Exposure to BDE-47 Alters Thyroid Hormone Levels and Gene Transcription in the Hypothalamic-Pituitary-Thyroid Axis of Zebrafish Larvae (Danio Rerio)
Xin Ren1,2, Hang Zhao1,2 and Xuesong Zhao1,2*
1Key Laboratory of Environmental Materials and Pollution Control, Education Department of Jilin Province, Siping, 136000, China
2College of Environmental Science and Engineering, Jilin Normal University, Haifeng Street, Tiexi Dist, Siping, 136000, China
*Address for Correspondence: Xuesong Zhao, College of Environmental Science and Engineering, Jilin Normal University, Siping, 136000, China, Phone: +86 04343292207; Fax: +86 04343292207; E-mail: [email protected]
Submitted: 25 August 2017; Approved: 23 September; Published: 26 September 2017
Citation this article: Ren X, Zhao H, Zhao X. Exposure to BDE-47 Alters Thyroid Hormone Levels and Gene Transcription in the Hypothalamic-Pituitary-Thyroid Axis of Zebrafish Larvae (Danio Rerio). Adv J Toxicol Curr Res. 2017;1(1): 023-032.
Copyright: © 2017 Zhao X, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: BDE-47; Hypothalamic-Pituitary-Thyroid Axis; Gene Transcription and Protein Expression; Thyroid Disruption; Zebrafish Larvae
Download Fulltext PDF
2,2’,4,4’-Tetrabromodi-phenyl ether (BDE-47) has the potential to disrupt thyroid hormone homeostasis, but the molecular mechanisms underlying this process have not yet been clarified. In the present study, zebrafish embryos were BDE-47 (0, 1, 5, or 10 µg/L) from fertilization to 14 d thereafter (dpf). The whole-body content of Thyroid Hormones (THs) and the transcription of genes and proteins related to the Hypothalamic-Pituitary-Thyroid (HPT) axis were analyzed. Abnormal morphologies and inhibition of body-weight gain have been associated with decreased levels of Thyroid Hormones (THs) in zebrafish larvae. BDE-47 exposure (up to 10 µg/L) significantly reduced Thyroxine (T4), indicating thyroid-related endocrine disruption. The gene expression levels of CRH and TSHβ were significantly induced by BDE-47 exposure (5 and 10 µg/L). The transcription of genes involved in the synthesis of TH proteins, NIS, TG, Pax8 and Nkx2.1 were significantly up-regulated by BDE-47 exposure (5 and 10 µg/L). Furthermore, the protein level of TG was significantly up-regulated in a concentration-dependent manner. The expression of Deio2 was also significantly up-regulated in BDE-47-treated groups (5 and 10 µg/L), possibly as a compensatory response to the decreased T4 levels. The transcription of TRα and TRβ was down-regulated in a concentration-dependent manner. However, BDE-47 exposure downregulated both the transcriptional and protein levels of TTR. The exposure to BDE-47 (10 µg/L) significantly increased the transcription of the UGT1 gene. The results described above that BDE-47 can alter gene and protein expression in the HPT axis, which may subsequently contribute to BDE-47-induced thyroid disruption.
Introduction
Polybrominated Diphenylethers (PBDEs) are extensively used as Brominated flame Retardants (BFRs) in a variety of consumer products, such as textiles, upholstery foam and electronic devices. PBDEs are hydrophobic and lipophilic and share many characteristics with Persistent Organic Pollutants (POPs). Consequently, they tend to be biomagnified in the food chain (e.g., BDE-47, 99, 100, 153, and 154). [1] In rivers around e-waste areas and industrial parks of Guangdong, South China, the measured concentrations of PBDEs in water and sediment were 24.4 ng/L and 4250 ng/g, respectively. [2,3] In the Pearl River, the measured concentrations of BDE-209 in the water and sediment were 65 ng/L and 7340 ng/g, respectively. [4] Increasing concentrations in a wide variety of wildlife species worldwide, as well as in the breast milk, blood and other tissues of humans, have focused worldwide attention on the potential health effects of PBDEs. [5] Several studies report potential oxidative damage, reproductive toxicity, neurological toxicity, and endocrine toxicity after PBDE exposure in rodents. [6] Additionally, in humans, BDE-47 and BDE-99 were found to cause developmental neurotoxicity in vitro by reducing the migration distance of human neural progenitor cells, an effect that could be reduced by adding T3. [7]
Of the various PBDEs, the PBDE congeners found in biota and human samples are predominantly 2,2’,4,4’-tetraBDE (BDE-47), followed by 2,2’,4,4’5-pentaBDE (BDE-99), 2,2’,4,4’6-pentaBDE (BDE-100), and 2,2’,4,4’5,5’-hexa BDE(BDE-153). [8] The present study is focused on BDE-47 not only because it is preferentially detected in human tissue, [9] breast milk, [10] and serum [11] but also because it can be transferred to the fetus through the maternal circulatory system or to the infant through breast milk. [12] PBDEs have emerged as a major environmental pollutant, raising concerns about possible adverse health effects to wildlife and humans. Although BDE-47 is ubiquitous in the environment, its toxicological effects are not well understood.
