Effect Of Methyl- Propyl (MP), Methyl-Butyl (MB), and Methyl-Propyl-Butyl (MPB) Mixtures on the Proliferation, DNA Fragmentation and Estradiol Secretion of MCF-7 Breast Cancer Cells and MCF-10A Normal Breast Epithelial Cells
Ewa Lucja Gregoraszczuk*
Department of Physiology and Toxicology of Reproduction, Institute of Zoology and Biomedical Research, Jagiellonian University in Kraków, Gronostajowa 9, 30-387 Krakow, Poland
*Address for Correspondence: Ewa Lucja Gregoraszczuk, Department of Physiology and Toxicology of Reproduction, Institute of Zoology and Biomedical Research, Jagiellonian University in Kraków, Gronostajowa 9, 30-387 Krakow, Poland, E-mail: [email protected]
Submitted: 18 August 2017; Approved: 06 September; Published: 08 September 2017
Citation this article: Gregoraszczuk EL. Effect Of Methyl- Propyl (MP), Methyl-Butyl (MB), and Methyl-Propyl-Butyl (MPB) Mixtures on the Proliferation, DNA Fragmentation and Estradiol Secretion of MCF-7 Breast Cancer Cells and MCF-10A Normal Breast Epithelial Cells. Adv J Toxicol Curr Res. 2017;1(1): 001-006.
Copyright: © 2017 Gregoraszczuk EL. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: MCF-1 Cells; MCF-10A Cells; Mixtures of Parabens; Single and Repeated Exposure; Proliferation; DNA Fragmentation; Estradiol Secretion
Download Fulltext PDF
Since parabens are used as preservatives in cosmetics as mixtures, here we aimed to compare the effects of Methyl- Propyl (MP), Methyl-Butyl (MB), and Methyl-Propyl-Butyl (MPB) on MCF-7 (breast cancer cells) and MF-10A (non-cancer breast epithelial cells) proliferation (Alamar Blue assay), DNA fragmentation ( ELISA), and estradiol (E2) secretion (ELISA). Cells were exposed to mixtures at a concentration of 20 N, either alone or supplemented with parabens together with 10 nM 17β-estradiol. ICI 182,780 (100 nM) was used to demonstrate the involvement of Estradiol Receptor (ER) in the action of the paraben mixture. For the MCF-7 cells, all mixtures increased cell proliferation and inhibited DNA fragmentation. No synergistic action with E2 was observed, and only the MPB increased E2 secretion by MCF-7 cells. For the MCF-10A cells, none of the mixtures affected cell proliferation, and only MPB decreased DNA fragmentation. No mixture affected the secretion of estradiol in either cell line. The ICI 182,780 inhibitory effect on parabens stimulated cell proliferation only in ER+ cells, thereby confirming their ER-mediated action. In conclusion, based on the presented results we suggest that simultaneous application of more than one paraben might promote the progression of malignancy but not initiation of cancer.
Introduction
Parabens, the alkyl esters of p-hydroxybenzoic acid occur naturally in some foods, including specific fruit juices and wine [1]. Used widely as antimicrobial preservatives can enter the body when pharmaceuticals, foods, and drinks containing parabens are swallowed or eaten [2]. Additionally, many products, such as makeup, moisturizers, hair-care products, and shaving creams, contain parabens [3-5]. When parabens are eaten, they are metabolised to Para-Hydroxybenzoic Acid (PHBA) in the intestine and subsequently re-esterified to another paraben or to the parent paraben [6]. When applied to the skin and absorbed into the body, bypass the metabolic process and enter the blood stream and body organs intact [7-9].
