Crocin Affects Passive Avoidance Memory Following Formaldehyde-Induced Neurotoxicity in a Rat Model
Mahsa Mashak shahabadi1,2, Seyyedeh Zahra Mousavi1,2, Seyyedeh Elaheh Mousavi3 and Majid Hassanpour Ezzati1,2*
1Department of Toxicology and Pharmacology, Faculty of Pharmacy, Pharmaceutical Sciences Branch, Islamic Azad University, Tehran, Iran
2Department of Physiology, Basic Sciences School, Shahed University, Tehran, Iran
3Department of Pharmacology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
*Address for Correspondence: Majid Hassanpour Ezzati, Shahed University, Opposite the Holy Shrine of Imam Khomeini, Khalij Fars Expressway, Tehran, Iran, Tel: +982151212242, E-mail: hassanpour@shahed.ac.ir
Submitted: 11 September 2017; Approved: 20 September; Published: 21 September 2017
Citation this article: Shahabadi MM, Mousavi SZ, Mousavi SE, Ezzati HM. Crocin Affects Passive Avoidance Memory Following Formaldehyde-Induced Neurotoxicity in a Rat Model. Adv J Toxicol Curr Res. 2017;1(1): 015-022.
Copyright: © 2017 Mousavi SE, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Neurotoxicity; Passive Avoidance Memory; Crocin; Nitric Oxide; Formaldehyde; Hippocampus; Rats
Download Fulltext PDF
Crocin, the main constituent of Crocus sativus L., protected against inflammation-induced neurotoxicity and improved learning behavior. Also, crocin inhibited production of inducible nitric oxide synthase. Studies also indicated an interaction between NO and Formaldehyde (FA) -induced neurotoxicity. In this study, effect of crocin on memory and body weight changes in neurotoxicity induced by FA was evaluated in a rat model. Moreover, the possible involvement of NO on protective effects of crocin was investigated. FA (5 mg/kg) was administered intraperitoneally for 5 consecutive days. Effect of crocin post-treatment (25, 50 and 100 mg/kg, i.p.) on FA neurotoxicity was evaluated on passive avoidance memory. To determine the contribution of NO, a NO synthesis precursor, L-arginine (L-Arg) 100 mg/kg, i.p.; and a nonselective NO synthase inhibitor N (G)-Nitro-L-Arginine Methyl Ester, (L-NAME) (10 mg/kg, i.p.) were used. Then, the hippocampus was evaluated histologically. FA caused weight loss and increased mortality rate. It also impaired fear memory. On the contrary, crocin (25 mg/kg, i.p.) reduced mortality rate, weight loss and hippocampal tissue damage induced by FA. Further, it improved the memory impairment by reducing the latency time and the hippocampus cell loss. Moreover, L-NAME reversed the protective effects of crocin, nevertheless; L-Arg enhanced the effects of crocin. Pretreatment with crocin protected the central and peripheral damages. Exogenous NO ameliorated the neuroprotective effect of crocin; as a result, crocin exerted its protective effects through NO up-regulation. Results of the present study suggest that inhibition of NO synthesis may exaggerate the neurotoxic effects of FA.
Introduction
Formaldehyde, HCHO, (FA) is a pungent, irritant and colorless gas which is found in the nature in domestic air, cigarette smoke and the polluted atmosphere of cities due to the incomplete burning of organics, photochemical smog and release from HCHO-containing products [1,2]. It is a small molecule (M.W. 30.026 g/mol) and is able to permeate the Blood–Brain Barrier (BBB) [3]. Long term exposure to FA may cause irreversible neurotoxicity [4] and is related to brain cancer (astrocytoma) [5]. Its neurotoxicity includes demyelization of hippocampal neurons [6] and impairment of the hippocampus [7], neurofilament protein changes [8], hyperphosphorylation of Tau protein [9]. Excess endogenous FA is a pathogenic factor in aged-dependent memory decline. Not only suppresses hippocampal Long-Term Potentiation (LTP) by blocking the N-Methyl-D-Aspartate (NMDA) receptors, FA also persistently induces spatial memory deterioration decreasing the receptors expression [10]. Furthermore, inhaled FA caused behavioral and memory disorders in rats and classified as ‘probably neurotoxic’ [11,12]. In the limbic system, the hippocampus plays a critical role in learning and memory [13] and increasing studies showed that inhaled FA exposure negatively affects the ability of spatial learning and memory in rats [14,15], suggesting that FA has harmful effects on the hippocampal functions.
Crocus sativus L. (Saffron) and its active components including crocin, crocetin etc. act as an antidote in different intoxications induced by natural toxins including snakebites, mycotoxins and endotoxins [16]. Crocin, one of main active components of saffron, is a carotenoid pigment and has the structure of crocetin di-gentiobiose ester [17]. Crocin exhibits a variety of pharmacological effects including improvement of learning behavior previously impaired by ethanol or scopolamine [18,19], anti-hyperlipidemic effect [20], anti-atherosclerotic property [21], protection against inflammation-induced neurotoxicity [22], inhibition of reperfusion-induced oxidative/nitrative injury in the ischemic brain [23] and protective effects against cisplatin-induced oxidative stress and nephrotoxicity [24]. Crocin is an effective antioxidant and its gentiobiose terminals affect its antioxidant performance [25]. Chronic oral crocin pretreatment (100 mg/kg) improved cognitive performance in Morris water maze task in Streptozotocin (STZ) -lesioned rats [26].