Due to their structural similarity to Polychlorinated Biphenyls (PCBs) and Thyroid Hormones (TH), PBDEs may act as TH disruptors. Studies of oral administrations of both commercial DE-71 and BDE-47 for 4 d decreased the total serum TH levels in rats [13]. In addition, PBDEs possess endocrine-disrupting properties that may predispose fish to such negative effects. For example, long-term exposure of fathead minnows (Pimephales promelas) to a dietary mixture of BDE-47 consistently showed decreased plasma T4 and elevated TSHβ mRNA levels at the end of 56d. [14] Moreover, reduced plasma T4 levels were noted in zebrafish embryos (Danio rerio) that were exposed to DE-71 and BDE-209 from fertilization to 14 d thereafter (dpf). [15,16].
In teleost fish, the biosynthesis, secretion, and metabolism of TH are controlled by the Hypothalamic-Pituitary-Thyroid (HPT) axis. The hypothalamus secretes Thyrotropin-Releasing Hormone (TRH) to stimulate Thyroid-Stimulating Hormone (TSH) secretion and regulate TH synthesis within the HPT axis. [17] The thyroid plays an important role in normal development, growth, physiological function, and metabolism in fish. With the heavy production and global use of consumer products, a large number of contaminants have recently emerged that interrupt the functions of TH by affecting the HPT axis via disruptions in the synthesis, regulation, metabolism, and action of TH. Recently, an in vivo model for testing endocrine disruption in the synthesis, regulation, metabolism, and action of TH. Recently, an in vivo model for testing endocrine disruption of THs was developed in zebrafish larvae. Yu et al, [15] and Chen et al, [16] have shown that the highest PBDE congeners (e.g., BDE-209) and a mixture of lower PBDE congeners (DE-71) can affect T4 levels and alter gene transcription in the HPT axis. The results from these studies suggest that the series of genetic responses in the HPT axis can potentially be used in evaluating the biological effects of PBDEs. A recent study also showed that BDE-47 can affect mRNA expression in the thyroid hormone pathway in the zebrafish embryos (Danio rerio). [18] Nevertheless, the available data on BDE-47 exposure in fish were inadequate for a comprehensive assessment of thyroid endocrine disruption, and the underlying molecular mechanism is not well understood.
Therefore, in the present study, we employed zebrafish embryos as a model to evaluate the responses of the HPT axis to BDE-47 exposure and to identify the potential mechanisms of BDE-47-induced endocrine toxicity in zebrafish embryos/larvae. We examined the expression and protein profiles of HPT genes and determined the levels of T4 in zebrafish embryos/larvae that were exposed to BDE-47.
Materials and Methods
Chemicals
BDE-47 (purity: 99%) was obtained from ChemServices (West Chester, PA) and a stock solution of BDE-47 (10.0 mg/mL) was prepared by dissolution in Dimethyl Sulfoxide (DMSO) (purity >99.9%, Sigma, St. Louis, MO, USA) and was stored at 4°C. All other chemicals used in the present study were of analytical grade.
Zebrafish maintenance and embryo exposure
Adult zebrafish (AB strain, 4 months old) were maintained at 28°C with a photoperiod of 14/10 h light/dark, and embryo exposure was performed according to a previous study. [19] Briefly, the embryos were collected 2 h post-fertilization (hpf) and examined under a stereomicroscope. After a series of exposure suspensions (0, 1, 5 and 10 µg/L) dispersed into a culture medium (consisting of 64.75 mg/L NaHCO3, 5.75 mg/L KCl, 123.25 mg/L MgSO4·7H2O, and 294 mg/L CaCl2·2H2O), the 600 normal embryos were randomly distributed into glass fish tanks containing 1 L of different concentrations of BDE-47. There were three replicates for each concentration exposure. During the experimental period, 50% of the exposure solution was renewed daily, and the zebrafish larvae were fed with cultured live fairy shrimp twice daily. The control and exposure embryos received 0.003% (V/V) DMSO. The embryos/larvae were examined under a stereo microscope (Olympus, Japan) equipped with a digital camera for morphological abnormalities, and the survival, malformation and hatching rates were also recorded at specified time points (t = 24, 48, 72, 96 hpf). Ten individual larvae from each beaker (n = 3) were randomly sampled, anesthetized in MS-222 and photographed with a digital camera. The length of each fry along the body axis from the anterior-most part of the head to the tip of the tail was measured from these digital images using Image ProPlus software (Media Cybernetics, Bethesda, MD, USA). The selected exposure concentrations of BDE-47 were previously ascertained by performing a range-finding study; this study revealed that, after exposure to the lowest concentration of BDE-47 (1 µg/L), the malformation rates trended toward an increase, but the tendency was not statistically significant. The exposure time (14 dpf) in our experiment was based on the timing of exogenous hormone exposure used by Crane et al. [20] in fathead minnows, and subsequently applied to other studies in zebrafish larvae. [15,16] After 14 dpf, the larvae were randomly sampled, immediately frozen in liquid nitrogen, and stored at -80°C for further thyroid hormone assays and gene and protein expression.
Gene expression
The procedures for RNA extraction and gene expression patterns were performed as previously described. [19] Briefly, total RNA was extracted from 40 homogenized zebrafish larvae using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. The primer sequences for SYBR green assays (Toyobo, Tokyo, Japan) for CRH, TSHβ, NIS, TG, NKx2.1, TTR, Deio1, Deio2 and β-actin are shown in table 1. To remove genomic DNA contamination, total RNA was digested using RNase-free DNase I (Promega Madison, WI, USA) and then purified. The total RNA recovered from the DNase I digestion was measured at 260 and 280 nm using a spectrophotometer (M2, Molecular Devices, Sunnyvale, CA, USA). The 260 nm reading was used to estimate the concentration of total RNA. The RNA quality in each sample was verified by measuring the 260/280 nm ratios, as well as by 1% agarose-formaldehyde gel electrophoresis with ethidium bromide staining. The synthesis of cDNA was performed using 2 µg total RNA from each sample mixed with 500 ng of random primer (Takara, Kyoto, Japan) using M-MLV Reverse Transcriptase (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Quantitative PCR was analyzed with an ABI PRISM 7500 Sequence Detector System (Perkin-Elmer Applied Biosystems, Foster City, CA, USA).