It has been unequivocally demonstrated in vivo and in vitro, in various screening tests, that parabens have endocrine-disrupting activity and may represent a potential risk to human health [10]. Parabens have been detected in human fluids, including blood [11], urine [12], milk [13], and in human breast tumors [14] in different populations. There is much published data concerning the action of single parabens on breast cancer cell proliferation [15-19]. Our last published data [20] showed that exposure to a single paraben at concentrations from 2 nM to 200 nM increased MCF-7 breast cancer cell proliferation and MCF-10A normal breast epithelial cell proliferation, suggesting that parabens might initiate the process of MCF-10A transformation. Moreover, we showed previously that exposure to low concentrations of parabens increases estradiol secretion in MCF-7 cells while decreasing it in MCF-10A cells. This result correlated with the gene and protein expression of cytochrome P450 aromatase (CYP19A1) in MCF-7 and MCF-10A cells and suggested that parabens induce the proliferation of these two cell lines via different mechanisms [20].
Charles and Darbre [21] demonstrated that parabens are present in human breast tissue, either alone or in combination, at concentrations sufficient to stimulate the proliferation of MCF-7 cells in vitro, and that the functional consequences of the presence of parabens in human breast tissue should be assessed on the basis of all five parabens rather than individual parabens. To our knowledge, there is only one published study that has addressed the effect of the combination of ethyl- and propylparaben on the proliferation of MCF-7 cells [21]. The study showed that the mixtures had similar EC50 values to the individual compounds, suggesting a lack of synergy.
Based on these publications, and fact that parabens can enter the body by ingestion, and dermal contact people are exposed to combination of different parabens, in the present study we decided to compared the effect of mixture on cell proliferation and assessed their possible interaction with 17β-estradiol on breast cancer cells (MCF-7 cell line) and normal breast epithelial cells (MCF-10A cell line). Moreover, which have not been evaluated till now, compare action of single and repeated exposure. Using ICI 182,780, a blocker of the estradiol receptor, we tested the hypothesis that paraben mixtures may induce cell proliferation via activation of the estradiol receptor.
Materials and Methods
Reagents
Dulbecco’s Modified Eagle’s Medium without phenol red (DMEM), Dulbecco’s modified Eagle’s medium, Ham’s F12 medium (1:1) without phenol red (DMEM/F12), charcoal-dextran, insulin, hydrocortisone, fulvestrant (ICI 182,780), and Epidermal Growth Factor (EGF) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Fetal bovine serum (FBS, heat-inactivated), horse serum (HS, heat-inactivated), penicillin, streptomycin, and trypsin-EDTA were obtained from PAA Laboratories GmbH (Colbe, Germany). Phosphate-Buffered Saline (PBS) was purchased from Biomed (Lublin, Poland). The compound 17β-estradiol (E2) was obtained from Steroids, Inc. (Newport, RI, USA). Methyl paraben, n-propylparaben, and n-butylparaben (Sigma Chemical Co., St. Louis, MO, USA) were dissolved in absolute ethanol. The final concentration of ethanol in the medium was 0.1%. Ethanol at this concentration has no effect on cell proliferation or apoptosis (data not shown). Alamar Blue was obtained from Invitrogen (Carlsbad, CA).
Cell culture
MCF-7 is an Estrogen Receptor (ER) positive cell line. The MCF-10A human breast epithelial cell line is not tumorigenic and does not express estrogen receptor [23]. Both cell lines were obtained from the American Type Culture Collection (Rockville, MD, USA). MCF-7 cells were cultured under the same conditions as described earlier [20]. MCF-7 cells were seeded in 96-well culture plates at a density of 9 × 103 cells per well, whereas MCF-10A cells were seeded at a density of 12 × 103 cells per well. Individual parabens, at a concentration of 20 nM, were used as controls for the activity of the mixtures. The concentrations of parabens were based on the data of Darbre et al. [9], who reported that the mean concentration of parabens in human breast tumors was 20.6 ± 4.2 ng per gram of tissue and performed dose response experiments ranging from 0.2 nM to 2 µM [20]. For the three prepared mixtures [Methylparaben + Propylparaben (MP), methylparaben + butylparaben (MB), and Methylparaben + Propylparaben + Butylparaben (MPB)], each compound was added at a concentration of 20 nM.
