Toxic Effects of Two New Phospholipases A2 Isolated from Bothrops pauloensis Snake Venom
Priscila Randazzo-Moura1,3, Georgina Sucasaca Monzon1, Thalita Rocha2,4*, Luis Alberto Ponce-Soto2, Sergio Marangoni2, Maria Alice da Cruz-Höfling2 and Lea Rodrigues-Simioni1
1Department of Pharmacology, Faculty of Medical Sciences, State University of Campinas (UNICAMP), Alexander Fleming Street, FCM10, Zip-code: 13083-887, Campinas - SP, Brazil
2Department of Biochemistry and Tissue Biology, Institute of Biology, State University of Campinas (UNICAMP), 255 Monteiro Lobato Street, Zip-code: 13083-862, Campinas, SP, Brazil
3Laboratory of Pharmacology, Faculty of Medical Sciences and of Health, Pontifical Catholic University of Sao Paulo (PUC)., 290 Dr Jose Ermirio de Moraes Square, Zip-code 18030-095, Sorocaba - SP, Brazil
4Multidisciplinary Research Laboratory, Sao Francisco University (USF). 218 Francisco de Assis Avenue, Jd. São José, Zip-code: 12916-900, Bragança Paulista - SP, Brazil.
*Address for Correspondence: Thalita Rocha, 218 Francisco de Assis Avenue, Jd. São José, Zip-code: 12916-900, Bragança Paulista - SP, Brazil, Zip-code: 12916-900, Tel: +(55) 11 2454-8000; E-mail: [email protected]
Submitted: 18 October 2017; Approved: 30 October 2017; Published: 31 October 2017
Citation this article: Randazzo-Moura P, Monzon GS, Rocha T, Ponce-Soto LA, Marangoni S, et al. Toxic Effects of Two New Phospholipases A2 Isolated from Bothrops pauloensis Snake Venom. Adv J Toxicol Curr Res. 2017;1(2): 069-073.
Copyright: © 2017 Rocha T, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Bothrops pauloensis; Lys 49; Asp 49; Neurotoxicity; Myotoxicity; Edematogenic Effect; Membrane Resting Potential
Download Fulltext PDF
Bothropic venoms have several substances necessary to serpents’ survival, among them Phospholipase A2 (PLA2) enzymes. The hydrolysis of sn-2 from membrane phospholipids and the release of lysophospholipids and fatty acids induces in the substrate a wide variety of pharmacological effects. Bp-12 (13,789.56 Da molecular mass) and Bp-13 (14,035.62 Da molecular mass) toxins isolated by chromatographic system in reverse phase HPLC on μ-Bondapack C18 column from Bothrops pauloensis snake venom belong to PLA2 family (Lys-49 and Asp-49, respectively). Electrophysiological studies in mouse Phrenic Nerve-Diaphragm Preparation (PND) showed a progressive sarcolemma depolarization, reaching values from -83.9 ± 1 mV (control) until -20.2 ± 1 mV (Bp-12) and -45.0 ± 1.5 mV (Bp-13) after 90 min (n = 3-6; p < 0.05). Histological analysis of mice PND and in vivo, rat paw edema formation and mice serum Creatine Kinase (CK) release were approaches to study the toxins’ toxicity. The PLA2s Bp-12 and Bp-13 (50 μg/mL) caused ~ 30% of damage (27.3 ± 1.1% and 30.6 ± 5% of respectively (p ˂ 0.05); the pathological states of fibers included fiber edema, hypercontration of sarcomeres and myofibrils clumping and condensation. After 60 minutes the CK levels were 827 ± 92.4 U/L for Bp-12 and 1440 ± 129 U/L for Bp-13 (n = 5-6) compared to control (71.4 ± 14.1 U/L, p ˂ 0.05). The induction of edema of paw was toxins concentration-dependent for Bp-12 and Bp-13, respectively [2.5 μg/paw (0.44 ± 0.02 mL and 0.45 ± 0.07 mL), 5 μg/paw (0.59 ± 0.03 and 0.47 ± 0.04 mL) and 10 μg/paw (0.67 ± 0.07 and 0.45 ± 0.06 mL)] . The results lead us to conclude that Bp-12 and Bp-13 are myotoxins for mammalian and might contribute to the pharmacological and pathological effects of the crude venom.