Gene names, accession numbers, and forward and reverse primer sequences are listed in table 1. The thermal cycle was as follows: initial denaturation for 10 min at 95 °C followed by 39 cycles of 95 °C for 30 s, 60 °C for 20 s and 72 °C for 1 min. Quantitative values were obtained from the Threshold Cycle (CT) number, which is the increase in the detected signal that is associated with an exponential growth of the PCR product. For each selected gene, RT-PCR reactions were performed on three replicate samples. The expression level of each target gene was normalized to its β-actin mRNA content. The fold change of the tested genes was analyzed using the 2-ΔΔCt method. [21].
Thyroid hormone extraction and measurement
The methods for extraction of whole body THs were modified from Crane et al. [20], which used fathead minnows. Briefly, 100 zebrafish larvae from each group were homogenized in 0.5 mL of ice-cold methanol and centrifuged at 10 000 × g for 15 min at 4 °C. Then, the homogenates were disrupted by intermittent sonic oscillation for 5 min on ice and then vortexed vigorously for 10 min. After centrifugation at 5000 × g for 15 min at 4 °C, the supernatants were collected, and the pellets were re-extracted with 0.5 mL of methanol and then centrifuged. The freshly collected supernatant was combined with the original supernatant and vacuum-dried overnight at room temperature. The samples were re-dissolved in 50 µL of methanol, 200 µL of chloroform, and 50 µL of 0.11 M barbital buffer (pH 8.6). The mixture was vortexed vigorously for 3 min and centrifuged at 3500 × g for 15 min at 4 °C. The upper layer was carefully collected and then vacuum-dried overnight at room temperature. The extracts were stored at -80 °C until the Radioimmunoassay (RIA) was performed. The efficiency of the TH extraction was determined by adding 100 µL of 125I-radiolabeled T3 or T4 to each larval homogenate prior to extraction. The RIA was performed with commercial kits (Beijing North Institute of Biotechnology, China) according to the manufacturer’s instructions. The minimum detectable levels of T3 and T4 in the radioimmunoassay were 0.5 ng/mL and 20 ng/mL, respectively. For both T3 and T4, the inter-assay Coefficients of Variance (CV) were < 10%.
Protein extraction and western blot analysis
Protein extraction was performed using commercial kits (KeyGEN BioTECH, Nanjing, China) according to the manufacturer’s instructions. Briefly, 100 zebrafish larvae from each treatment were homogenized in 0.5 mL of lysis buffer containing proteinase inhibitors (1%, v/v), phosphatase inhibitor (0.1%, v/v) and Phenylmethanesulfonyl fluoride (PMSF) (1%, v/v). Next, the samples were centrifuged at 1000 g for 10 min at 4°C. The supernatants were collected and stored at -80 °C for further western blot analysis.
In our study, Thyroglobulin (TG) and Transthyretin (TTR) protein levels were analyzed. Protein concentrations were analyzed. Protein concentrations were determined using the Bradford method. The western blot analysis was conducted according to a previous study. [12] Briefly, approximately 40 µg of each protein sample was separated by SDS-PAGE and then transferred to a nitrocellulose membrane (Amersham Biosciences, USA). The nitrocellulose membrane was blocked for 1 h with 5% non-fat dried milk in Tris-Buffered Saline (TBS) and incubated with primary antibody against TG (Sigma, St.Louis, MO, USA)/TTR (Abcam, USA) at 37 °C for 2 h. The blots were washed six times for 30 min with Tris-Buffered Saline Tween-20 (TBST) and then incubated with AP-conjugated secondary antibody at 37°C for 1 h. The blots were washed six times and then developed using a BCIP/NBT Kit (AMRESCO, USA). In this study, rat GAPDH antibody (ProteinTech, USA) was used to detect proteins in zebrafish.
Statistical analysis
The mean values of TH content and genes expression were calculated from the replicates, and the mean ± standard errors (S.E.M) were expressed. The normality of the data was verified using the Kolmogorov-Smirnov test, and the homogeneity of variances was analyzed by Levene’s test. The differences between the control and each exposure group were evaluated by the independent-sample t test using SPSS 17.0 software (SPSS, Chicago, IL, USA). Differences were considered significant or highly significant at p < 0.05 and p < 0.01, respectively.
Results
Developmental toxicity
Exposure to 1 and 5 µg/L of BDE-47 did not affect the body weight, while exposure to 10 µg/L of BDE-47 significantly inhibited body weight gain (body weight, 0.35 ± 0.04 mg) relative to the control (body weight, 0.39 ± 0.02 mg) (p < 0.05). There were no significant effects on body length after exposure to 1, 5 and 10 µg/L BDE-47 relative to the control group at 14 dpf. The malformation rates were significantly increased in embryos exposed to 10 g/L of BDE-47 (p 0.05), while the recorded survival rates and hatching rates of embryos exposed to 0, 1, 3, and 10 µg/L of BDE-47 showed no significant differences after 14 d of exposure (table 2).