To evaluate the activity of a single exposure, the compounds were added at the beginning of the experiment. After 3 days, the culture medium was replaced with fresh medium without compounds and incubated for an additional 3 days.
To evaluate the activity of repeated exposure, the compounds were added at the beginning of the experiment and then every 48 h for 6 days. Thus, the compounds were added three times during the experiment.
To evaluate the potential interaction of parabens with 17β-estradiol (E2), single parabens and in the mixture were added in combination with 10 nM 17β-estradiol and 100 nM ICI 182, 780.
To evaluate their action on estradiol secretion, cells were exposed to mixtures or individual parabens (20 nM) for 72 h, after which the supernatants were collected and stored at -20°C
Cell proliferation
The Alamar Blue Colorimetric Assay (Invitrogen, Carlsbad, CA) was used to evaluate cell proliferation. Metabolically active cells convert resazurin to the fluorescent metabolite resorufin, and the fluorescence reading of the sample is proportional to the number of living cells. After 6 days of culture, Alamar Blue stock solution was aseptically added to the wells in amounts equal to 10% of the well volume and incubated for 5 h with the cells. Resazurin reduction was measured with an excitation wavelength of 540 nm and an emission wavelength of 590 nm in a FLUOROmicroplate reader (BIO-TECH Instruments, Winooski, VT, USA).
DNA fragmentation
A cellular DNA Fragmentation ELISA kit (Roche Applied Science, Mannheim, Germany) was used to determined DNA fragmentation. This assay is based on the quantitative detection of bromodeoxyuridine (BrdU)-labelled DNA fragments. Briefly, after exposure to BrdU for 18 h, cells were reseeded into a cultured medium with 0.1% BSA in a 96-well microplate (MCF-7 cells at 2 × 104 cells/well, MCF-10A cell at 2, 3 × 104 cells/well) and treated for 24 h with the test compounds (methylparaben, n-propylparaben and n-butylparaben in dose 20 nM, and 10 nM 17β-estradiol). After 24 h, the cells were washed with Phosphate Buffered Saline (PBS) and lysed with the kit buffer according to the manufacturer’s instructions. The cytoplasmic fraction was transferred separately into an anti-DNA pre-coated microtiter plate and analyzed using the ELISA procedure as recommended by the manufacturer. DNA fragmentation was measured spectrophotometrically at 450 nm using an ELISA reader (ELx808 BIO-TEK Instruments, Winooski, VT, USA). The results presented are the mean value of three independent experiments.
Estradiol secretion
The estradiol concentration was measured using the enzyme immunoassay DRG Estradiol ELISA (DRG Instruments GmbH, Marburg, Germany) according to the manufacturer’s instructions. The sensitivity threshold of the assay was 9,714 pg/ml; the intra-assay variation was 4.13–6.81%, the inter-assay variation was 7.25–9.39%, and the linear range was 9,714–2000 pg/ml. The absorbance values were measured at a wavelength of 450 nm using an ELISA reader (ELx808 BIO-TEK Instruments, USA).
Statistical analysis
Data were expressed as the mean ± standard error (SD) from three independent experiments performed in quadruplicate. The statistical analyses were performed using Graph Pad Prism 5. The data were analyzed by two-way Analysis Of Variance (ANOVA) followed by Tukey’s Honestly Significant Differences (HSD) multiple range test. All data are expressed as the mean ± SD. Groups that are significantly different from each other (p < 0.05) are indicated in the figures with different letters. Data points with the same letters are not significantly different.
Results
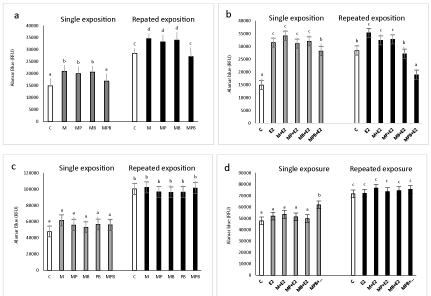
Effect of single and repeated exposure to paraben mixtures on basal and 17β-estradiol-stimulated MCF-7 cell proliferation
Single and repeated exposure to 17β-estradiol alone (10 nM) caused a statistically significant increase in MCF-7 cell proliferation (33% above control, and 25% above control; respectively).
