In vitro Cytotoxic Effects of Extracts of Fourteen Medicinal Plants of Nigerian Origin on Vero Cells
Obi RK1*, Chikwendu CI1 and Shenge JA2
1Department of Microbiology, School of Biological Sciences, Federal University of Technology, Owerri, P.M.B 1526, Owerri, Imo State, Nigeria
2Virology Unit, Department of Biological Sciences, Dominican University, Ibadan, Oyo State, Nigeria
*Address for Correspondence: Obi RK, Department of Microbiology, School of Biological Sciences, Federal University of Technology, Owerri, P.M.B 1526, Owerri, Imo State, Nigeria, Tel: 234-080-387-575-15; ORCID: 000-000-016-524-694X; E-mail: [email protected]
Submitted: 08 February 2020; Approved: 18 February 2020; Published: 03 March 2020
Citation this article: Obi RK, Chikwendu CI, Shenge JA, In vitro Cytotoxic Effects of Extracts of Fourteen Medicinal Plants of Nigerian Origin on Vero Cells. Adv J Toxicol Curr Res. 2020;4(1): 001-010.
Copyright: © 2020 Obi RK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Cell line; Toxicity; Probit; Medicinal plants; Inhibitory concentration; Herbarium; Vero
Download Fulltext PDF
Background and Objective: Studies on the efficacy and safety of medicinal plants have indicated that there are many phytochemicals that have cytotoxic, genotoxic, and carcinogenic effects. The aim of the present study was therefore to evaluate the non-specific toxicity of extracts of fourteen (14) edible medicinal plants indigenous to Nigeria using African green monkey kidney (Vero) cells.
Materials and Methods: Different parts of some of the plants were collected from the wild in Ijegun and Ikotun communities, while others were bought from Mushin market, Lagos State, South Western Nigeria. They were subjected to Soxhlet extraction using analytical grade methanol and dried in vacuo, under pressure. The resulting extracts were then subjected to cytotoxic analysis using Vero cells. Using probit, the concentration of the extracts that inhibited Cytopathic Effect (CPE) on Vero cells by 50% (IC50), was calculated.
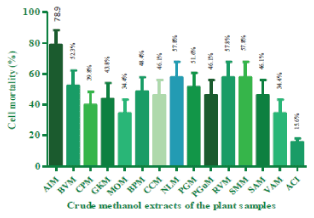
Results: Results showed that the highest percentage of cytotoxic activity of the methanol extracts of the plants was produced by A. indica (78.9%) and IC50 of 0.011 μg μL-1. G. kola with 43.8% cytotoxic activity produced IC50 of 0.090 μg μL-1. The result also showed that the extracts with the least percentage toxicities were M. oleifera and V. album. Acyclovir, an orthodox drug, was found to be milder on the Vero cells with 15.6% and IC50 of 4.56 μg μL-1.
Conclusion: The cytopathic activities exhibited by the methanol extracts of the fourteen medicinal plants used in this study shows that they are very toxic to mammalian cells.
Introduction
Many plants produce toxic secondary metabolites as natural defense mechanisms from adverse conditions. In some plant species, these toxic substances are not distinguished from therapeutically bioactive components [1]. Due to the fact that plants do not move about, they have developed different strategies that will enable them to co-exist with herbivores and potentially pathogenic microorganisms in their environment. In view of this, they synthesize a large number of bioactive compounds known as phytoanticipins that are released in response to specific environmental stimuli [2]. Some of these phytochemicals produced by plants against herbivorous insects also end up being harmful to humans, probably because certain biological similarities between humans and insects [3].
The primary aim of toxicological assessment of any herbal medicine is to identify adverse effects and to determine limits of exposure level at which such effects occur. Two important factors which are taken into consideration in evaluating the safety of any herbal drug are the nature and significance of the adverse effect and the exposure level where the effect is observed [4]. Another important objective of toxicity analysis is the detection of toxic plant extracts or compounds in the early and late stages of drug discovery and development from plant sources [5]. This facilitates the identification of toxic metabolites which can be discarded or modified during the process and create an opportunity for extensive evaluation of safer, promising alternatives [6]. For certain compounds, modifications such as dosage reduction, chemical group or structural adjustments may improve their tolerability [7].