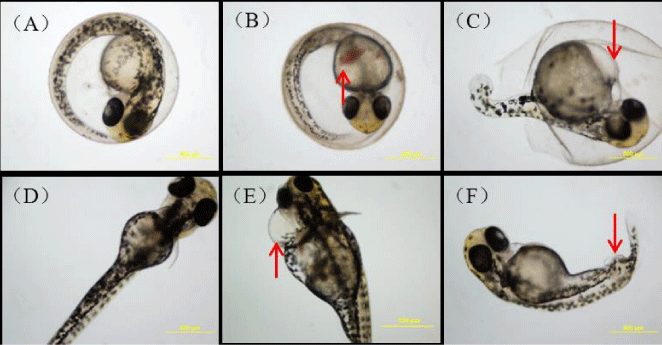
The zebrafish embryos treated with 10 µg/L BDE-47 showed a suite of abnormalities including including hyperemia, yolk sac edema, pericardial edema, and spinal curvature (figure 1).
Whole-body T3 and T4 contents
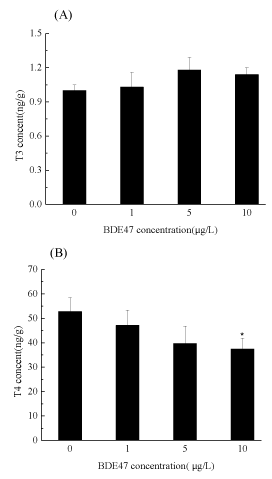
At the end of the exposure, the T3 and T4 contents of the whole body in the control group were determined to be 1.14 and 52.81 ng/g, respectively. There were no significant effects of exposure to 1, 5 and 10 µg/L BDE-47 on T3 relative to the control (figure 2A). No significant difference was observed in the T4 content in the zebrafish embryos exposed to 1 and 5 µg/L BDE-47, but a significantly decrease in the T4 level (37.49 ± 4.38) was detected in zebrafish embryos exposed to 10 µg/L BDE-47, compared with the control (figure 2B).
mRNA expression profiles of selected genes
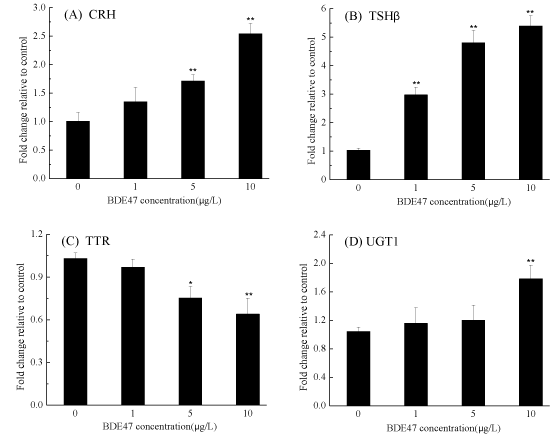
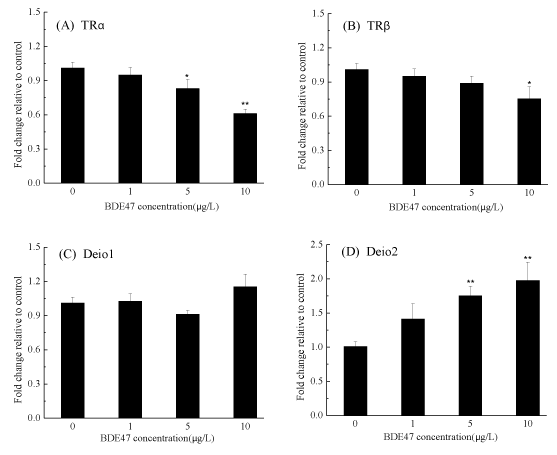
Several genes involved in the regulation, transport, binding and metabolism of THs were examined. The transcription of the Corticotropin-Releasing Hormone (CRH) gene increased in a dose-dependent manner and was significantly up-regulated by 1.71- and 2.54-fold after exposure to 5 and 10 µg/L BDE-47, respectively, compared with the control group (p < 0.01) (figure 3A), while thyroid stimulating hormone (TSHβ) gene transcription was significantly up-regulated in a dose-dependent manner relative to the control (p < 0.01) (figure 3B). It was noted that the transcription of TTR was significantly down-regulated by 0.75- and 0.64-fold in the groups exposed to 5 and 10 µg/L of BDE-47, respectively p<0.05 (figure 3C). A small but significant downregulation of hepatic uridine diphosphoglucuronosyl transferase (UGT1ab) transcription was observed in the zebrafish embryos exposed to 10 µg/L of BDE-47 (1.78-fold) (p<0.01) (figure 3D, figure 4).
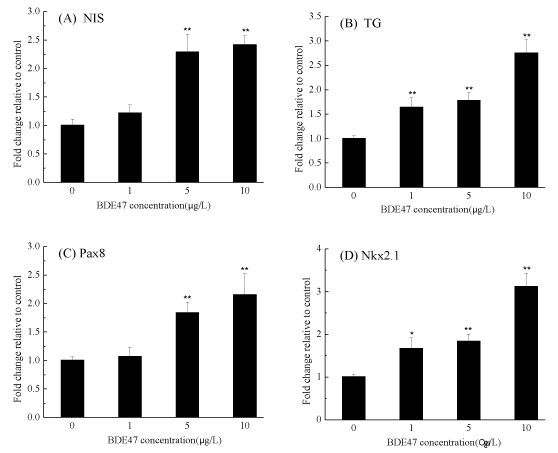
Transcription of the sodium/iodide symporter (NIS) gene was significantly up-regulated by 2.29- and 2.42-fold in the groups exposed to 5 and 10µg/L BDE-47 (p < 0.01) (figure 4A). Upon treatment with 1, 5, and 10 µg/L BDE-47, the expression of the TG gene was significantly up-regulated by 1.65-, 1.78- and 2.76-fold , respectively in a dose-dependent manner (p < 0.01) (figure 4B). When examining the marker genes involved in thyroid development and growth, Pax8 transcription was significantly upregulated by 1.84- and 2.16-fold in the zebrafish embryos exposed to 5 and 10 µg/L BDE-47, respectively (figure 4C), while Nkx2.1 was significantly increased by 1.67-, 1.84- and 3.13-fold in the zebrafish embryos treated with 1, 5, and 10 µg/L BDE-47, respectively (p < 0.05) (figure 4D, figure 5).