Both single and repeated exposure to mixtures of two parabens (MP and MB) caused a significant increase in the proliferation of MCF-7 breast cancer cell. No significant effect on cell proliferation was observed upon exposure to three other parabens mixture (MPB) (Figure 1a). After co-treatment with 17β-estradiol (E2), no significant effect on MCF-7 breast cancer cell proliferation after single exposure was observed; however, after repeated exposure, MPB decreased E2-stimulated MCF-7 cell proliferation (Figure 1b).
17β-Estradiol alone at a concentration of 10 nM did not affect the proliferation of MCF-10A cells. Except for MPB, which stimulated cell proliferation after a single exposure, none of the mixtures increased basal MCF-10A cell proliferation, and none of the mixtures had any effect on 17β-estradiol-stimulated proliferation of MCF-10A cells (Figures 1c, d).
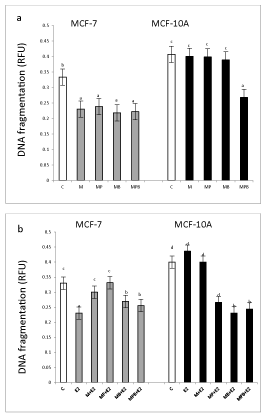
Effect of paraben mixtures on basal and 17β-estradiol stimulated DNA fragmentation in MCF-7 and MCF-10A.
In MCF-7 cells, methyl paraben and all tested mixtures (with the same intensity) decreased DNA fragmentation (Figure 2a). In MCF-10A, except for the MPB mixture that decreased DNA fragmentation below control levels, none of the other treatments affected DNA fragmentation.
Exposure to E2 decreased DNA fragmentation in the MCF-7 but not MCF-10A cells (Figure 2b). All tested mixtures of parabens (with different intensity) reversed E2 action on DNA fragmentation, with M and MP returning DNA fragmentation to control levels and MB and MPB to 30% below control levels (Figure 2b).
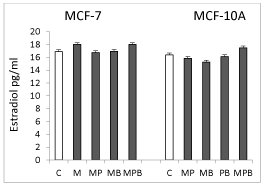
Effect of paraben mixtures estradiol secretion by MCF-7 and MCF-10A cells
None of the tested mixtures affected the secretion of estradiol by MCF-7 or MCF-10A cells (Figure 3).
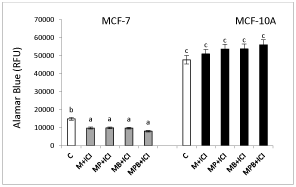
Effect of estradiol receptor blocker (ICI 182,780.) on paraben mixtures stimulated MCF-7 and MCF-10A cells proliferation
ICI 182,780 decreased all paraben mixture-stimulated proliferation of MCF-7 cells (Figure 3). In MCF-10A cells, ICI 182,780 did not affect cell proliferation (Figure 4).
Discussion
Our results demonstrate the different effects of paraben mixtures on cell proliferation and DNA fragmentation in cancer and non-transformed breast cells. Moreover, our data suggest that paraben mixtures induce cell proliferation directly via the ER but are not active on estradiol secretion.