Cytotoxicity assays are performed to predict potential toxicity, using cultured cells which may be normal or transformed cells. These tests normally involve incubation of cultured cells and plant extracts, to detect how basal or specialized cell functions may be affected by the substance, before safety studies could be performed using whole organisms. This can also provide insight toward the carcinogenic and genotoxic effect of herb-derived compounds and extracts [8]. The ability of a plant extract to inhibit cellular growth and viability can also be ascertained as an indication of its toxicity [9]. Culturing cells with cytotoxic compound can lead to necrosis involving loss of membrane integrity and rapid death as a result of cell lysis. Furthermore, active cell division may completely stop, leading to a decrease in cell viability, or the cells can activate a genetic program of controlled cell death or apoptosis [10]. Evidence of cell necrosis is manifested by rapid swelling, loss of membrane integrity, shut down of metabolism and release cellular contents into the environment [11]. Apoptosis is characterized by well-defined cytological and molecular events including a change in the refractive index of the cell, cytoplasmic shrinkage, nuclear condensation and cleavage of DNA into fragments [12].
In view of the fact that many plants produce toxic and medicinally useful secondary metabolites in their tissues, this study was designed to evaluate the cytotoxicity of partially purified extracts of fourteen medicinal plants indigenous to Nigeria, on uninfected Vero cells.
Materials and Methods
Study design
This is an In vitro tissue culture study that involved the collection, methanol extraction, and cytotoxic analysis of fourteen medicinal plants of Nigerian origin using Vero cells. Initial tissue culture study was carried out at the Virology Laboratory in the Department of Medical Microbiology and Parasitology, College of Medicine, University of Lagos from Jan 2013-Oct 2014. Methanol extraction was done at the Pharmocognosy Department, School of Pharmacy University of Lagos from Oct 2013-June 2016. Evaluation of cellular toxicity was done at the Department of Virology, College of Medicine, and University of Ibadan from Oct 2014-Oct 2016.
Collection and preparation of plant samples
Different plant parts of the selected fourteen (14) medicinal plants were collected from Ijegun, Okota, and Mushin Market, all in Lagos State, South Western Nigeria, between October, 2012 and July, 2014. The plants were authenticated a Taxonomist at the Herbarium Unit of the Department of Botany, University of Lagos, where voucher specimens were deposited. The voucher numbers were: Azadirachta indica (A. Juss.) LUH 5502, Bambusa vulgaris (Shrad.) LUH 5493, Bryophyllum pinnatum (Lam.) LUH 5500, Citrus paradisi (Macfad.) LUH 7659, Cymbopogon citratus (D.C) Stapf. LUH 5496, Garcinia kola (Heckel.) LUH 7658, Moringa oleifera (Lam.) LUH 5498, Newbouldia laevis (P. Beauv.) LUH 5696, Piper guineense (Schumah.) LUH 5501, Psidium guajava (L.) LUH 8146, Rauvolfia vomitoria (Afzel.) LUH 5503, Senna alata (L.) Roxb. LUH 5496, Spondias mombin (L.) LUH 5492, Viscum album (L.) LUH 8252. Table 1 shows the medicinal uses of the plants, their local, common and species names [13,14].
Extraction and concentration of plant materials
Each of the plant parts was dried at 25°C for 7 days. The samples were blended to coarse powder using a laboratory milling machine (Christy and Norris, England). The method of extraction used was hot continuous extraction using the Soxhlet extractor (Thermo Fisher Scientific, USA), using 250 mL of methanol (Emsure, Merck KGaA, Germany) as described by Velmurugan, et al [15]. After extraction, the solvent was concentrated in vacuo at 45°C using a rotary evaporator (Heidolph Laborata 4010 Digita, USA).
Reconstitution of extracts/fractions
Dried/pasty (10 mg) solid plant extracts were introduced into sterile calibrated 50 mL centrifuge tubes. They were then reconstituted in 0.5% Dimethyl Sulfoxide (DMSO) (Sigma) and shaken vigorously to ensure complete dissolution and brought to a final volume of 10 mL with the addition of 9.95 mL of sterile distilled water. They were subsequently filtered; first, with 0.45 µM and then with 0.22 μM membrane syringe filters (Cell Treat USA). A 100 µL of each extract concentration was thereafter used at the concentrations of 1, 0.5, 0.25, 0.125, 0.063, 0.031, 0.016 and 0.008 μg/μL, to evaluate the cellular toxicity of the extracts on Vero cells using 96-well plates [16].