Introduction
Snake venom Phospholipases A2 (PLA2s) constitute an important group of molecules that display several pharmacological activities [1-4]. They are included in two broad categories, D49 and K49: D49 with an aspartate amino acid at position 49 and, consequently, calcium-dependent catalytic activity, and K49, with a lysine amino acid at position 49 and, consequently, catalytically inactive.
Envenomation caused by Bothrops snakes bites is often characterized by a complex series of local pathophysiological and systemic hemodynamic and hemostatic disturbances [5,6]. Some of their isolated components were shown to affect the dynamics of neuromuscular junction physiology in vitro [7-14].
In this work, we unravel some of the effects of two myotoxins isolated from Bothrops pauloensis snake venom which had been characterized biologically as a Lys49 PLA2 (Bp-12) and Asp49 PLA2 (Bp-13). The purpose was to assess the relative contribution of each toxin for the in vitro toxicity for the whole venom.
Materials and Methods
Animals
Male Swiss mice (18-25g) and male Wistar rats (160-180g) were obtained from the Multidisciplinary Center for Biological Investigation at the State University of Campinas (CEMIB-UNICAMP). The animals were housed in a temperature-controlled room (25 ± 3°C) with a 12 hour light/dark cycle and access to water and food ad libitum. The experimental protocols were approved by the institutional Committee for Ethics in Animal Use (CEUA/UNICAMP, Protocols 939-2 and 1266-1) and were done in accordance with the ethical guidelines of the Brazilian Society of Laboratory Animal Science (SBCAL).
Toxins
Bp-12 and Bp-13 toxins were isolated from Bothrops pauloensis snake venom (Batatais Serpentarium, São Paulo State, Brazil) as described by [12] and [15]. All chemicals and reagents used in this research were of analytical or sequencing grade.
Intracellular recordings
Male Swiss mice were sacrificed by halothane inhalation and the hemidiaphragm along with its phrenic-nerve was removed and mounted as previously described by Bulbring for rats [16].
Membrane Resting Potential (RP) of phrenic nerve-diaphragm preparations was measured through a high input impedance electrometer (World Precision 750, Sarasota, FL, USA) by using conventional microelectrode techniques [17]. The dissected muscle was mounted in an aerated chamber (95% O2 - 5 % CO2) with Tyrode solution (composition in mM: NaCl 137, KCl 2.7, CaCl2 1.8, MgCl2 0.49, NaH2PO4 0.42, NaHCO3 11.9 and glucose 11.1; pH 7.4, 25-28°C), with or without Bp-12 and Bp-13 (50 µg/mL) addition. The glass microelectrode filled with 3 M KCl (resistance 10 – 20 MΩ) was inserted into a muscle fiber at an endplate region. The membrane RP was recorded on an oscilloscope (Tektronix, Beaverton, OR, USA). The RP recordings were taken at the end-plate regions in the absence (control) or presence of toxins.
Myonecrosis evaluation: morphological and biochemical assays
Myotoxicity was assessed in hemidiaphragm muscle from mouse Phrenic Nerve-Diaphragm preparations (PND), after 120 min incubation in Tyrode solution (control) and Bp-12 or Bp-13 (50 µg/mL) toxins. Following the incubation period, the muscles were washed and then transferred to 4% paraformaldehyde fixative for 24 h, washed three times in saline solution, dehydrated in ethanol series (70%, 80%, 90% and 100% x 3; 30 min each bath), embedded and included in historesin (Leica Nublock/Heidelberg, Germany). Sections (2 µm thick) were obtained using a Leica RM 2035 microtome and stained with Toluidine Blue (TB) for light microscopy (Olympus BX51 light microscope, Japan) analysis. Muscle images were captured using a digital imaging system (Image Pro Plus 6.0, Mídia Cybernetics Inc., USA).
The percentage of damaged fibers was counted in three non-overlapping, non-adjacent sections per animal in a total of 15 sections per toxin- and Tyrode-incubated preparations (n = 5 mice/Bp-12/Bp-13/Tyrode). Percentage of muscle damage was assessed by multiplying the number of damaged fibers by 100 and dividing it by the total number of fibers present in the whole sectional muscle area. Damaged fibers were qualified according to the pathologic state and normal fibers were those with polygonal morphology and normal myofibrils distribution/organization.
