis. Res Vet Sci. 2000; 69: 89-93. https://goo.gl/QUJFf4
- Qureshi MA, Brake J, Hamilton PB, Hagler WM Jr, Nesheim S. Dietary exposure of broiler breeders to aflatoxin results in immune dysfunction in progeny chicks. Poult Sci. 1998; 77: 812-9. https://goo.gl/zkQtQT
- Kiran MM, Demet O, Ortatath M, Oguz H. The preventive effect of polyvinyl–polypyrrolidone on aflatoxicosis in broilers. Avian Pathol. 1998; 27: 250-5. https://goo.gl/NVrsmT
- Omar NA. Effect of some aflatoxins on a lymphatic organ (spleen) of male albino rats (histopathological study). Egyptian Journal of Hospital Medicine. 2012; 48: 357-367. https://goo.gl/SbVgou
- Chen K, Peng X, Fang J, Cui H, Zuo Z, Deng J, et al. Effects of dietary selenium on histopathological changes and T cells of spleen in broilers exposed to aflatoxin B1. Int J Environ Res Public Health. 2014; 11: 1904-13. https://goo.gl/mNRz4s
- Abo-Norag M, Edrington TS, Kubena LF, Harvey RB, Phillips TD. Influence of hydrated sodium calcium aluminosilicate and virginamycin on aflatoxicosis in broiler chicks. Poult Sci. 1995; 74: 626-32. https://goo.gl/PNwUMM
- Jindal N, Mahipal SK, Mahajan NK. The effects of hydrated sodium calcium aluminosilicate on prevention of aflatoxicosis in broilers. Indian Journal of Animal Science. 1993;63: 649–652.
- Miazzo R, Rosa CA, De Queiroz Carvalho EC, Magnoli C, Chiacchiera SM, Palacio G, et al. Efficacy of synthetic zeolite to reduce the toxicity of aflatoxin in broiler chicks. Poult Sci. 2000; 79: 1-6. https://goo.gl/53CXs7
- Santurio JM, Mallmann CA, Rosa AP, Appel G, Heer A, Dageforde S. Effect of sodium bentonite on the performance and blood variables of broiler chickens intoxicated with aflatoxin. Br Poult Sci. 1999; 40: 115-9. https://goo.gl/apou56
- Edrington TS, Kubena LF, Harvey RB, Rottinghaus GE. Influence of super activated charcoal on the toxic effects of aflatoxin or T-2 toxins in growing broilers. Poult Sci. 1997; 76: 1205-11. https://goo.gl/MrWUwD
- Aravind KL, Patil VS, Devegowda G, Umakantha B, Ganpule SP. Efficacy of esterified glucomannan to counteract mycotoxicosis in naturally-contaminated feed on performance, serum biochemical and haematological parameters in broilers. Poult Sci. 2003; 82: 571-6.
- Dawson AK. Mycotoxin-binding agents in nutritional strategies for improving animal performance and health. In: Biotechnology in the feed Industry. Proceedings of the Alltech’s 1999 Asia Pacific Lecture Tour: Under the Microscope. (Lyons TP and KA Jacques, eds). Alltech (UK), Stamford, UK: Focal Points for the New Millennium; 1999. p: 65-74. https://goo.gl/sgX1U5
- Ordinance, 44/20.04.2006 for veterinary medical requirements to animal rearing facilities, Official Gazette 41 Appendix 8.
- Ordinance No. 20 of 01.11.2012 on minimum requirements for protection and humane treatment of experimental animals and the requirements for the use, breeding and / or delivery of the animals, State Gazette, 87, 9.11.2012.
- Nilipour AH. Mycotoxins, an insidious global concern. World Poultry. 2002; 2: 18-20.
- Denli M, Okan F, Doran F. Effect of conjugated linoleic acid (CLA) on the performance and serum variables of broiler chickens intoxicated with aflatoxin B1. South African Journal of Animal Science. 2004; 34: 97-103. https://goo.gl/64kZof
- Tessari EN, Oliveira CA, Cardoso AL, Ledoux DR, Rottinghaus GE. Effects of aflatoxin B1 and fumonisin B1 on body weight, antibody titres and histology of broiler chicks. Br Poult Sci. 2006; 47: 357-64. https://goo.gl/XHA6sq
- Rawal S, Kim JE, Coulombe R Jr. aflatoxin B1 in poultry: Toxicology, metabolism and prevention. Res Vet Sci. 2010; 89: 325-31. https://goo.gl/gxgGSg
- Sehu A, Cakir S, Cengiz O, Essiz D. MYCOTOX and aflatoxicosis in quails. Br Poult Sci. 2005; 46: 520-4. https://goo.gl/aqsW4E
- Perozo F, Rivera S. Effect of aflatoxin B1 exposure and selenium supplementation on immune response in broilers. Indian Veterinary Journal. 2003; 80: 1218-1221.