Evaluation of cellular toxicity
Preparation of cell line: The method used was based on cellular morphologic changes [17]. Vero cells were prepared at a density of 8 x104 cells mL-1 in a 10% MEM in 75 cm2 tissue culture flasks (Cell Treat, USA). A 100 μL of this cell suspension (containing 8000 cells) was then dispensed into each well of a flat bottomed 96-well tissue culture plate (Cell Treat, USA) and incubated for 24 hours at 37°C. The 10% MEM was aspirated thereafter and discarded.
Preparation and two-fold dilution of plant extract: Eight sterile test tubes labeled with the code name for each extract was arranged on a rack to carry out a 2 fold serial dilution. Unto each tube was introduced 1 mL (1000 μL) of the 1% MEM to serve as diluent. Then 1 mL (1000 μL) of each fully thawed extract was introduced into the first tube containing no medium labeled as Neat (undiluted (100%)) while the remaining 1 mL (1000 μL) was introduced into the second tube containing the 1% MEM diluent and labeled ½ (50%). After proper mixing, 1 mL (1000 μL) of the extract and medium mixture was aspirated and introduced into the third tube labeled ¼ (25%). The dilution continued to the last bottle labeled 1/128 (0.8%).
Preparation of positive: The positive control, Virest 200 mg, a brand of Acyclovir (Hovid Bhd, Malaysia), was prepared by dissolving 200 mg of the tablet in 200 mL of Phosphate Buffered Saline (PBS) and filtered using 0.22μm of 250 mL (Nalgene filter, USA) fixed to electrically controlled suction pump. Then 1 mL of the filtered drug was dispensed into 9 mL of PBS in a sterile universal bottle. A single two-fold serial dilution of the drug was carried out to evaluate its toxicity on the Vero cells.
Cytotoxicity activity: The 96 well tissue culture plates were also labeled with the different dilution and code name of each extract. Then using different pipette tips, 100 μL of each extract dilution was introduced into each of the 96-wells containing the Vero cells in duplicates. The last row of wells containing cells but no extracts was used as negative control, the row containing solvent only was used as solvent control, while the row containing the Virest drug and cell was used as positive control.
The plates were labeled and incubated at 37°C in a 5% CO2 incubator and moisture. Cell viability was monitored every day for 14 days to observe any possible immediate changes in morphology (CPE) compared with the control wells containing only medium and no extract, using an inverted microscope (Inverskop 40C, USA) [18,19]. Complete (100%) CPE was scored as 4+ (100%); 3+ (75%); 2+ (50%); 1+ (25%) and 0 (0%) when there was no CPE.
Statistical analyses
Results were presented as Standard Error of Mean (SEM). Data were assessed by one-way Analysis of Variance (ANOVA) followed by Duncan multiple comparison, Turkey’s multiple comparison and student’s t-test. All statistical analysis were performed at the p < 0.05 level of significance. All the statistical analysis were done using GraphPad software version 5.01 (GraphPad Software Incorporated, U.S.A, 2007).
Results
Evaluation of cellular toxicity
Cytotoxicity activity: The toxicity of fourteen crude extracts were evaluated using Vero cells. All the extracts produced varying degrees of toxicity on the cell. The result in figure 1 showed that the highest percentage of cytotoxic activity of the crude extracts of the plants was produced by A. indica (78.9%) while G. kola produced 43.8% cytotoxic. The result also showed that the extracts with the low percentage toxicities on Vero cells were M. oleifera and V. album with 34.4%. Acyclovir, an orthodox drug, was found to be milder on the Vero cells with 15.6%.
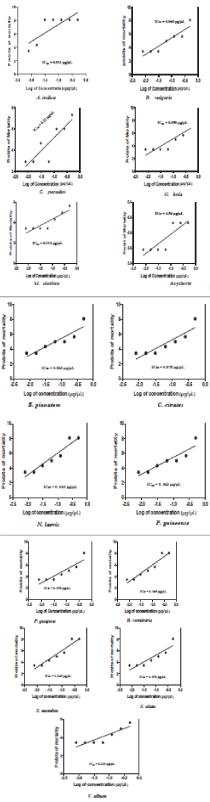
Fifty percent (50%) inhibitory concentration (IC50): The result of the IC50 of the extracts showed that A. indica with 0.011 μg μL-1 was the most toxic. Garcinia kola showed 0.090 μg μL-1. Moringa oleifera and Viscum album were the least toxic extracts at equal IC50 of 0.313 μg μL-1. Acyclovir being a processed drug exhibited very mild toxicity on the Cell at 4.56 μg μL-1.