In zebrafish, two isoforms of TRs, TRα and TR, have been identified. The transcription of TRα was decreased in a dose-dependent manner and was significantly down-regulated upon exposure to 5 and 10 µg/L BDE-47 by 0.83- and 0.61-fold, respectively (p < 0.05) (figure 5A). A small but significant down-regulation of TRβ expression was observed in the group treated with 10 µg/L BDE-47 (p < 0.05) (figure 5B). The mRNA expression of two deiodinase isoforms (Deio1 and Deio2) showed different trends. The transcription of Deio1 was not altered in any BDE-47-treated groups (figure 5C), while the transcription of Deio2 was upregulated significantly in the groups treated with 5 and 10 µg/L BDE-47 by 1.75- and 1.98-fold, respectively (p < 0.01) (figure 5D).
Protein abundance
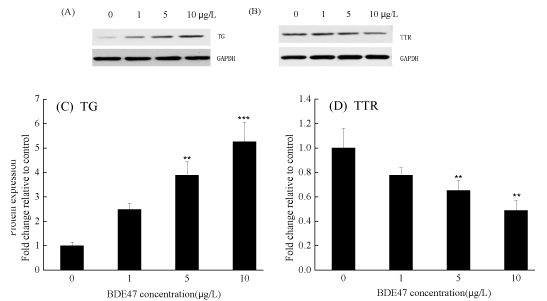
Exposure to BDE-47 up-regulated TG protein expression in a concentration-dependent manner; significant up-regulation was observed after exposure to 5 and 10 µg/L of BDE-47 (p < 0.01) (figure 6A and C). However, the transcription of TTR protein was down-regulated in a concentration-dependent manner, and a significant down-regulation was observed in the 5 and 10 µg/L BDE-47 treated groups compared with the control (p < 0.05) (figure 6B and D).
Discussion
The thyroid system plays an important role in the regulation of differentiation, growth, metabolism, and salinity adaptation in fish. This study employed developing zebrafish larvae to assess the thyroid endocrine disruption caused by exposure to BDE-47 and to ascertain the possible biological mechanisms of this disruption. qPCR assays were employed to detect changes in a series of genes related to thyroid synthesis, transport, binding, and regulation. In the zebrafish larvae, both THs and genes involved in thyroid function were affected by BDE-47 exposure. The results indicated that the HPT axis in zebrafish larvae can be used to evaluate the potential mechanisms of thyroid endocrine disruption for lower PBDE congeners.
Limited studies on the effect of PBDEs on growth parameters have reported that the growth and metamorphosis of African clawed frogs were inhibited by DE-71 (a commercial PBDE mixture) and BDE-47 exposure. [22] The authors suggested that the results may be due to the disruptive effects of PBDE on TH homeostasis. THs play a critical role not only in Anuran metamorphosis but also in the development and growth of zebrafish. In support of the above point, exposure to different concentrations of BDE-209 and DE-71 significantly altered T4 levels accompanied by growth retardation in zebrafish larvae. [15,16] However, in the present study, increased malformation rates and inhibited body weight gain were observed with decreased T4 levels, but the survival rates and body lengths of the zebrafish larvae were not significantly affected. Our results suggest that the concentration range of this study was insufficient to induce death, but BDE-47 could lead to abnormal morphologies and developmental retardation by reducing TH levels in zebrafish larvae. Barja-Fernández have also found that the gills, intestine and liver of turbot (Psetta maxima) showed abnormal histopathology after BDE-47 treatment. [23].
In fish, T4 and T3 are the main forms of TH secreted from the thyroid gland and play major roles in development and growth. In the present study, T4 levels decreased in a dose-dependent manner with BDE-47 exposure. This result is in agreement with several previous reports on thyroid disruption with decreased T4 upon BDE-47 exposure in rodents [9] as well as DE-71 exposure in adult zebrafish. [24] Although decreased T4 levels are generally observed in mammals and fish exposed to PBDEs, the T3 levels did not significantly increase in our study, suggesting a conversion from T4 to T3 or T3 metabolism is not affected by BDE-47. In fish, Deio1 can convert T4 to T3 by removing iodine from the outer ring of T4. [25] In the present study, the Deio1 expression was not altered in any BDE-47-treated groups, which suggested that T3 synthesis or metabolism was not altered by BDE-47 exposure.