In addition, C. sativus L. extracts reduced some stress biomarkers in a rat model [27]. Oral administration of extract of C. sativus L. (CSE) prevented the impairment of learning in ethanol-treated mice [28]. Furthermore, CSE prevented ethanol-induced inhibition of hippocampal LTP in anesthetized rats [29]. Post-training administration of CSE successfully counteracted extinction of recognition memory in the normal rat and pre-training treatment significantly antagonized the scopolamine-induced performance deficits in the step-through passive avoidance test [30].
Nitric oxide (NO), a soluble, short-lived, and freely diffusible gas, is an important intracellular messenger in the brain [31]. Supposedly, NO participates in the mechanisms of synaptic plasticity in the hippocampus [32] and is important in cognition [33]. Reportedly, exceeding NO concentrations is associated with neuronal damage [34] and mitochondrial dysfunction [35]. Accordingly, the saffron extract (200, 400 and 800 µg/ml) had a cytotoxic effect on Hepatocellular Carcinoma Cell Line (HepG-2) and laryngeal carcinoma cell line (Hep-2) and this effect was probably related to a decrease in the NO concentration [36]. In the isolated rat heart, cardioprotective effect of crocin against ischemia/reperfusion injury may possibly be explained by regulating Endothelial Nitric Oxide Synthase (eNOS) and Inducible Nitric Oxide Synthase (iNOS) expressions [37]. Crocin also inhibited iNOS expression and NO production via down-regulation of nuclear factor kappa B (NF-κB) activity in Lipopolysaccharide (LPS) -stimulated macrophages. It provided a novel molecular mechanism for the inhibitory effects of crocin against endotoxin-mediated inflammation [38]. In another study, crocin protects the brain against excessive oxidative stress in transient global ischemia in mice. Oral administration of crocin (20, 10 mg/ kg) significantly inhibited the increased NO content in a dose-dependent manner and NOs activities [23].
Regarding the above investigations, in this study, effects of crocin on some central and peripheral physiological factors such as, fear memory, hippocampus function and body weight changes in FA-induced neurotoxicity are evaluated in a rat model. Further, the possible involvement of NO the modification effects of crocin is explored.
Materials and Methods
Animals
Seventy two adult male Wistar rats (weighing 200–250 g) assigned as the main study group and forty two rats as the pilot study. The animals were maintained under controlled temperature (23 ± 2oC) and light conditions (light, 07.00–19.00 hrs) Food (standard pellet diet) and tap water were supplied ad libitum.
The pilot study
In order to find the suitable dose for FA administration to induce neurotoxicity, and also which dose (or doses) of crocin may probably reduce the FA drawbacks on mortality rate, weight loss and memory impairment. Fifty four rats were randomly divided into the 9 groups. FA (10 and 5 mg/kg) and crocin (25, 50 and 100 mg/kg) were administered (i.p.) to five groups. One group only received saline as vehicle. Three remaining groups received both FA (5 mg/kg) and crocin at different doses 25, 50 and 100 mg/ in a 3-week period.
Main study’s animal groups
According to the pilot study, FA at dose 5 mg/kg was selected appropriate to be used throughout the study. Then about 84 intact rats were divided into 12 groups of 6-8. The rats in group I (n= 7) were used as the control. The rats in group II (n= 7) were injected intraperitoneally (i.p.) with FA (5 mg/ kg) in 5 consecutive days [39]. Crocin was injected at doses 25, 50 and 100 mg/kg (i.p.) to three groups. Crocin also was injected at doses 25, 50 and 100 mg/kg (i.p.) 30 min following FA (5 mg/ kg) to 3 other groups [40].
To determine the contribution of NO in crocin reduction in FA-induced central and peripheral consequences, L-arginine (L-Arg), NO synthesis precursor (100 mg/kg, i.p.), and a nonselective NO synthase inhibitor N (G)-Nitro-L-Arginine Methyl Ester (L-NAME) (10 mg/kg, i.p.) were co-administered 30 min prior crocin treatment (25 mg/kg, i.p.) Two groups also were received L-Arg and L-NAME as well as crocin treatment (25 mg/kg, i.p.) with a 30 min interval [41].
Passive shock avoidance test (shuttle box)
The apparatus (shuttle box) consisted of equal sized light and dark compartments (20 × 80 × 20 cm). The two compartments were separated by a guillotine door (7 × 9 cm) that could be raised to 10 cm. Floor of the dark compartment consisted of a stainless steel shock grid floor. Electric shocks were delivered to the grid floor with a stimulator (50 Hz, 1.5 s, 1.5-2 mA intensity). This test has two stages, instruction and memory test [42,43].
The passive avoidance test was conducted according to previous studies [42,44]. In acquisition trail, each rat was placed in the illuminated compartment, and 10 s later, the guillotine door was raised and the rats were allowed to move freely into the dark chamber. Upon entering to the dark compartment, the door was closed, and a 50 Hz, 1 mA constant current shock was applied for 2 s. Each rat was retrained in the apparatus and received foot-shock, if it entered the dark compartment in 120 s. Acquisition trail was terminated when the rat remained in the light compartment for 120 s. In the next step, the retention trail was screened on the day 15th, 24 hrs following the acquisition trial, by placing the animal into the light compartment and recording latency to enter the dark compartment. This escape latency served as a measure of retention performance of the step-through avoidance response. If the rats did not enter the dark compartment during 300 s, successful acquisition of passive avoidance response would be recorded. Short latencies indicate poor retention compared to significant longer latencies.