Myonecrosis was also assayed by measuring the CK serum activity in mice after time-points of gastrocnemius Intra-Muscular (i.m) injection of Phosphate Buffered Saline (PBS) (control - 50 μL), Bp-12 and Bp-13 (50 μg/50 μL) toxins. After 30 minutes, 1, 3, 6, 9 and 12 hours of i.m. injection, the mice were deeply anesthetized and blood samples were collected from the caudal vein into heparinized capillary tubes. Immediately after sampling, the blood was centrifuged (4oC, 3000 rpm/10 min) for serum separation and colorimetric determination of CK activity using CK-NAC kit (Bioclin/Quibasa, Belo Horizonte, MG, Brazil). The serum CK activity was expressed as international units/liter (U/L), with 1 unit of activity causing the phosphorylation of 1 nmol of creatine (substrate) per minute, at 37oC.
Measurements of rat paw edema
Male Wistar rats (120-180 g) were used for measuring the hind paw edema induced by a single sub-plantar injection (0.1 mL) of the Bp-12 or Bp-13 [dissolved in 0.9 % (w/v) sterile saline]. The paw volume was measured 15, 30, 60, 120 and 180 minutes after injection of dose range of toxins (2.5, 5 and 10 µg/paw) using a hydroplethysmometer (model 7150, Ugo Basile, Varese, Italy). The results were expressed as the increase in paw volume (mL) calculated by subtracting the basal volume measured in the contralateral paw. In some cases, the area under the time-course curve (AUC, mL min) was calculated using the trapezoidal rule, which works by approximating the region under the graph of the function f(x) as a trapezoid and calculate its area.
Statistical analysis
Results were reported as mean ± S.E.M. and the statistical comparison were obtained using one-way ANOVA followed by the Tukey and Kruskal-Wallis post hoc test. The Dunn’s multiple test was also performed and several experimental groups were compared to the control group (p < 0.05 indicated statistical significance).
Results
Electrophysiology
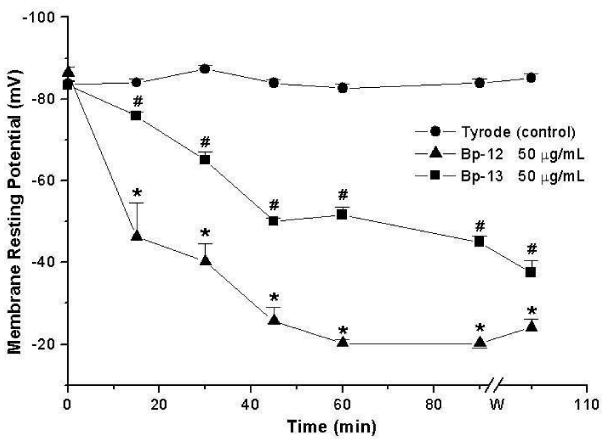
The measurement of the membrane Resting Potential (RP) of PND incubated with Lys49 PLA2 Bp-12 or Asp49 PLA2 Bp-13 (50 μg/mL) showed a progressive sarcolemma depolarization, reaching values of -20 ± 1 mV (Bp-12) and -45 ± 1.5 mV (Bp-13) after 90 minutes (n = 3-6) which differed significantly from the obtained for control -84 ± 1 mV at the same condition (p < 0.05, Figure 1).
Myonecrosis evaluation- Histology
Figure 2 shows cross-section of mouse hemidiaphragm muscle fibers after 120 min of the incubation with Tyrode solution (control) or PLA2 Bp-12 or PLA2 Bp-13 toxins. Control muscle fibers displayed a normal polygonal cross profile shape, even distribution of myofibrils and intact sarcolemma; no damaged fiber was observed (Figure 2A). Preparations incubated with (50 μg/mL) of Lys49 PLA2 Bp-12 or Asp49 PLA2 Bp-13 showed 27.3 ± 1.1% and 30.6 ± 5.1% of fibers with different pathological states, respectively (Figures 2B and 2C, respectively; p < 0.05 compared to control – 1.1 ± 0.2). Figure 2B and 2C illustrates altered fibers whose pathological states include rounded polygonal profile typical of osmotic disturbance and sarcolemma impairment, sarcoplasmic area devoid of myofibrils intermingled with fibrils densely clumped and hypercontracted.