All paraben mixtures had a stimulatory action on basal cancer cell proliferation, which was accompanied by an inhibitory effect on DNA fragmentation. However, no synergistic effect with E2 was detected, neither on proliferation or DNA fragmentation. The observed effect of the mixture of MCF-7 cells was comparable to the action of individual compounds previously described by us [20]. To our knowledge, there is only one published study that has investigated the effect of the combination of ethyl- and propylparaben on the proliferation of MCF-7 cells [21]. Van Meeuwen et al. [22] found that the mixtures had similar EC50 values to the individual compounds, confirming the lack of synergy. Our results related to the interaction with the estradiol are in line with the findings of Van Meeuwen et al.[22], who examined the activity of methyl-, ethyl-, propyl-, butyl-, and benzylparaben in combination with endogenous estradiol (E2) on MCF-7 cell proliferation, and indicated that parabens contribute only marginally, if at all, to the total circulating estrogens. Also, Byford et al. [17] failed to observe any effect on MCF-7 cell proliferation when combining single parabens with 17β-estradiol. Moreover, the same authors noted that higher concentrations of n-propylparaben and n-butylparaben antagonized 17β-estradiol after 12 days of culture, which is consistent with our observation concerning the mixture of three parabens and E2. The combination of isobutylparaben and 17β-estradiol also failed to affect the proliferation of the MCF-7 and ZR-75-1 cell lines, both ER-dependent breast cancer lines, as described by Darbre et al. [16]. Van Meeuwen et al.[22] examined the activity of methyl-, ethyl-, propyl-, butyl-, and benzylparaben in combination with endogenous estradiol (E2) on MCF-7 cell proliferation and found that parabens contribute only marginally, if at all, to the total circulating estrogens. Other data showed a lack of additives when combining phthalates [23] or tetrachlorobiphenyls [24] with 17β-estradiol. Using ICI 182, 780, we show a decrease in the proliferation of MCF-7 cells exposed to all mixtures. This decrease was equal in magnitude to that observed in cells treated with 17β-estradiol, thus confirming that parabens act via the ER receptor in MCF-7 cells. Parabens have been found to similarly induce both ESR1 and ESR2, with a possibly stronger effect on ESR1 [25, 26]. Byford et al. [17] demonstrated that the proliferative effect of methyl-, ethyl-, propyl-, and butylparaben could be inhibited by the pure anti-estrogen ICI 182, 780. The same results for isobutylparaben and benzylparaben were described by Darbre et al. [8, 16]. Studies conducted with individual parabens describe decreased MCF-7 cell proliferation in the presence of an ER blocker [27].
In non-transformed cells, none of the tested paraben mixtures affected cell proliferation, and only BP and MPB decreased DNA fragmentation. When testing co-treatments of paraben mixtures with E2, PB and MPB enhanced proliferation, and all mixtures decreased DNA fragmentation. However, unlike in cancer cells, no effect on MCF-10A cell proliferation was noted after co-treatment of E2 and all parabens mixtures with ICI 182, 780. To our knowledge, the data presented here are the first showing the action of paraben mixtures on non-transformed breast cells. Taking into consideration the elevated physiological levels of estradiol in the menstrual cycle and in pregnancy, as well as in frequently applied contraceptive agents, extra exposure to paraben mixtures might result in breast cancer initiation.
Interestingly, in contrast to the stimulatory effect of single parabens on the CYP19A1 transcript levels, CYP19A1 protein expression, and estradiol secretion in the MCF-7 cell line, and the opposite inhibitory effects of parabens in MCF-10 cells line [27] we observed no effect of any of the investigated paraben mixtures in MCF-10A cells and a stimulatory action on estradiol secretion by MCF-7 cells only when using a combination of three parabens. This is a well-known phenomenon and has been described previously in the case of the action of xenobiotics. This is in agreement with our previously published studies, which show that the effects of artificial mixtures are different from those predicted based on the sum of the effects of individual congeners [28,29].
In summary, 1) The fact that mixtures of parabens in normal breast cells have no effect on cell proliferation and, additionally, did not potentiate the action of E2, seem not to be harmful to breast cells; 2) The mixture of two or three parabens, based on their stimulation of proliferation and inhibition of tumour cell DNA fragmentation, might promote the progression of malignancy; however, this does not seem to occur by stimulating estradiol secretion or by potentiating endogenous estradiol, but probably via activation of estradiol receptors.