Histological assessments
Nissl staining: The day after the behavioral test session (day 11 of the study), the rats were deeply anesthetized with ether and sacrificed. The brains have been fixed via transcardial perfusion of Phosphate Buffered Saline (PBS, 0.1 M, pH 7.4) followed by 200-250 mL paraformaldehyde solution (1%) [45]. The brains were removed and post-fixed by fixative at 4°C for one week, then, dehydrated using a series of alcohols and xylene. Finally, the paraffin embedded blocks were prepared and cut coronally on a microtome at a thickness of 8 μm and mounted onto the silan-coated slides. Before staining, the sections on the glass slides were deparaffinized and then hydrated using a series of alcohols and distilled water, and finally stained using Cresyl violet for general histology. The soma diameter (in μm) of pyramidal neurons was assessed from the photos taken from CA1 pyramidal layer under 40X light microscopy. To quantify the soma diameters, a pyramidal-like neuron was randomly selected from pyramidal layer of the CA1 region from each slice and an average was taken from each neuron’s long, short, and an intersected oblique axes defined on its soma using Image J software by an experimenter blind to the experimental groups [46].
Statistical analysis: Comparisons are performed using by GraphPad Prism 5. Two-way ANOVA statistical analysis followed by Bonferroni’s complementary analysis. P-value < 0.05 is considered to be a significant difference.
Results
Mortality rates of the treated animals in the pilot study are demonstrated in table 1. The animals were monitored during a three-week period. It is evident that FA (10 mg/kg, i.p.) cause 100% mortality rate compared to saline; 83.33% (P < 0.001) in the first week of the study and the remaining 16.67% in the second week was perished. As a result, FA was administered in a reduced concentration 5 mg/kg all over the study. Accordingly, FA (5 mg/kg) resulted only 50% mortality rates in the first week compared to saline (P < 0.01). On the other hand, crocin 50 mg/kg caused 16.66% mortality in the week, however; crocin at doses 25 and 100 mg/kg did not cause death. While crocin at doses 50 and 100 caused 50% and 16% mortality in the FA-received animals (5 mg/kg) in the first week, respectively; crocin 25 mg/kg successfully eliminated mortality in the FA-received animals (5 mg/kg) compared to groups which only received FA (5 mg/kg), P < 0.001.
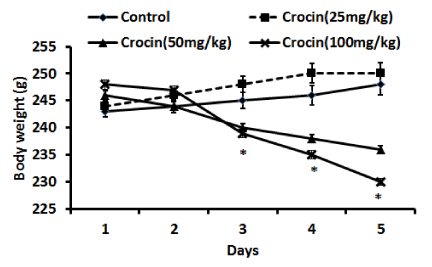
In (figure 1), effects of crocin treatments (25, 50, and 100 mg/kg, i.p.) on body weight of the animals during 5 days are given. As can be observed, crocin at doses 50, and 100 mg/kg lowered the weighs in a significant manner (P < 0.01) compared to saline-received animals. This change started from day 3 and continued to day 5. Nevertheless, crocin (25 mg/kg) did not change the weights and the animals had a normal weights’ range.
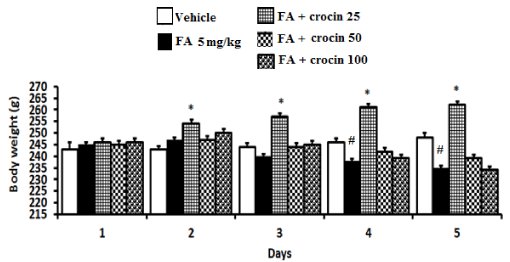
(Figure 2), Effect of crocin (25, 50 and 100 mg/kg, i.p.) post-treatment on body weights of the FA-induced neurotoxicity rats during the 5-day period (n= 6). Two-way ANOVA. #(P < 0.01) significantly different from the vehicle. *(P < 0.01) significantly different from FA.
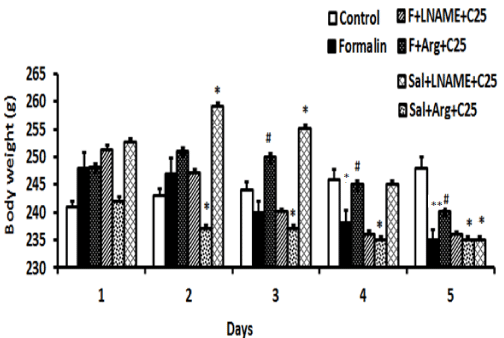
(Figure 3) demonstrates effect of pretreatment with L-NAME (10 mg/kg, i.p.) and L-Arg (100 mg/kg, i.p.) on body weights of crocin (25 mg/kg, i.p.) treated rats received FA during the 5-day period (n= 5) is illustrated in figure 3. As can be observed, FA significantly reduced the body weights compared with control on days 4 and 5, P < 0.05 and P < 0.01, respectively. Moreover, L-Arg in combination with crocin (25 mg/kg, i.p.) decreased the body weights in comparison with FA from day 2 to day 5, P < 0.05, P < 0.05, P < 0.05 and P < 0.01, respectively. Conversely, when L-Arg administered in combination with FA and crocin, it increased the body weight on days 3, 4 and 5, P < 0.05, P < 0.05, and P < 0.05, respectively. On the other hand, L-NAME in combination with crocin (25 mg/kg, i.p.) increased the body weights from day 1 to day 3, P < 0.05, P < 0.05, P < 0.05 and P < 0.05, respectively. However, it reduced the body weight on day 5, P < 0.05. Similarly, when L-NAME was administered in combination with FA and crocin, it decreased the body weight on days 3, 4 and 5, P < 0.05, P < 0.05, and P < 0.05, respectively.