Level toxicity: the level of toxicity represents the extent at which the extracts caused cytopathic effect on Vero cell line. The result in table 2 represents the level of toxicity and the maximum concentration at which the extracts showed no evident CPE on the cell line. Methanol extract of A. indica showed its Maximum Non-Toxic Concentration (MNTC) at 0.008 μg μL-1. In G. kola, methanol extract, the MNTC was 0.063 μg μL-1. The methanol extracts of M. oleifera and V. album also showed an MNTC of 0.063 μg μL-1. Acyclovir was mild on the cell throughout but had its MNTC at 0.063 μg μL-1.
Discussion
Medicinal plants are typically presumed to be non-toxic and regarded safe due to their natural origin and long use in traditional medicine to treat various forms of diseases [20,21]. However, scientific studies on efficacy and safety of some medicinal plants indicated that there are many phytochemicals that have cytotoxic, genotoxic, and carcinogenic effects when used for longer periods [22,23] and even at shorter times. Cytotoxic agents unselectively kill and damage both normal and cancerous cells by interfering with either, the cellular process or mechanical process [24]. Cytotoxicity testing is therefore important for the sole purpose of determining the potential toxicity of the compounds being studied [25].
This study demonstrated cytotoxicity indices as a measure of cell mortality as observed under the inverted microscope. The reduction in viable cell numbers was visibly evident with all the extracts after incubation. Similarly, the morphological effects were very prominent with all the extract-treated cells manifesting extensive rounding, shrinking, and vacuolation. The extracts and fractions were able to inhibit the proliferation of Vero cells at IC50 values as recommended by the National Cancer Institute (NCI) for crude extracts. The recommended guideline set the IC50 limit of activity for crude plant extracts at < 20 µg/mL after an exposure time of 72 hours [26]. The lower the IC50 values of a given crude extract, the higher will be its toxicity potential [27]. This observation is in agreement with the findings of this present study which showed that the IC50 of 4.56 µg/µL recorded for Acyclovir, a processed and purified drug, was far higher than that recorded for all the crude extracts and their fractions. In addition, a higher IC50 and low MNTC indicated that the extracts could be considered safe to be applied directly to mammalian cells without activation of cell death, or indirectly, as pharmaceutical raw materials for drug production [26].
Previously, studies have confirmed the high toxicity of methanol extracts to cancer cell lines (HeLa), and attributed this to its toxic target to their cell membrane [28,29]. As reported by Sentkowska, et al. [30], the high toxicity of methanol extracts could be due to the polar nature of methanol and its capability to extract various polar and sometimes non-polar bioactive compounds with varying toxicities. Methanol molecule consists of a single atom of a tetraedric carbon, linked to 3 hydrogens, and a hydroxyl (OH) group. The -OH group is polar in nature, and the three hydrogens, the water-insoluble hydrocarbon chain. This gives methanol dual properties, and the capability to dissolve both polar and non-polar molecules [31].
The cytotoxicity activities of the extracts ranged from high, mid, and low. The potentiality (78.9%) of the crude leaf extract of A. indica as a highly active cytotoxic agent against Vero cells was revealed in its very low IC50 of 0.011 µg µL-1. The cytotoxic properties of A. indica could be attributed to its possession of methyl stearate, which Bai and Krishnakumar [32] reported to possess antiproliferative and cytotoxic properties. The toxicity profile of A. indica has also been reported by several other workers [33-35]. Although the toxicity of this plant was reported to be harmful to mammalian cells, it has been shown to be beneficial as a pesticide [36] and a larvicide [37,38]. A. indica has also been reported to be toxic to spermatocytes [39].
The percentage cytotoxicity of seed extract of G. kola (43.8%) and IC50 of 0.090 µg µL-1 produced marked apoptotic effect on Vero cells. One of the constituents of G. kola which could have been responsible for this cytopathic effect on Vero cell is methyl stearate, reported to possess antiproliferative effect on mammalian cells. The toxicity profile of G. kola on mammalian cells has further been manifested in its ability to antagonize ovulation, and prevent progesterone production and fetal implantation in pregnant mammals [40]. In addition, the ability of G. kola to interfere with sperm production in both men and animals due to its toxic profile to mammalian cells has been extensively documented [41]. Other workers have reported caution in the use of G. kola [42] due to its high toxic activity to mammalian cells.