Highlight
17β-estradiol caused an increase in cell proliferation and a decrease in DNA fragmentation in MCF-7 but not MCF-10A cells.
Paraben mixtures increased proliferation of MCF-7 but not MCF-10A cells.
None of the mixtures affected the secretion of estradiol in either cell line.
ICI 182, 780-mediated inhibition of parabens stimulated cell proliferation only in MCF-7 cells.
Acknowledgment
Authors thanks Dr. Anna Wróbel for performing experiments and analysis of results. This study was supported by the National Sciences Centre from 2011 to 2014 as project DEC-2011/01/N/NZ7/00015.
- Soni MG, Carabin IG, Burdock GA. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem Toxicol 2005; 43: 985-1015. https://goo.gl/Y6YWf3
- Liao C, Liu F, Kannan K.Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ Sci Technol. 2013; 47: 3918-25. https://goo.gl/XDzGsz
- Loretz L, Api AM, Barraj L, Burdick J, Davis de A, Dressler W, et al. Exposure data for personal care products: hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem. Toxicol. 2006; 44: 2008-18. https://goo.gl/N4d46p
- Andersen A. Final amended report on the safety assessment of methyl paraben, ethyl paraben, propyl paraben, isopropyl paraben, butyl paraben, isobutyl paraben and benzyl paraben as used in cosmetic products. Int J Toxicol. 2008; 27: 1-82. https://goo.gl/msv8dp
- Yazar K, Johnsson S, Lind ML, Boman A, Liden C. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis 2011; 64: 265-72. https://goo.gl/fA4r5K
- Lakeram M, Paine AJ, Lockley DJ, Sanders DJ, Pendlington R, Forbes B. Transesterification of p-hydroxybenzoate esters (parabens) by human intestinal (Caco-2) cells. Xenobiotica 2006; 36: 739-49. https://goo.gl/sw5T4u
- Okereke JN, Udebuani AC, Ezeji EU, Obasi KO, Nnoli MC. Possible Health Implications Associated with Cosmetics: A Review. Science J of Pub Health. 2015; 17: 58-63. https://goo.gl/w3owp1
- Darbre PD, Byford JR, Shaw LE, Hall S, Coldham NG, Pope GS, et al. Estrogenic activity of benzyl paraben. J Appl Toxicol. 2003; 23: 43-51. https://goo.gl/iPztBA
- Darbre PD, Aljarrah A, Miller WR,Coldham NG, Sauer MJ, Pope GS. Concentrations of parabens in human breast tumors. J. Appl Toxicol. 2004; 24: 5–13. https://goo.gl/dQxe4u
- Darbre PD, Harvey PW. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxicol. 2008; 28: 561-578. https://goo.gl/A4GJy5
- Sandanger TM, Huber S, Moe MK, Braathen T, Leknes H, Lund E. Plasma concentrations of parabens in postmenopausal women and self-reported use of personal care products: the NOWAC post genome study. J Expo Sci Environ Epidemiol. 2011; 21: 595-600. https://goo.gl/DCwi3r
- Xiaoyun Ye, Amber M Bishop, John A Reidy, Larry L Needham, Antonia M Calafat. Parabens as urinary biomarkers of exposure in humans. Environ Health Perspect. 2006; 114: 1843-6. https://goo.gl/EUPXXS
- Schlumpf M, Kypke K, Wittassek M, Angerer J, Mascher H, Mascher D. Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: correlation of UV filters with use of cosmetics. Chemosphere. 2010; 81: 1171-1183. https://goo.gl/agAnCA
- Barr L, Metaxas G, Harbach CA, Savoy LA, Darbre PD. Measurement of paraben Concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J Appl Toxicol. 2012; 32: 219-32. https://goo.gl/HPs24n