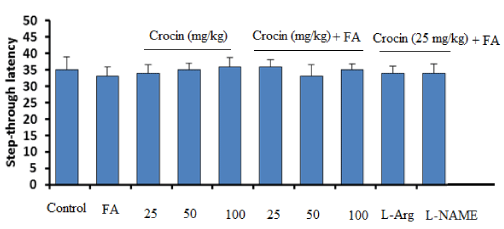
(Figure 4) presents the latency times of different groups to entering from the light compartment to the dark compartment on day 1 of the shuttle box experiment (acquisition response) in passive avoidance test. Statistical analysis revealed no significant differences among the groups.
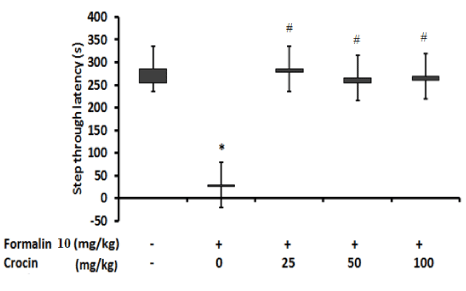
(Figure 5) represents effect of crocin at doses 25, 50 and 100 mg/kg on the latency times of groups receiving FA to entering from the light compartment to the dark compartment on day 2 of the shuttle box experiment (retention response) in passive avoidance test. The statistical analysis revealed marked differences in FA group compared with vehicle and the rats entered the dark compartment in a significantly shorter time (P < 0.05). Conversely, FA groups receiving crocin 25, 50 and 100 mg/kg did not have any significant difference compared with vehicle group, however; crocin 25, 50 and 100 mg/kg were markedly different from FA group and hade a considerably higher latencies, P < 0.05.
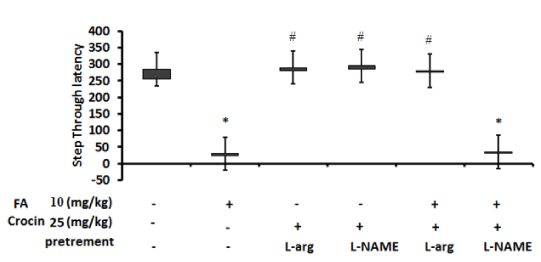
(Figure 6) is a representative of L-NAME and L-Arg pretreatment effects on crocin at dose 25 mg/kg on the latency times of groups receiving FA and crocin or crocin and vehicle in passive avoidance test to entering from the light compartment to the dark compartment on day 2 of the experiment (retention response). As can be understood, FA group and FA + L-NAME + crocin (25 mg/kg) group were significantly different from vehicle group and entered the dark chamber in a lower times. Conversely, the group received L-Arg + crocin (25 mg/kg), the group received L-NAME + crocin (25 mg/kg) and the group received FA+ L-Arg + crocin (25 mg/kg) all were markedly different from FA group and showed considerably higher latencies.
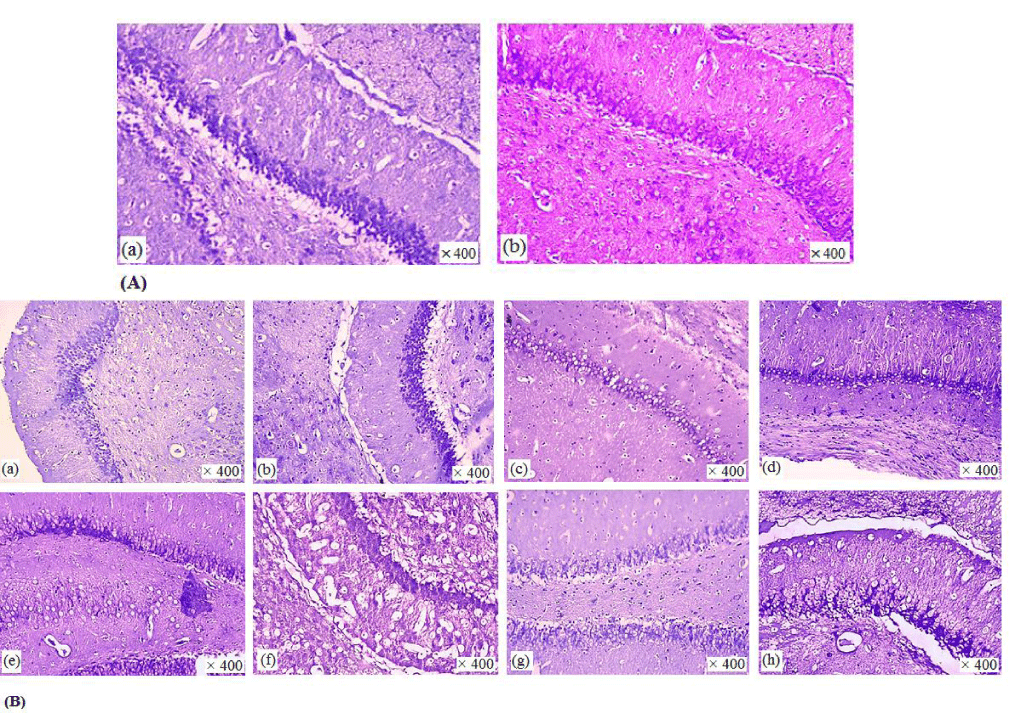
(Figure 7) illustrates photomicrographs of Nissl staining of CA1 pyramidal neurons in different animal groups. Microscopic examination showed remarkable morphological details compared with the control group. Further, Nissl stain showed that the neuronal morphology was affected after FA treatment for 5 days and there was a significant confusion and shrink of neuron morphology in FA-treated group. In fact, FA damaged the entire pyramidal and granular cells layer. On the other hand, crocin’s post- treatment at different doses was protective with different degrees. L-Arg pretreatment treatment also could enhance protective effect of crocin on hippocampal tissue.