Myonecrosis evaluation – Creatine kinase
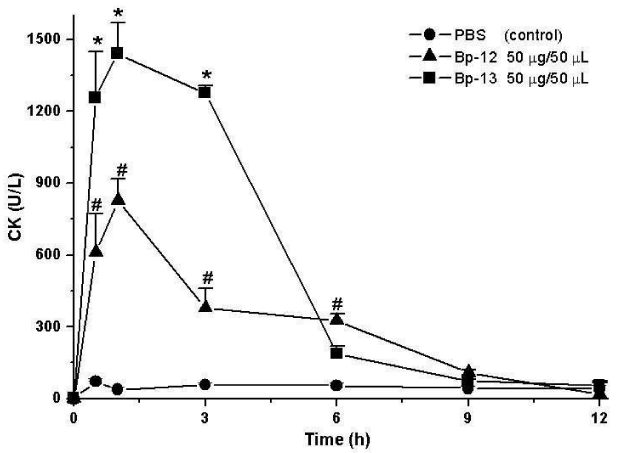
Creatine Kinase (CK) release was evaluated to substantiate the myonecrosis observed in histological section. Figure 3 shows that after 60 minutes of incubation the CK serum levels were 71.4 ± 14 U/L for control mice gastrocnemius injected with sterile saline solution (0.9%) and 827 ± 92.4 U/L for Lys49 PLA2 Bp-12 and 1440 ± 129 U/L for Asp49 PLA2 Bp-13 (n = 5-6; p < 0.05).
Paw edema formation
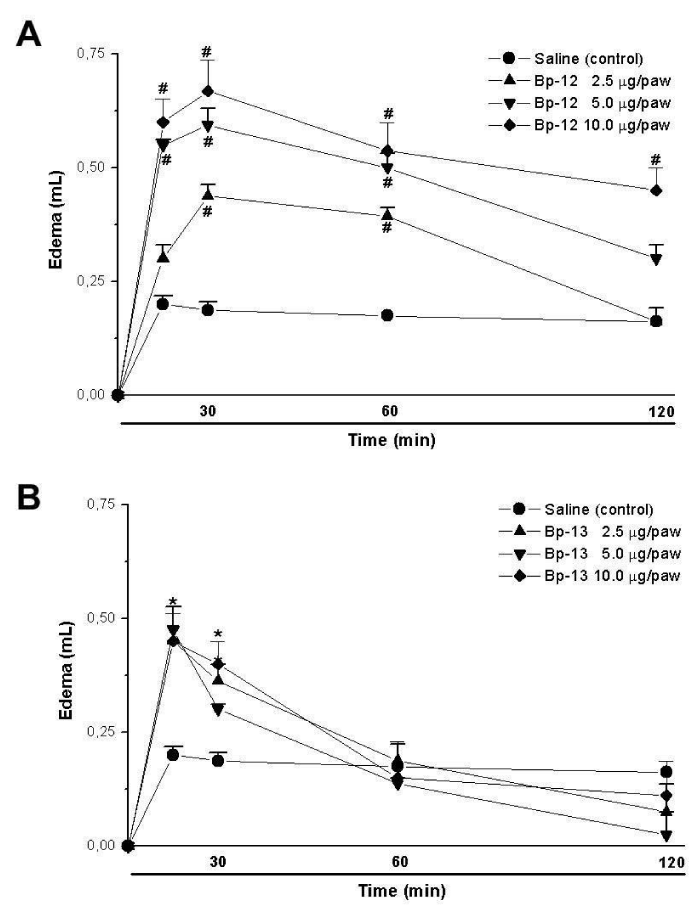
Paw edema induction was observed after 30 minutes of 2.5 μg/paw (0.44 ± 0.02 mL), 5 μg/paw (0.59 ± 0.03 mL) and 10 μg/paw (0.67 ± 0.07 mL) for Lys49 PLA2 Bp-12 injection (n = 5) (Figure 4A). However, for Asp49 PLA2 Bp-13 the paw edema was observed after 15 minutes, showing 0.45 ± 0.07 mL (2.5 μg/paw), 0.47 ± 0.01 mL (5 μg/paw) and 0.45 ± 0.06 mL (10 μg/paw) of edema (n = 5) (Figure 4B).