Although leaf extract M. oleifera was not as toxic as A. indica, it still produced remarkable cytopathic effect on Vero cells, manifested in its percentage toxicity of 34.4% and IC50 of 0.313 µg µL-1. Jafarain, et al. [43] has previously reported the cytotoxicity of leaf extract of M. oleifera on HeLa cells and attributed this to the presence of phenols in the plant tissues. It is possible also that the presence of this phytochemical, which has been previously identified to be a constituent in Nigerian M. oleifera plants, contributed in no small way to the cytopathic activities of M. oleifera observed in this study. In a similar manner, Kasolo, et al. [44] reported that M. oleifera leaf extract possess phytochemicals which could cause toxicity to mammalian kidney, liver and heart if half lethal doses are administered orally to Swiss Albino mice for thirty days.
Several other reports have been made about the cytotoxic activities of the crude extracts of the plants used in this study. According to Reppas [45], two adult cattle died of myocardial degeneration within 48 h after they were fed with a large amount of B. pinnatum (L.), probably due to the presence cardiac glycosides and bufadienolide in the plant tissues. Similarly the fruits of most V. album (L.) species were described as toxic berries which could lead to vomiting, hypotension, cerebral dysfunctions and death by a heart attack if ingested in large quantity [46]. However very little information is available on the cytotoxicity of N. laevis to Mammalian cells which this study has also shown to be very toxic.
Conclusion
This toxicity study has shown that, although all the plants are edible, they should be consumed with caution since it was observed that their methanol extracts could damage mammalian cells. However this high cytopathic activity of the crude extracts against a transformed cell line could be exploited in drug production against cancerous cells.
- Verma S, Singh SP. Current and future status of herbal medicines. Veterinary world. 2008; 1: 347-350. http://bit.ly/3a7eDdn
- Kennedy DO, Wightman EL. Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. Advances in Nutrition. 2011; 2: 32-50. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22211188
- Nassel DR, Winther AM. Drosophila neuropeptides in regulation of physiology and behavior. Prognostic Neurobiology. 2010; 92: 42-104. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22211188
- Amster E, Tiwari A, Schenker MB. Case report: Potential arsenic toxicosis secondary to herbal kelp supplement. Environmental Health Perspectives. 2007; 115: 606-608. http://bit.ly/391Wttq
- Gow PJ, Connelly NJ, Hill RL, Crowley P, Angus PW. Fatal fulminant hepatic failure induced by a natural therapy containing kava. Med J Aust. 2003; 178: 442-443. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12720510
- Gamaniel KS. Toxicity from medicinal plants and their products. Nig J Nat Prod and Med. 2000; 4: 4-8. http://bit.ly/2TcHkyG
- Celik MM, Karakus A, Zeren C, Demir M, Bayarogullari H, Duru M, Al M. Licorice induced hypokalemia, edema, and thrombocytopenia. Hum Exp Toxicol. 2012; 31: 1295-1298. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22653692
- Mosihuzzaman, M. Herbal medicine in healthcare-an overview. Nat Prod Commun. 2012; 7: 807-812. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22816312
- Abe K, Matsuki N. Measurement of cellular 3-(4,5-dimethylthiazol–2-y-l)-2,5-diphenyltetrazolium (MTT). Neuroscience Research. 2000; 38: 325-329. http://bit.ly/2T0mMdR
- Unterthiner T, Mayr A, Klambauer G, Steijaert M, Ceulemans H, Wegner JK, et al. Deep learning as an opportunity in virtual screening. Workshop on deep learning and representation learning (NIPS2014). 2014. http://bit.ly/2HZ70tj
- Unterthiner T, Mayr A, Klambauer G, Hochreiter S. Toxicity prediction using deep learning. Front Environ Sci. 2015; 230-236. http://bit.ly/2w5wP8A
- Fan F, Wood KV. Bioluminescent assays for high-throughput screening. Assay Drug Dev Technol.2007; 5: 127-136. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17355205
- Odugbemi TO, Akinsulire OR. Medicinal plants by species names in outlines and pictures of medicinal plants from Nigeria. 1st edition. Nigeria: University of Lagos Press; 2006. pp. 73-161. https://stanford.io/2T1iN0L
- Ohemu TL, Agunu A, Olotu PN, Ajima U, Dafam DG, Azila JJ. Ethnobotanical survey of medicinal plants used in the traditional treatment of viral infections in Jos, Plateau state, Nigeria. International Journal of Aromatic Plants. 2014; 4: 74-81. http://bit.ly/2T35Q6G
- Velmurugan S, Babu MM, Punitha SMJ, Viji VT, Citarasu T. Screening and characterization of antiviral compounds from Psidium guajava Linn. Root bark against white spot syndrome virus. Indian Journal of Natural Products and Resources 2012; 3: 208-214. http://bit.ly/2PGjSsT
- Beltran OMS. Investigation of the anti-mycobacterial and cytotoxic effect of three medicinal plants used in the traditional treatment of tuberculosis in northern Mexico and the Southwest United States available at 2008.