Discussion
In the present study, the neural protective effect of crocin was demonstrated in FA-induced neurotoxicity in male rats. FA in dose 20 mg/kg proved toxic and lethal for the animals and virtually, 100% of the animals were lost during the 3-weeks course of the pilot study. So that, FA 10 mg/kg with 50 % mortality rate, was injected to the rats in the “main study” groups as the effective dose. In addition, the mortality rate was markedly decreased upon crocin treatment (25 mg/kg). Moreover, crocin (25 mg/kg) considerably reversed the fetal effect of FA 10 mg/kg. In the main study (5 days), while, crocin in dose 25 mg/kg increased the body weight, crocin in high doses (50 and 100, mg/kg) decreased the body weights. The animals receiving crocin (25 mg/kg) not only had a normal weights’ range, but also did not show any mortality. While L-NAME pretreatment in combination with crocin could reverse the protective effect of crocin (25 mg/kg) on weight loss, L-Arg in combination with crocin could cause weight modification. In behavioral passive avoidance test, no significant changes were observed between groups in acquisition step. On the other hand, while FA group showed considerably lower latencies compared with control, crocin pretreatments influenced the latency times in the retention step in comparison to FA and the animals had significantly higher latency times. On the whole, the pathologic data were in accordance with behavioral outcomes.
Virtually, consistent with our investigation, clinical abnormalities, significant mortality and body weight loss were observed in the 40 ppm groups in B6C3F1 mice exposed to chronic FA vapor. Inhalation exposures to 10, 20, and 40 ppm of FA vapor induced histologic lesions in the upper respiratory system and concentrations of 40 ppm were lethal [47]. Also, the Intracerebroventricular (I.C.V.) injection of FA in rats impaired the function of learning and memory and increased the formation of apoptosis and lipid peroxidation in the hippocampus. Moreover, FA exposure inhibited the expression of cystathionine β-synthase, the major enzyme responsible for Endogenous Hydrogen Sulfide (H2S) generation in the hippocampus of rats [48]. Although we administered FA through i.p. route, its toxic effects were observed in the memory, vitality, the body weight and the hippocampus tissue of the animals. Male Balb/c mice were exposed to gaseous FA for 7 days (8 h/d) with (0, 0.5 and 3.0 mg/m) and the toxicity of FA on mouse nervous system was related to NO/cGMP and cAMP signaling pathways [49].
In a clinical study, saffron extract administered at one dose had body weight reducing and satiating effects in mildly overweight healthy women [50]. In line with our study, effects of saffron and crocin on some metabolic factors such as body weights were shown. In one experiment, saffron extract (25, 50, 100, 200 mg/kg, p.o.) had anti-obesity and anorectic effects in obese Wistar rats. Their results suggested that chronic saffron and crocin reduce the body weight, food intake and blood leptin levels significantly. Crocin (5, 15, 30, 50 mg/kg, p.o.) may be one of the active constituents involved in the effects of saffron [51]. Saffron extract (40 and 80 mg/kg) significantly decreased food consumption in obese rats. Crocin (80 mg/kg) showed a significant decrease on rate of body weight gain, total fat pad and weight ratio of epididymal fat to body. Furthermore, crocin (80 mg/kg) significantly reduced plasma levels of Triacylglycerol (TG) and total cholesterol (TC) [52]. Similarly, oral chronic saffron extract (25, 50, 100, 200 mg/kg) and crocin (5, 15, 30, 50 mg/kg) significantly reduced body weight, food intake and leptin levels in obese Wistar rats [51]. In our study, crocin at high doses (50 and 100 mg/kg, i.p.) decreased the body weights, however; at low dose (25 mg/kg, i.p.) it could enhance the animal’s weights.