Discussion
Classical manifestation of Bothropic envenomation is local and systemic effects, the magnitude of which could cause local and systemic myolysis, hemodynamic disturbances and renal dysfunction [18]. Although envenoming by Bothrops produces no neurotoxic clinical signs, venoms from several species can cause neuromuscular blockade in vitro and produce signs of peripheral muscular weakness in chickens and mice. Up to the present time, only B. jararacussu and B. pauloensis species were satisfactorily investigated regarding this activity, including the isolation of neurotoxic and myotoxic components [7,14,19,20,21]. These authors showed that the presynaptic effects from B. pauloensis venom were mainly due to the presence of phospholipases as one of its components.
PLA2s are among the most abundant components of snake venoms, showing a wide range of activity even presenting conserved structures [22]. Understanding the structural basis for their diverse toxic activities, including neurotoxicity and myotoxicity, is still a challenging task. In this work, two new toxins, Bp-12 (Lys49) and Bp-13 (Asp49) from B. pauloensis snake venom were pharmacologically characterized. These toxins induced neuromuscular blockade in mouse phrenic nerve-diaphragm preparations [12,15]. The same was observed for Lys 49 myotoxin II (BnSP-7), another PLA2 also isolated from B. pauloensis venom, which displayed neurotoxic activity upon chicken biventer cervicis preparations [14].
Suggested that neuwieditoxin-I and II, another Asp49 PLA2s isolated from B. pauloensis venom, were probably responsible for the presynaptic neurotoxicity in vitro. As reported in the present research, Bp-13 can also participate of the neurotoxic venom effect [7].
The majority of PLA2 bothropic myotoxins, as Lys49 PLA2 Bp-12 and Asp49 PLA2 Bp-13, affect the membrane resting potential, in contrast to BthTX-I from B. jararacussu and BaTx from B. alternatus which at low concentrations do not change this parameter [11,23].
The morphological analysis combined with CK release was performed for a more consistent assessment of myotoxicity. Histological analysis after Bp-12 and Bp-13 diaphragm muscle exposure showed signified muscle damage, displaying hypercontracted, edematous and vacuolated fibers, which include both toxins into the myotoxic phospholipase group. Such results are in accordance to those observed for BthTX-I [11,24] and BnpTX-I and –II, Bp-PLA2 [4,25].
In the present research, CK data confirmed the histological results, as also reported for other bothropic toxins [4,10,23-26]. In addition to morphological analysis and determination of CK serum, both Bp-12 and Bp-13, induced footpad edema as reported to BbTX-I and –II, from B. brazili [10], BmjeTX-I and –II, from B. marajoensis [26], as well as 6-1, 6-2 and Bj-VII from B. jararacussu [27]. The mechanism of edema formation by PLA2 may be explained by phospholipid hydrolysis, probably due to liberation of precursors of a variety of eicosanoids and platelet activator factors [28].
The results presented for Bp-12 and Bp-13 did not show a marked difference between both toxins, even considering the fact that they are Lys-49 and Asp-49, respectively, inferring that they act by different mechanisms exerted by molecular regions distinct from the enzymatic sites.
Conclusion
Taken together, these results characterized biologically Lys49 PLA2 Bp-12 and Asp49 PLA2 Bp-13 as classical myotoxins considering membrane resting depolarization, morphological and biochemical damage, and proinflammatory effects.
Acknowledgements
The authors thank Mr. Paulo A. Baldasso for technical assistance. This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and was part of G.S.M MSci. Thesis supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The work was also supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant no. 08/57906-3); M.A.C.H. is a 1A level Research Fellow from CNPq.