- Celik I, Oguz H, Demet O, Dönmez HH, Boydak M, Sur E. Efficacy of polyvinylpolyrrolidane in reducing the immunotoxicity of aflatoxin in growing broilers. Br Poult Sci. 2000; 41: 430-9. https://goo.gl/k3bKMW
- Nahed AO. Effect Of Some Aflatoxins On A Lymphatic Organ (Spleen) Of Male Albino Rats (Histopathological Study). The Egyptian Journal of Hospital Medicine. 2012; 48: 357 - 367. https://goo.gl/VqMq6L
- Sakhare PS, Harne SD, Kalorey DR, Warke SR, Bhandarkar AG, Kurkure NV. [Effect of Toxiroak® polyherbal feed supplement during induced aflatoxicosis, ochratoxicosis and combined mycotoxicoses in broilers]. Veterinarski Arhiv. 2007; 77: 129–146. https://goo.gl/ovYPSW
- Anandkumar C, Theophilus, Balachandran C. Pathological Effect of Citrinin and Aflatoxin in Broiler Chicken. Pharmaceutical Science. 2014; 4: 1-14.
- Balachandran C, Parthasarathy, K.R. A, Sundararaj A. Experimental study on pathology of cyclopiazonic acid mycotoxicosis in broiler chicken. Indian Veterinary Journal. 1998; 75: 693-697.
- Hashem MA, Mohamed MH. Haemato-biochemical and pathological studies on aflatoxicosis and treatment of broiler chicks in Egypt. Vet Ital. 2009; 45: 323-37. https://goo.gl/T4Cuuw
- Gabal MA, Azzam AH. Interaction of aflatoxin in the feed and immunisation against selected infectious diseases in poultry. II. Effect on one-day-old layer chicks simultaneously vaccinated against Newcastle disease infectious bronchitis and infectious bursal disease. Avian Pathol. 1998; 27: 290-5. https://goo.gl/qBmR94
- Ibrahim IK, Shareef AM, Al-Joubory KM. Ameliorative effects of sodium bentonite on phagocytosis and Newcastle disease antibody formation in broiler chickens during aflatoxicosis. Res Vet Sci. 2000; 69: 119-22. https://goo.gl/WX9aE4
- Ortatatli M, Oguz H, Hatipoglu F, Karaman M. Evaluation of pathological changes in broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Res Vet Sci. 2005; 78: 61-8. https://goo.gl/EWXW9b
- Sur E, Donmez HH, Boydak M, Ataman MB. Effects of glucomannan on the sacculus rotundus and peripheral blood lymphocytes in New Zealand rabbits during aflatoxicosis. The Scientific World Journal. 2012 Under publication. https://goo.gl/Deud9b
- Valchev I, Zarkov I, Grozeva N, Nikolov Y. Effects of aflatoxin В1 on production traits, humoral immune response and immunocompetent organs in broiler chickens. Agricultural Science and Technology. 2014; 256-62. https://goo.gl/UNHnmj
- Kumar R, C. Balachandran. Pathological changes in broiler chicken fed aflatoxin and cyclopiazonic acid. XV Annual Conference of Indian Association of Veterinary Pathologists. 1998; 9-11.
- Prabu PC, Dwivedi P, Sharma AK. Toxicopathological studies on the effects of aflatoxin B, ochratoxin A and their interaction in New Zealand White rabbits. Exp Toxicol Pathol. 2013; 65: 277-86. https://goo.gl/NSdo3k
- Busby W.F. and Wogan, G.N. Aflatoxins. In: Chemical Carcinogens. Searle S.E. Ed., ACS Monograph 182, pp. 945n1136, American Chemical Society, Washington D.C. 1984.
- Verma RJ, Nair A. Ameliorative effect of vitamin E on aflatoxin-induced lipid peroxidation in the testis of mice. Asian Journal and Andrology . Asian J Androl. 2001; 3: 217-21. https://goo.gl/fYDpwk
- Wangikar PB, Dwivedi P, Sinha N, Sharma AK, Telang AG. Teratogenic effects in rabbits of simultaneous exposure to ochratoxin A and aflatoxin B1 with special reference to microscopic effects. Toxicology. 2005; 215: 37-47. https://goo.gl/G2dPML
- Mohammed AM, MetwallyNS. Antiaflatoxicogenic activities of some aqeous plant extracts against AFB1 induced Renal and Cardiac damage. Journal of Pharmacology and Toxicology.2009; 4; 1-16. https://goo.gl/LcgQAM
- Sharma V, Sharma C, Paliwai R, Prachetal, Sharma S. Ameliorative Effects of Curcuma Longa and Curcumin on aflatoxin B1 Induced Serological and Biochemical Changes In Kidney of Male Mice. Asian Journal of Biochemical and Pharmaceutical Research. 2011; 1: 338-351.
- Verma RJ, Chaudhari SB. Altered acidic and basic protein concentrations in skeletal muscle of aflatoxinfed rabbits. Medical Science Research. 1999; 27: 427-428.
- Darwish HR, Omara EA, Abdel-Aziz KB, Farag LM, Nada SA, Tawfek NS. Saccharomyces cerevisiae modulates Aflatoxin-induced toxicity in male Albino mice. 2011; 3: 32-43. https://goo.gl/coiq3P
- Ortatatli, M, Oguz H. Ameliorative effects of dietary clinoptilolite on pathological changes in broiler chickens during aflatoxicosis. Res Vet Sci. 2001; 71: 59-66. https://goo.gl/nFEMdK
- Kana JR, Ngoula F, Tchoffo H, Tadondjou CD, Sadjo YR, Teguia A, et al. Effect of biocharcoals on hematological, serum biochemical and histological parameters in broiler chickens fed aflatoxin B1-contaminated diets. J Anim Sci Adv.2014; 4: 939-948. https://goo.gl/xoJWGi


Sign up for Article Alerts