Protective effects of carotenoids from saffron on neuronal cell damage in rodent models were investigated and crocin was the most potent antioxidant that combats ischemic stress-induced neuron death by increasing glutathione levels [53,23]. Moreover, saffron extract improved impairments of learning behaviors in mice, and prevented ethanol-induced inhibition of hippocampal Long-Term Potentiation (LTP), a form of activity-dependent synaptic plasticity that may underlie learning and memory. This effect of saffron was attributed to crocin [18].
Recently, the protective effect of chronic oral crocin (100 mg/kg, p.o.) against i.c.v. STZ-induced oxidative damage in rat striatum has been shown [54]. The active constituents of saffron, crocin and crocetin markedly suppressed LPS-induced nitrite release and reactive oxygen species production from rat microglial cells. Crocin and crocetin considerably reduced the production of some pro-inflammatory molecules in the culture media of primary microglia [22]. Furthermore, treatment with crocins (30 mg/kg and 15 mg/kg, i.p.) attenuated scopolamine (0.2 mg/kg)-induced spatial memory performance deficits in rat [19].
C. sativus L. extract (150 and 450 mg/kg, i.p.) attenuated morphine-induced memory impairment on passive avoidance learning in mice [55]. In an animal study, chronic restraint stress (6 h/day) rats that received saffron extract (30 mg/kg) or crocin (15 and 30 mg/kg) had significantly higher levels of lipid peroxidation products, higher activities of antioxidant enzymes and lower total antioxidant reactivity capacity [56].
The results of our study suggest that crocin post-treatment may prevent FA-induced neuronal damage in the hippocampus and restores weight loss in NO-dependent manner.
Conclusion
Crocin, active constituents of saffron extract, might be useful as a treatment for neurodegenerative disorders associated with memory impairment such as Alzheimer’s disease. These observations indicate that crocin may prevent the impairment of learning and memory as well as the oxidative stress damage to the hippocampus induced by chronic stress. Thus, it may be useful in pharmacological alleviation of cognitive deficits. Nevertheless, further study is needed to strengthen these outcomes.
- Simith AE. Formaldehyde. Occup Med. 1992; 42: 83–88. https://goo.gl/x52dCU
- Franklin P, Dingle P, Stick S. Raised exhaled nitric oxide in healthy children is associated with domestic formaldehyde levels. Am J RespirCrit Care. 2000; 161: 1757-1759. https://goo.gl/UAspmZ
- Gronvall JL, Garpenstrand H, Oreland L, Ekblom J. Autoradiographic imaging of formaldehyde adducts in mice: possible relevance for vascular damage in diabetes. Life sciences. 1998; 63: 759-768. https://goo.gl/KeFMaV
- Kilburn KH. Neurobehavioral impairment and seizures from formaldehyde. Arc Environ Health. 1994; 49: 37-44. https://goo.gl/fSVvjq
- Stroup NE, Blair A, Erikson GE. Brain cancer and other causes of death in anatomists 2. J Natl Cancer Inst. 1986; 77: 1217-1224. https://goo.gl/VAVJYW
- Perna RB, Bordini EJ, Deinzer-Lifrak M. A case of claimed persistent neuropsychological sequelae of chronic formaldehyde exposure: clinical, psychometric, and functional findings. Arch Clin Neuropsychol. 2001; 16: 33-44. https://goo.gl/gviinm
- Gurel A, Coskun O, Armutcu F, Kanter M, Ozen OA. Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: biochemical and histological studies. J Chem Neuroa nat. 2005; 29: 173-178. https://goo.gl/EsKz1u
- He R, Lu J, Miao J. Formaldehyde stress. Sci China Life Sci. 2010; 53: 1399-1404. https://goo.gl/iZXDC2
- Lu Y, Li T, Qureshi HY, Han D, Paudel HK. Early growth response 1 (Egr-1) regulates phosphorylation of microtubule-associated protein tau in mammalian brain. J BiolChem. 2011; 286: 20569-20581 https://goo.gl/7E5sMX
- Tong Z, Han C, Luo W, Wang X, Li H, Luo H, et al. Accumulated hippocampal formaldehyde induces age-dependent memory decline. Age (Dordr). 2013; 35: 583-596. https://goo.gl/TrSYyK
- Sorg B, Hochstatter T. Behavioral sensitization after repeated formaldehyde exposure in rats. Toxicol Ind Health. 1999; 15: 346-355. https://goo.gl/meVW9L
- Pitten FA, Kramer A, Herrmann K, Bremer J, Koch S. Formaldehyde neurotoxicity in animal experiments. Pathol Res Pract. 2000; 196: 193-198. https://goo.gl/G5WYWB
- Morris RGM, Garrud P, Rawlins JA, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982; 297: 681-683. https://goo.gl/RsLhA6