- -Vega S, Ponce-Soto LA, Martins-de-Souza D, Marangoni S. Biochemical and pharmacological characterization of PhTX-I a new myotoxic phospholipase A2 isolated from Porthidium hyoprora snake venom. Comp Biochem Physiol C Toxicol Pharmacol. 2011; 154: 108-119. https://goo.gl/hqhF6q
- Montecucco C, Gutiérrez JM, Lomonte B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: common aspects of their mechanisms of action. Cell Mol Life Sci. 2008; 65: 2897-2912. https://goo.gl/9kj78g
- Ponce-Soto LA, Barros JC, Marangoni S, Hernandez S, Dal Belo CA, Corrado AP, et al. Neuromuscular activity of BaTX, a presynaptic basic PLA2 isolated from Bothrops alternatus snake venom. Comp Biochem Physiol C Toxicol Pharmacol. 2009; 150: 291-297. https://goo.gl/NJuaau
- Rodrigues RS, Izidoro LFM, Teixeira SS, Silveira LB, Hamaguchi A, Homsi-Brandeburgo MI, et al. Isolation and functional characterization of a new myotoxic acidic phospholipase A2 from Bothrops pauloensis snake venom. Toxicon. 2007; 50: 153-165. https://goo.gl/eSU2GZ
- Gutiérrez JM, Ownby CL. Skeletal muscle degeneration induced by venom phospholipases A2: insights into the mechanisms of local and systemic myotoxicity. Toxicon. 2003; 42: 915-931. https://goo.gl/zZXN6v
- Lomonte B, Angulo Y, Calderón L. An overview of Lysine-49 phospholipase A2 myotoxins from crotalid snake venoms and their structural determinants of myotoxic action. Toxicon. 2003; 42: 885-901. https://goo.gl/SQqVJk
- Borja-Oliveira CR, Kassab BH, Soares AM, Toyama MH, Giglio JR, Marangoni S, et al. Purification and N-terminal sequencing of two presynaptic neurotoxic PLA2 Neuwieditoxin-I and Neuwieditoxin-II, from Bothrops neuwiedi pauloensis (jararaca-pintada) venom. J Venom Anim Toxins Incl Trop Dis. 2007; 13: 103-121. https://goo.gl/LnTHxh
- Cogo JC, Prado-Franceschi J, Giglio JR, Corrado AP, Cruz-Höfling MA, Donato JL, et al. An unusual presynaptic action of Bothrops insularis snake venom mediated by phospholipase A2 fraction. Toxicon. 1998; 36: 1323-1332. https://goo.gl/EdwsUC
- de Moraes DS, Abreu VA, Rostelato-Ferreira S, Leite GB, Cruz-Höfling MA, Travaglia-Cardoso SR, et al. Neuromuscular activity of Bothrops alcatraz snake venom in chick biventer cervicis preparations. Toxicon. 2012; 59: 294-299. https://goo.gl/A4u3za
- Huancahuire-Vega S, Ponce-Soto LA, Martins-de-Souza D, Marangoni S. Structural and functional characterization of Brazilitoxins II and III (BbTX-II and -III), two myotoxins from the venom of Bothrops brazili snake. Toxicon. 2009; 54: 818-827. https://goo.gl/zTDEPg
- Oshima-Franco Y, Leite GB, Dal-Belo CA, Hyslop S, Prado-Franceschi J, Cintra AC, et al. The presynaptic activity of bothropstoxin-I, a myotoxin from Bothrops jararacussu snake venom. Basic Clin Pharmacol Toxicol. 2004; 95: 175-182. https://goo.gl/YTm861
- Randazzo-Moura P, Ponce-Soto LA, Rodrigues-Simioni L, Marangoni S. Structural characterization and neuromuscular activity of a new Lys49 phospholipase A2 homologous (Bp-12) isolated from Bothrops pauloensis snake venom. Protein J. 2008; 27:355-362. https://goo.gl/M3YXCx
- Rodrigues VM, Marcussi S, Cambraia RS, de Araújo AL, Malta-Neto NR, Hamaguchi A, et al. Bactericidal and neurotoxic activities of two myotoxic phospholipases A2 from Bothrops neuwiedi pauloensis snake venom. Toxicon. 2004; 44: 305-314. https://goo.gl/j9RNiN