It has been proposed that CRH and TSH function as common regulators of the thyroidal axis as feedback mechanisms that are triggered by changes in the concentrations of circulating THs in fish. [26] In the present study, the CRH and TSHβ genes were upregulated significantly in the zebrafish larvae exposure to 5 and 10 µg/L BDE-47. Previous studies suggest that THs modulate the expression of TRH and TSH in mammals via a negative feedback mechanism; therefore, the modulation of the transcription of the CRH and TSH genes is influenced by TH levels. [17] A similar feedback mechanism was also found in recent studies on fish. Lema et al, [14] have demonstrated decreased plasma levels of T4 accompanying the enhanced expression of TSHβ in adult fathead minnows after a low dietary dose of BDE-47. Another recent study also showed that exposure to DE-71 and BDE-209 reduced the levels of T4 in zebrafish and was associated with the elevated expression of CRH and TSHβ. [15,16] Therefore, this study indicated that the elevated mRNA expression of CRH and TSHβ can be attributed to reduced negative feedback from the hypothalamus and pituitary due to decreased levels of T4.
In our experiment, the expression of genes involved in TH synthesis (e.g., NIS and TG) and thyroid development and growth (e.g., Nkx2.1a and Pax8) was significantly upregulated in the zebrafish larvae exposed to 5 and 10 µg/L BDE-47 compared with the control. As thyroid transcription factors, Pax8 protein is essential for the late differentiation of the follicular cells, and Nkx2.1 (or TTF1) plays an essential role in thyroid development in zebrafish. [27] The transcriptional up-regulation of the Pax8 and Nkx2.1 genes suggests that BDE-47 induces gene expression and that thyroid growth and development were promoted to compensate for the depressed T4 levels. The product of Sodium/Iodide Symporter (NIS) is an integral membrane protein involved in TH synthesis and in transporting sodium and iodide across the basolateral plasma into thyroid follicle cells. TG is a dimeric protein produced by and used entirely within the thyroid gland and can be used by the thyroid gland to produce the THs. [28] The transcription of the NIS and TG genes is regulated by thyroid transcription factor (TTF-1, TTF-2) and Pax8 and is stimulated by TSH. [29] The increased gene transcription of NIS and TG observed in the present study may be controlled by Nkx2.1a and Pax8 to compensate for the reduced T4 levels. A significant up-regulation in transcription of these genes has also been reported in zebrafish larvae exposed to DE-71 and BDE-209 exposure to zebrafish larvae concomitant with decreased T4 levels. [15,16] Furthermore, increased protein expression levels of TG were observed in the present study. Thus, the increased transcription and translation of TG further support the occurrence of a compensatory response to lowered T4 levels at the TH synthesis stage.
TTR, the TH binding marker gene, has been proposed as a key TH carrier protein that regulates the supply of the hormone to various target tissues and plays an essential role in the thyroid axis in fish. [28] In the present study, the expression of TTR at both the transcriptional and protein levels was significantly down-regulated. It has been suggested that environmental contaminants, due to their structure-activity resemblance to THs, may affect circulating TH levels by competing for their binding sites on transport proteins and interfering with TH homeostasis by binding with TTR. [23] Therefore, although the potential mechanisms for reductions in TTR are not well understood, the inhibition of TTR mRNA and protein levels in the present study suggests that BDE-47 could influence TH binding proteins and could interfere with TH homeostasis.
In vertebrates, the activities of Deio1 and Deio2 are critical for the regulation of circulating and peripheral TH levels. In fish, Deio1 plays a minimal role in plasma TH homeostasis, while it has a considerable influence on iodine recovery and TH degradation; additionally, Deio2 converts T4 into a more active form, i.e., T3. [30] In the present study, we found a significant increase in Deio2 gene expression in the zebrafish larvae exposed to 5 and 10 µg/L BDE-47, while the transcription of the Deio1 gene showed no significant difference at level of BDE-47 exposure. It seems probable that the increase in the transcription of Deio2 is at least partly responsible for the reduction in the circulating T4 levels and will have a significant impact on tissue TH homeostasis. A similar study showed that Deio1 may be more readily responsive to changes in thyroid levels than Deio2, [31] and the results showed that decreased Deio2 gene expression reduced the conversion of T4 to T3. Walpita et al. have demonstrated that the developmental delay in zebrafish embryos/larvae is induced by Deio2 knockdown and not Deio1 knockdown, which induced abnormal developmental progression, [32] suggesting that Deio2 might be the major contributor to TH activation in developing zebrafish embryos/larvae.
In zebrafish, THs act by binding to specific nuclear receptors (TRs) and are necessary for embryogenesis and larval development. There are two major thyroid Hormone Receptor (TR) isoforms (TRα and TRβ) that bind T3 and mediate TH-regulated gene expression. [28] Several environmental contaminants interfere with the regulatory network of TH by binding to the TR and then inducing variations in the expression of TR subtypes, which depend on tissue-specific and developmental state-specific functions. A previous study had shown that, in fathead minnows, the dietary intake of BDE-47 did not affect the TR transcription levels in the liver but did decrease TRβ mRNA levels in the brain. [14] However, the mRNA expression of TRα and TRβ were both downregulated significantly in this experiment. Additionally, both TRα and TRβ mRNA expression in Sebastiscus marmoratus embryos was down-regulated by Pyrene. [33] Moreover, microcystin- leucine-arginine also inhibited the transcription of TRα and TRβ in zebrafish embryos. Furthermore, decreased TRα and TRβ could lead to the failure of THs to bind and activate the appropriate response cascades. [34] Thus, we speculate that the decreased TRα and TRβ mRNA expression observed in this study might play an important role in BDE-47-induced thyroid disruption; the inhibited expression further demonstrates that BDE-47 impaired the TH signaling, thereby influencing the embryonic development and growth.