- Lu Z, Li CM, Qiao Y, Yan Y, Yang X. Effect of inhaled formaldehyde on learning and memory of mice. Indoor air. 2008; 18: 77-83. https://goo.gl/nK5E4C
- Liu Y, Ye Z, Yang H, Zhou L, Fan D, He S, et al. Disturbances of soluble N-ethylmaleimide-sensitive factor attachment proteins in hippocampal synaptosomes contribute to cognitive impairment after repetitive formaldehyde inhalation in male rats. Neuroscience. 2010; 169: 1248-1254. https://goo.gl/iEAQkK
- Razavi M, Hosseinzadeh H. Saffron as an antidote or a protective agent against natural or chemical toxicities. DARU. 2015; 23: 31. https://goo.gl/mMz9fn
- Ochiai T, Ohno S, Soeda S, Tanaka H, Shoyama Y, Shimeno H. Crocin prevents the death of rat pheochromyctoma (PC-12) cells by its antioxidant effects stronger than those of α-tocopherol. Neurosci Lett. 2004; 362: 61-64. https://goo.gl/aqou6Y
- Abe K, Saito H. Effects of saffron extract and its constituent crocin on learning behaviour and long‐term potentiation. Phytother Res. 2000; 14: 149-152. https://goo.gl/8LRGPL
- Pitsikas N, Zisopoulou S, Tarantilis PA, Kanakis CD, Polissiou MG, Sakellaridis N. Effects of the active constituents of Crocus sativus L., crocins on recognition and spatial rats’ memory. Behav Brain Res. 2007; 183: 141-146. https://goo.gl/GtJEz3
- Lee IA, Lee JH, Baek NI, Kim DH. Antihyperlipidemic effect of crocin isolated from the fructus of Gardenia jasminoides and its metabolite crocetin. Biol Pharm Bull. 2005; 28: 2106-2110. https://goo.gl/kWHZfU
- He SY, Qian ZY, Tang FT, Wen N, Xu GL, Sheng L. Effect of crocin on experimental atherosclerosis in quails and its mechanisms. Life sciences. 2005; 77: 907-921. https://goo.gl/mMSBZu
- Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010; 648: 110-116. https://goo.gl/81upzH
- Zheng YQ, Liu JX, Wang JN, Xu L. Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007; 1138: 86-94. https://goo.gl/kgpPmi
- Naghizadeh B, Mansouri SMT, Mashhadian NV. Crocin attenuates cisplatin-induced renal oxidative stress in rats. Food chem. Toxicol. 2010; 48: 2650-2655. https://goo.gl/hWBZ3E
- Akhtari K, Hassanzadeh K, Fakhraei B, Fakhraei N, Hassanzadeh H, Zarei SA. A density functional theory study of the reactivity descriptors and antioxidant behavior of Crocin. Comp TheorChem 2013; 1013: 123-129. https://goo.gl/kZjzQS
- Naghizadeh B, Mansouri MT, Ghorbanzadeh B, Farbood Y, Sarkaki A. Protective effects of oral crocin against intracerebroventricularstreptozotocin-induced spatial memory deficit and oxidative stress in rats. Phytomedicine. 2013; 20: 537-542. https://goo.gl/TnEsYs
- Mobarakeh JI, Fakhraei N, Sadr ZA, Hamidipour A, Mostafavi E, Hosseini RH, Sardari S. Effect of aqueous, ethanolic and acetonitrile Crocus sativus L. extracts on stress biomarkers in male rats. J Med Plant Res. 2013; 7: 3269-3279. https://goo.gl/PrZNvG
- Zhang Y, Shoyama Y, Sugiura M, Saito H. Effects of Crocus sativus L. on the ethanol-induced impairment of passive avoidance performances in mice. Biol Pharm Bull. 1994; 17: 217-221. https://goo.gl/GpiQLw
- Sugiura M, Saito H, Abe K, Shoyama Y. Ethanol extract of Crocus sativus L. Antag-onizes the inhibitory action of ethanol on hippocampal long term potentiation in vivo. Phytother Res. 1995; 9: 100-104. https://goo.gl/f1B25D
- Pitsikas N, Sakellaridis N. Crocus sativus L. extracts antagonize memory impairments in different behavioural tasks in the rat. Behav Brain res. 2006; 173: 112-115. https://goo.gl/MBP2Kg
- Garthwaite J. Glutamate, nitric oxide and cell-cell signaling in the nervous system. Trends Neurosci. 1991; 14: 60-67. https://goo.gl/7GsSYc
- Haley JE, Wilcox GL, Chapman PF. The role of nitric oxide in hippocampal long-term potentiation. Neuron. 1992; 8: 211-216. https://goo.gl/SjXEJo
- Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog. Neurobiol. 2001; 64: 51-68. https://goo.gl/8gDL9K
- Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci. 1991, 88: 6368-6371. https://goo.gl/JyYZa7
- Das I, Ramchand CN, Gliddon A, Hirsch SR. Nitric oxide, free radicals and polyamines may have a role in the membrane pathology of schizophrenia. Neuropsychobiology. 1998; 37: 65-67. https://goo.gl/pPKw5v
- Parizadeh MR, GhafooriGharib F, Abbaspour AR, TavakolAfshar J, Ghayour-Mobarhan M. Effects of aqueous saffron extract on nitric oxide production by two human carcinoma cell lines: Hepatocellular carcinoma (HepG2) and laryngeal carcinoma (Hep2). Avicenna J Phytomed. 2011; 1: 43-50. https://goo.gl/UPKw4u