- Soares AM, Guerra-Sá R, Borja-Oliveira CR, Rodrigues VM, Rodrigues-Simioni L, Rodrigues V, et al. Structural and functional characterization of BnSP-7, a Lys49 myotoxic phospholipase A2 homologue from Bothrops neuwiedi pauloensis venom. Arch Biochem Biophys. 2000; 378: 201-209. https://goo.gl/89GnS7
- Sucasaca-Monzón G, Randazzo-Moura P, Rocha T, Torres-Huaco FD, Vilca-Quispe A, Ponce-Soto LA, et al. Bp-13 PLA2: Purification and Neuromuscular Activity of a New Asp49 Toxin Isolated from Bothrops pauloensis Snake Venom. Biochem Res Int. 2015; 2015: 826059. https://goo.gl/8hgejm
- Bülbring E. Observations on the isolated phrenic-nerve diaphragm preparation of the rat. Br J Pharmacol. 1946; 1: 38-61. https://goo.gl/sEkfqu
- Fatt P, Katz B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951; 115: 320-370. https://goo.gl/KJeACG
- Cruz LS, Vargas R, Lopes AA. Snakebite envenomation and death in the developing world. Ethn Dis. 2009; 19: 42-46. https://goo.gl/GJBfpc
- Homsi-Brandeburgo MI, Queiroz LS, Santo Neto H, Rodrigues-Simioni L, Giglio JR. Fractionation of Bothrops jararacussu snake venom: partial chemical characterization and biological activity of Bothropstoxin. Toxicon. 1988; 26: 615-627. https://goo.gl/KjKweC
- Rodrigues-Simioni L, Borgese N, Ceccarelli B. The effects of Bothrops jararacussu venom and its components on frog nerve-muscle preparation. Neuroscience. 1983; 10: 475-489. https://goo.gl/XbWR2P
- Rodrigues VM, Marcussi S, Cambraia RS, de Araújo AL, Malta-Neto NR, Hamaguchi A, et al. Bactericidal and neurotoxic activities of two myotoxic phospholipases A2 from Bothrops neuwiedi pauloensis snake venom. Toxicon. 2004; 44: 305-314. https://goo.gl/j9RNiN
- Valentin E, Lambeau G. What can venom phospholipases A2 tell us about the functional diversity of mammalian secreted phospholipases A2? Biochimie. 2000; 82: 815-831. https://goo.gl/vdXrnG
- Ponce-Soto LA, Martins-de-Souza D, Marangoni S. Neurotoxic, myotoxic and cytolytic activities of the new basic PLA(2) isoforms BmjeTX-I and BmjeTX-II isolated from the Bothrops marajoensis (Marajó Lancehead) snake venom. Protein J. 2010; 29: 103-113. https://goo.gl/eb6rkg
- Randazzo-Moura P, Leite GB, Silva GH, Paffaro Júnior VA, Cintra ACO, Cruz-Höfling MA, et al. A study of the myotoxicity of Bothropstoxin-I using manganese in mouse phrenic nerve-diaphragm and extensor digitorum longus preparations. Braz J Morphol Sci. 2006; 23: 171-184. https://goo.gl/vd95k8
- Rodrigues VM, Marcussi S, Cambraia RS, de Araújo AL, Malta-Neto NR, Hamaguchi A, et al. Bactericidal and neurotoxic activities of two myotoxic phospholipases A2 from Bothrops neuwiedi pauloensis snake venom. Toxicon. 2004; 44: 305-314. https://goo.gl/j9RNiN
- Galbiatti C, Rocha T, Randazzo-Moura P, Ponce-Soto LA, Marangoni S, Cruz-Höfling MA, et al. Pharmacological and partial biochemical characterization of Bmaj-9 isolated from Bothrops marajoensis snake venom. J Venom Anim Toxins Incl Trop Dis. 2012; 18: 62-72. https://goo.gl/HVBgvX
- Bonfim VL, de Carvalho DD, Ponce-Soto LA, Kassab BH, Marangoni S. Toxicity of phospholipases A2 D49 (6-1 and 6-2) and K49 (Bj-VII) from Bothrops jararacussu venom. Cell Biol Toxicol. 2009; 25: 523-532. https://goo.gl/thqxL7
- Landucci EC, Castro RC, Pereira MF, Cintra AC, Giglio JR, Marangoni S, et al. Mast cell degranulation induced by two phospholipase A2 homologues: dissociation between enzymatic and biological activities. Eur J Pharmacol. 1998; 343: 257-263. https://goo.gl/yPMcef


Sign up for Article Alerts