- Park IW, Han C, Song X, Green LA, Wang T, Liu Y, et al. Inhibition of HIV-1 entry by extracts derived from traditional Chinese medicinal herbal plants. BMC Complement Altern Med. 2009; 9: 29. PubMed:https://www.ncbi.nlm.nih.gov/pubmed/19656383
- Ojo OO, Oluyege JO, Famurewa O. Antiviral properties of two Nigerian plants. African Journal of Plant Science. 2009; 3: 157-159. http://bit.ly/2w5B3gs
- Omilabu SA, Munir AB, Akeeb OO, Adesanya AB, Badaru SO. Antiviral effect of Hibiscus sabdariffa and Celosia argentea on measles virus. African Journal of Microbiology Research. 2010; 4: 293-296. http://bit.ly/2T12dOl
- Fennell CW, Lindsey KL, McGaw LJ, Sparg SG, Stafford GI, Elgorashi EE, et al. Assessing African medicinal plants for efficacy and safety and pharmacological screening toxicology. J Ethnopharmacol. 2004; 94: 205-217. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15325724
- Chen W, van Wyk B, Vermaak I, Viljoen A. Cape Aloes-A review of the phytochemistry, pharmacology and commercialisation of Aloe ferox. Phytochemistry Letters. 2012; 2: 1-12. http://bit.ly/2PrUve6
- Ernst, E. Risks of herbal medicinal products. Pharmacoepidemiol Drug Safety. 2004; 13: 767-771. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15386721
- Rietjens IM, Boersma MG, Van der Woude H, Jeurissen SMF, Schutte ME, Alink GM. Flavonoids and alkenylbenzenes: Mechanisms of mutagenic action and carcinogenic risk. Mutat Res. 2005; 574: 124-138. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15914212
- Samaha HS, Kelloff GJ, Steele V, Rao CV, Reddy BS. Modulation of apoptosis by sulindac, curcumin, phenylethyl-3-methylcaffeate and 6-phenylhexyl isothiocyanate: Apoptotic index as a biomarker in colon cancer chemoprevention and promotion. Cancer Research; 57: 1301-1305. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9102217
- Freshney RI. Culture of Animal Cell: A Manual of Basic Technique. 4th ed. New York: John Wiley and Sons Incorporated; 2000. pp. 329-339.
- El-Sayed S Abdel-Hameed, Salih A Bazaid, Mohamed M Shohayeb, Mortada M. El-Sayed, Eman A. El-Wakil. Phyto chemical studies and evaluation of antioxidant, anticancer and antimicrobial properties of Conocarpus erectus L. growing in Taif, Saudi Arabia. European Journal of Medicinal Plants. 2012; 2: 93-112. http://bit.ly/2wSbX51
- Mahavorasirikul W, Viyanant V, Chaijaroenkul W, Itharat A, Na-Bangchang K. Cytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells In vitro. BMC Complementary and Alternative Medicine. 2010; 10: 55. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20920194
- Vijayarathna S, Sasidharan S. Cytotoxicity of methanol extracts of Elaeis guineensis on MCF-7 and Vero cell lines. Asian Pacific Journal of Tropical Biomedicine. 2012; 2: 826-829. http://bit.ly/2vmIks1
- Hanisa H, Mohdazmi ML, Suhaila M, Hakim MN. Effects of Centellaasiatica L, Curcuma longa L, and Strobilanthescrispus L. extracts on 3 kidney cell lines: In vitro cytotoxicity analysis. International Journal of Pharmacy and Pharmaceutical Sciences. 2014; 6: 388-392. http://bit.ly/2TnquNP
- Sentkowska A, Biesaga M, Pyrzynska K. Application of hydrophilic interaction liquid chromatography for the quantification of flavonoids in genista tinctoria extract. Journal of Analytical Methods in Chemistry. 2016; 1-9. http://bit.ly/2Ps0zDq
- dos Santos PC. Why is methanol used as a first solvent for extraction purpose to look for bioactives in medicinal plants? 2014.