It has been suggested that Uridine Diphosphate-Glucuronosyltransferases (UGT) and Sulfotransferases (SULTs) play important roles in TH homeostasis via the major pathway for T4 conjugation. [34] Recently, zebrafish larvae exposed to DE-71 for 14 d also exhibited reduced levels of circulating T4, accompanied by increased UGT gene transcription. [15] In this study, we observed significant UGT1 mRNA expression upregulation, demonstrating that UDPGTs might play a role in reducing the levels of TH; thus, the decreased T4 levels may be directly related to the regulation of UGT1.
Conclusions
In summary, our results showed that exposing zebrafish larvae to BDE-47 induced abnormal morphologies and developmental retardation in larva development and influenced the levels of THs, indicating that BDE-47-induced thyroid endocrine disruption might contribute to abnormal morphologies in the early life stages of zebrafish. Moreover, the present study showed that BDE-47 can modulate the expression levels of certain genes encoding proteins involved in TH and TTR synthesis in the HPT axis of the zebrafish larvae. Hence, a short-term bioassay that involves qPCR analysis of gene expression following BDE-47 treatment in zebrafish larvae may provide a promising approach to evaluate thyroid endocrine disruption.
Acknowledgements
This project was supported by grants from the National Nature Science Foundation of China (21507039) Province Department of Education “Thirteen Five” scientific and technological research projects of Jilin, China (Kyrgyzstan UNESCO co-word [2016] No. 227).
- Voorspoels S, Covaci A, Jaspers VLB, Neels H, Schepens P. Biomagnification of PBDEs in three small terrestrial food chains. Environ Sci Technol. 2007; 41: 411-416. https://goo.gl/dg8T9f
- Wu JP, Luo XJ, Zhang Y, Luo Y, Chen SJ, Mai BX, et al. Bioaccumulation of Polybrominated Diphenyl Ethers (PBDEs) and Polychlorinated Biphenyls (PCBs) in wild aquatic species from an electronic waste (e-waste) recycling site in South China. Environ Int. 2008; 34: 1109-1113. https://goo.gl/zBxXBH
- Leung AO, Luksemburg WJ, Wong AS, Wong MH. Spatial Distribution of Polybrominated Diphenyl Ethers and Polychlorinated Dibenzo-p-dioxins and Dibenzofurans in Soil and Combusted Residue at Guiyu, an Electronic Waste Recycling Site in Southeast China. Environ Sci Technol. 2007; 41: 2730-2737. https://goo.gl/eJaHdr
- Guan YF, Wang JZ, Ni HG, Luo XJ, Mai BX, Zeng EY. Riverine inputs of polybrominated diphenyl ethers from the Pearl River Delta (China) to the coastal ocean. Environ Sci Technol. 2007; 41: 6007–6013. https://goo.gl/JKYJ4y
- Besis A, Samara C. Polybrominated Diphenyl Ethers (PBDEs) in the indoor and outdoor environments-A review on occurrence and human exposure. Environ Pollut. 2012; 169: 217-229. https://goo.gl/HwwCCc
- Wan B, GUO Lianghong. Research progress on the investigation of environmental toxicology of polybrominated diphenyl ethers. Enviro Chem. 2011; 1: 143-149. https://goo.gl/sMk4nT
- Schreiber T, Gassmann K, Götz C, Hübenthal U, Moors M, Krause G, et al . Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model: evidence for endocrine disruption. Environ Health Persp. 2010; 118: 572-578. https://goo.gl/uk5sbh
- Gómara B, Herrero L, Ramos JJ, Mateo JR, Fernández MA, García JF, et al. Distribution of polybrominated diphenyl ethers in human umbilical. Cord serum, paternal serum, maternal serum, placentas, and breast milk from Madrid population, Spain. Environ Sci Technol. 2007; 41: 6961-6968. https://goo.gl/UGu4BE
- Petreas M, She JW, Brown FR, Winkler J, Windham G, Rogers E, et al. High body burdens of 2,2',4,4'- tetrabromodiphenyl ether (BDE-47) in California women. Environ Health Persp. 2003; 111: 1175-1179. https://goo.gl/ioXZfz
- Tsydenova OV, Sudaryanto A, Kajiwara N, Kunisue T, Batoev VB, Tanabe S. Organohalogen compounds in human breast milk from Republic of Buryatia, Russia. Environ Pollut 2007; 146: 225-232. https://goo.gl/ig8huo
- Kim J, Kang JH, Park H, Baek SY, Kim YH, Chang YS. Assessment of Polybrominated Diphenyl Ethers (PBDEs) in serum from the Korean general population. Environ Pollut. 2012; 164: 46-52. https://goo.gl/2Dpk3W
- Schecter A, Pavuk M, Papke O, Ryan JJ, Birnbaum L, Rosen R. Polybrominated Diphenyl Ethers (PBDEs) in US mothers' milk. Environ Health Persp. 2003; 111: 1723-1729. https://goo.gl/z2vk5V
- Richardson VM, Staskal DF, Ross DG, Diliberto JJ; DeVito MJ, Bimbaum LS. Possible mechanisms of thyroid hormone disruption in mice by BDE 47, a major polybrominated diphenyl ether congener. Toxicol Appl Pharm. 2008; 226: 244-250. https://goo.gl/CgARVU