- Esmaeilizadeh M, Dianat M, Badavi M, Samarbaf-Zadeh A, Naghizadeh B. Effect of crocin on nitric oxide synthase expression in post-ischemic isolated rat heart. Avicenna J Phytomed. 2015; 5: 420-426. https://goo.gl/1WCT7J
- Kim JH, Park GY, Bang SY, Park SY, Bae SK, Kim Y. Crocin suppresses LPS-stimulated expression of inducible nitric oxide synthase by upregulation of heme oxygenase-1 via calcium/calmodulin-dependent protein kinase 4. Mediators Inflamm. 2014; 2014:728709. https://goo.gl/AHKtg1
- Zararsiz I, Kus I, Ogeturk M, Akpolat N, Kose E, Meydan S, et al. Melatonin prevents formaldehyde‐induced neurotoxicity in prefrontal cortex of rats: an immunohistochemical and biochemical study. Cell Biochem Funct. 2007; 25: 413-418. https://goo.gl/ooa97S
- Mehri S, Hosseinzadeh H, Abnous K. Crocin decreased acrylamide–induced neurotoxicity in Wistar rat through oxidative stress and apoptosis pathway. Res Pharm Sci. 2012; 7: 1009. https://goo.gl/Ex6agC
- Gaur V, Aggarwal A, Kumar A. Possible nitric oxide mechanism in the protective effect of hesperidin against ischemic reperfusion cerebral injury in rats. Indian J Exp Biol. 2011; 49: 609-618. https://goo.gl/E1uedt
- Baydas G, Ozer M, Yasar A, Tuzcu M, Koz ST. Melatonin improves learning and memory performances impaired by hyperhomocysteinemia in rats. Brain Res. 2005; 1046: 187-194. https://goo.gl/zVrrQ4
- Naylor AS, Persson AI, Eriksson PS, Jonsdottir IH, Thorlin T. Extended voluntary running inhibits exercise-induced adult hippocampal progenitor proliferation in the spontaneously hypertensive rat. J Neurophysiol. 2005; 93: 2406-2414. https://goo.gl/oXsJdW
- Pitchaimani V, Arumugam S, Thandavarayan RA, Thiyagarajan MK, Aiyalu R, Sreedhar R, et al. Nootropic activity of acetaminophen against colchicine induced cognitive impairment in rats. J ClinBiochemNutr. 2012; 50: 241-244. https://goo.gl/Pke7xM
- Saffarzadeh F, Eslamizade MJ, Ghadiri T, Modarres Mousavi SM, Hadjighassem M, Gorji A. Effects of TRPV1 on the hippocampal synaptic plasticity in the epileptic rat brain. Synapse. 2015; 69: 375-383. https://goo.gl/wZH842
- Eslamizade MJ, Madjd Z, Rasoolijazi H, Saffarzadeh F, Pirhajati V, Aligholi H, et al. Impaired memory and evidence of histopathology in CA1 pyramidal neurons through injection of Aβ1-42 peptides into the frontal cortices of rat. Basic ClinNeurosci. 2016; 7: 31-41. https://goo.gl/iiFHae
- Maronpot RR, Miller RA, Clarke WJ, Westerberg RB, Decker JR, Moss OR. Toxicity of formaldehyde vapor in B6C3F1 mice exposed for 13 weeks. Toxicology. 1986; 41: 253-266. https://goo.gl/R5gDoy
- Tang XQ, Zhuang YY, Zhang P, Fang HR, Zhou CF, Gu HF, et al. Formaldehyde impairs learning and memory involving the disturbance of hydrogen sulfide generation in the hippocampus of rats. J MolNeurosci. 2013; 49: 140-149. https://goo.gl/NEiLPr
- Cao FH, Cai J, Liu ZM, Li H, You HH, Mei YF, et al. Toxic effect of formaldehyde on mouse different brain regions. Sheng li xuebao. 2015; 67: 497-504. https://goo.gl/GveSEM
- Gout B, Bourges C, Paineau-Dubreuil S. Satiereal, a Crocus sativus L extract, reduces snacking and increases satiety in a randomized placebo-controlled study of mildly overweight, healthy women. Nutr Res. 2010; 30: 305-313. https://goo.gl/JkaLPx
- Kianbakht S, Hashem Dabaghian F. Anti-obesity and anorectic effects of saffron and its constituent crocin in obese Wistar rat. J. Med. Plants Res. 2015; 1: 25-33. https://goo.gl/bVNNQ4
- Mashmoul M, Azlan A, Yusof BNM, Khaza'ai H, Mohtarrudin N, Boroushaki MT. Effects of saffron extract and crocin on anthropometrical, nutritional and lipid profile parameters of rats fed a high fat diet. J Funct Foods. 2014; 8: 180-187. https://goo.gl/dntX14
- Ochiai T, Shimeno H, Mishima KI, Iwasaki K, Fujiwara M, Tanaka H, et al. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim Biophys Acta. 2007; 1770: 578-584. https://goo.gl/sSjN81
- Naghizadeh B, Mansouri MT, Ghorbanzadeh B. Protective effects of crocin against streptozotocin-induced oxidative damage in rat striatum. Acta Med Iran. 2014; 52: 101. https://goo.gl/RvWquA
- Naghibi SM, Hosseini M, Khani F, Rahimi M, Vafaee F, Rakhshandeh H, et al. Effect of aqueous extract of Crocus sativus L. on morphine-Induced memory impairment. Advances in pharmacological sciences. 2012; 2012: 494367. https://goo.gl/TRPW98
- Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F, et al. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol 2011; 667: 222-229. https://goo.gl/ZSr4FZ

Sign up for Article Alerts