- Bai VDM, Krishnakumar S. Evaluation of antimicrobial metabolites from marine microalgae Tetraselmis suecica using gas chromatography-mass spectrometry (GC-MS) analysis. Int J Pharm Pharm Sci. 2013; 5: 17-23. http://bit.ly/2T3F7qE
- Biu AA, Yusufu SD, Rabo JS. Phytochemical screening of Azadirachta indica (Neem) (Meliaceae) in Maiduguri, Nigeria. Bioscience Research Communications. 2009; 21: 281-283. http://bit.ly/2Tj6YBT
- Ashafa AOT, Orekoya LO, Yakubu MT. Toxicity profile of ethanolic extract of Azadirachta indica stem bark in male Wistar rats. Asian Pacfici Journal of Tropical Biomedicine. 2012; 2: 811- 817. http://bit.ly/2wcBp4F
- Bakr SA. Evaluation of acute toxicity of water extracts of Azadirachta indica leaves and seeds in rats. Pak J Biol Sci. 2013; 16: 697-700. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24505996
- Boeke SJ, Boersma MG, Alink GM, van Loon JJ, van Huis A, Dicke M, et al. Safety evaluation of neem (Azadirachta indica) derived pesticides. J Ethnopharmacol. 2004; 94: 25-41. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15261960
- Duru UN, Orji JC, Obi RK. Larvicidal potential of crude extracts of Azadirachta indica (Neem). World Journal of Pharmacy and Pharmaceutical Sciences. 2012; 1: 1188-1197.
- Sharma S, Senrung A, Singh AK. Toxic effect of neem, Azadirachta indica (A. Juss) foliage extracts against Diamondback Moth (DBM), Plutella xylostella (L.) (Lepidoptera, Plutellidae). Jbiopest. 2014; 7(supp.): 99-105. http://bit.ly/2Pu39Zq
- Tedeschi R, Alma A, Tavella L. Side-effects of three neem (Azadirachta indica A. Juss) products on the predator Macrolophus caliginosus Wagner (Het., Miridae). Applied Entomology. 2001; 125: 397-402. http://bit.ly/2v93H05
- Yakubu MT, Quadri AL. Safety evaluation of aqueous extract of Garcinia kola seeds in male Wistar rats. Iranian Journal of Toxicology. 2016; 10: 41-47. http://bit.ly/2wOZMpv
- Jegede AI, Ajadi MB, Akinloye O. Modulatory effects of kolaviron (Garcinia kola extract) on spermogram and reproductive system of adult male wistar rats in lead acetate. Journal of Toxicology and Environmental Health Sciences. 2013; 5: 121-130. http://bit.ly/3cctqW1
- Kagbo H, Ejebe D. Phytochemistry and preliminary toxicity studies of the methanol extract of the stem bark of Garcinia kola (Heckel). The Internet Journal of Toxicology. 2009; 7: 1-8. http://bit.ly/2T5F3GS
- Jafarain A, Asghari G, Ghassami E. Evaluation of cytotoxicity of Moringa oleifera Lam. callus and leaf extracts on Hela cells. Adv Biomed Res. 2014; 3: 194. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25337524
- Kasolo JN, Bimenya GS, Ojok L, Ogwal-okeng JW. Phytochemicals and acute toxicity of Moringa oleifera roots in mice. Journal of Pharmacognosy and Phytotherapy. 2011; 3: 38-42. http://bit.ly/2wRktRO
- Reppas GP. Bryophyllum pinnatum poisoning of cattle. Australian Journal of Veterinary Medicine. 1995; 72: 425-427. http://bit.ly/2Pt0zTr
- Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga, Latha L. Extraction, isolation and characterization of bioactive compounds from plants' extracts. African Journal of Traditional and Complement Alternative Medicine. 2011; 8: 1-10. http://bit.ly/386vCei

Sign up for Article Alerts