- Lema SC, Dickey JT, Schultz IR, Swanson P. Dietary exposure to 2,2 ',4,4 '-tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormone-regulated gene transcription in the pituitary and brain. Environ Health Persp. 2008; 116: 1694-1699. https://goo.gl/35kL2q
- Yu LQ, Deng J, Shi XJ, Liu CS, Yu K, Zhou BS. Exposure to DE-71 alters thyroid hormone levels and gene transcription in the hypothalamic-pituitary-thyroid axis of zebrafish larvae. Aquat Toxicol. 2010; 100: 376. https://goo.gl/iFtdQy
- Chen Q, Yu LQ, Yang LH, Zhou BS. Bioconcentration and metabolism of decabromodiphenyl ether (BDE-209) result in thyroid endocrine disruption in zebrafish larvae. Aquat Toxicol. 2012; 110: 141-148. https://goo.gl/mWaqeg
- Zoeller RT, Tan SW, Tyl RW. General background on the hypothalamic- pituitary-thyroid (HPT) axis. Crit Rev Toxicol. 2007; 37: 11-53. https://goo.gl/sMUUQX
- Chan WK, Chan KM. Disruption of the hypothalamic-pituitary-thyroid axis in zebrafish embryo-larvae following waterborne exposure to BDE-47, TBBPA and BPA. Aquat Toxicol. 2012; 108: 106-111. https://goo.gl/9JWL5B
- Zhao XS, Wang ST, Wu Y, You H, Lv LN. Acute ZnO nanoparticles exposure induces developmental toxicity, oxidative stress and DNA damage in embryo-larval zebrafish. Aquat Toxicol. 2013; 136-137: 49-59. https://goo.gl/aT7Z2r
- Crane HM, Pickford DB, Hutchinson TH, Brown JA. Developmental changes of thyroid hormones in the fathead minnow, pimephales promelas. Gen Comp Endocr. 2004; 139: 55-60. https://goo.gl/WwU8TV
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2^(-DeltaDelta Ct) method. Methods 2001; 25: 402-408. https://goo.gl/ftEKpZ
- Balch GC, Velez Espino LA, Sweet C, Alaee M, Metcalfe CD. Inhibition of metamorphosis in tadpoles of Xenopus laevis exposed to polybrominated diphenyl ethers (PBDEs). Chemosphere. 2006; 64: 328-338. https://goo.gl/AnS4Nd
- Barja Fernández S, Míguez JM, Álvarez Otero R. Histopathological effects of 2,2',4,4'-tetrabromodiphenyl ether (BDE-47) in the gills, intestine and liver of turbot (Psetta maxima). Ecotox Environ Safe. 2013; 95: 60-68. https://goo.gl/kcPe5c
- Yu L, Lam JC, Guo Y, Wu RS, Lam PK, Zhou B. Parental Transfer of polybrominated diphenyl ethers (PBDEs) and thyroid endocrine disruption in zebrafish. Environ Sci Technol. 2011; 45: 10652-10659. https://goo.gl/h6g5Ko
- Walpita CN, Crawford AD, Janssens EDR, Van der Geyten S, Darras V.M. Type 2 iodothyronine deiodinase is essential for thyroid hormone-dependent embryonic development and pigmentation in zebrafish. Endocrinology. 2009; 150: 530-539. https://goo.gl/CdCgrk
- Geven EJ, Flik G, Klaren PH. Central and peripheral integration of interrenal and thyroid axes signals in common carp (Cyprinus carpio L.). J Endocrinol. 2009; 200: 117-123. https://goo.gl/F84RTE
- Wendl T, Lun K, Mione M, Favor J, Brand M, Wilson SW, et al. pax2.1 is required for the development of thyroid follicles in zebrafish. Development. 2002; 129: 3751-3760. https://goo.gl/iMfgR6
- Porazzi P, Calebiro D, Benato F, Tiso N, Persani L. Thyroid gland development and function in the zebrafish model. Mol Cell Endocrinol. 2009; 312: 14-23. https://goo.gl/8hbgC9
- Kambe F, Nomura Y, Okamoto T, Seo H. Redox regulation of thyroid- transcription factors, Pax-8 and TTF-1, is involved in their increased DNA-binding activities by thyrotropin in rat thyroid FRTL-5 cells. Mol. Endocrinol. 1996; 10: 801-812. https://goo.gl/o7WA1A
- Orozco A, Valverde RC. Thyroid hormone deiodination in fish. Thyroid. 2005; 15: 799-813. https://goo.gl/qM9bZ9
- Shi X J, Liu C S, Wu GQ, Zhou BS. Waterborne exposure to PFOS causes disruption of the hypothalamus-pituitary-thyroid axis in zebrafish larvae. Chemosphere. 2009; 77: 1010-1018. https://goo.gl/cVX5GC
- He CY, Zuo ZH, Shi X, Sun LB, Wang CG. Pyrene exposure influences the thyroid development of Sebastiscus marmoratus embryos. Aquat Toxicol. 2012; 124: 28-33. https://goo.gl/2Jp2XQ
- Yan W, Zhou YX, Yang J, Li SQ, Hu DJ, Wang JH, et al. Waterborne exposure to microcystin-LR alters thyroid hormone levels and gene transcription in the hypothalamic-pituitary-thyroid axis in zebrafish larvae. Chemosphere. 2012; 87: 1301-1307. https://goo.gl/MKa1v9
- Hood A, Klaassen CD. Differential effects of microsomal enzyme inducers on in vitro thyroxine (T-4) and triiodothyronine (T-3) glucuronidation. Toxicol Sci. 2000; 55: 78-84. https://goo.gl/kGfYWX

Sign up for Article Alerts