- Okubo T, Yokoyama Y, Kano K, Kano I. ER-dependent estrogenic activity of Parabens assessed by proliferation of human breast cancer MCF-7 cells and expression of ERalpha and PR. Food Chem Toxicol. 2001; 39: 1225-32. https://goo.gl/GXsVKd
- Darbre PD, Byford JR, Shaw LE, Horton, RA. Oestrogenic activity of isobutylparaben in vitro and in vivo. J Appl Toxicol. 2001; 22: 219-26. https://goo.gl/2kEK2c
- Byford JR, Shaw LE, Drew MG, Pope GS, Sauer MJ, Darbre PD. Oestrogenic activity of parabens in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol. 2002; 80: 49-60. https://goo.gl/nFk1YR
- Pugazhendhi D, Pope GS, Darbre PD. Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines. J Appl Toxicol. 2005; 25: 301-9. https://goo.gl/h4N5FL
- Vanparys C, Maras M, Lenjou M, Robbens J, Van Bockstaele D, Blust R, et al. Flow cytometric cell cycle analysis allows for rapid screening of estrogenicity in MCF-7 breast cancer cells. Toxicol in Vitro. 2006; 20: 1238-48. https://goo.gl/whMk1P
- Wróbel A, Gregoraszczuk EŁ. Effects of single and repeated in vitro exposure of three forms of parabens, methyl-, butyl- and propylparabens on the proliferation and estradiol secretion in MCF-7 and MCF-10A cells. Pharmacol Rep. 2013; 65: 484-93. https://goo.gl/prSRU9
- Charles AK, Darbre PD. Combinations of parabens at concentrations measured in human breast tissue can increase proliferation of MCF-7 human breast cancer cells. J Appl Toxicol. 2013; 33: 390-8. https://goo.gl/hKLDF9
- van Meeuwen JA, van Son O, Piersma AH, de Jong PC, van den Berg M. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol Appl Pharmacol. 2008; 230: 372-82. https://goo.gl/f5Trgg
- Jobling S, Reynolds T, White R, Parker MG, Sumpter JP. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995; 103: 582-7. https://goo.gl/cnByKw
- Nesaretnam K, Dils R, Darbre PD. 3, 4,3’,4’-tetrachlorobiphenyl acts as an oestrogen and can promote breast tumor growth. Biochem Soc Trans. 1996; 24: 361S. https://goo.gl/nFmbQz
- Gomez E, Pillon A, Fenet H, Rosain D, Duchesne MJ, Nicolas JC, et al. Estrogenic activity of cosmetic components in reporter cell lines:parabens, UV screens, and musks. 2005; 68: 239-51. https://goo.gl/8V6iqu
- Watanabe Y, Kojima H, Takeuchi S, Uramaru N, Ohta S, Kitamura S. Comparative study on transcriptional activity of 17 parabens mediated by estrogen receptor α and β and androgen receptor. Food Chem Toxicol. 2013; 57: 227-34. https://goo.gl/U2cJXH
- Wróbel AM, Gregoraszczuk EŁ. Differential effect of methyl-, butyl- and propyl paraben and 17β-estradiol on selected cell cycle and apoptosis gene and protein expression in MCF-7 breast cancer cells and MCF-10A non-malignant cells. J Appl Toxicol. 2014; 34: 1041-50. https://goo.gl/Pu8LSK
- Gregoraszczuk EL, Ptak A, Karniewska M, Ropstad E. Action of defined mixtures of PCBs, p,p'-DDT and its metabolite p,p'-DDE, on co-culture of porcine theca and granulosa cells: steroid secretion, cell proliferation and apoptosis. Reprod Toxicol. 2008; 26: 170-4. https://goo.gl/La6bEh
- Gregoraszczuk EŁ, Rak A, Kawalec K, Ropstad E. Steroid secretion following exposure of ovarian follicular cells to single congeners and defined mixture of polybrominated dibenzoethers (PBDEs), p,p'-DDT and its metabolite p,p'-DDE. Toxicol Lett. 2008; 178: 103-9. https://goo.gl/Cmd7pi

Sign up for Article Alerts