Exfoliated Deciduous Tooth as the Source of Stem Cells: A Technique for Proliferation and Harvesting in vitro
Shouvik Mandal1*, Bani Bandana Ganguly2 and Nitin N Kadam3
1Department of Conservative Dentistry & Endodontics, MGM Dental College & Hospital, Navi Mumbai, India
2MGM Center for Genetic Research & Diagnosis, MGM New Bombay Hospital, Navi Mumbai, India
3Department of Pediatrics, MGM Medical College & Hospital, Navi Mumbai, India
*Address for Correspondence: Shouvik Mandal, Conservative Dentistry & Endodontics, B-4, Lane-8, Sector -9, CBD Belapur, Navi Mumbai-400614, Maharashtra, India, Tel: +91 9821107993; E-mail: [email protected]
Submitted: 14 September 2017; Approved: 24 October 2017; Published: 26 October 2017
Citation this article: Mandal S, Ganguly BB, Kadam NN. Exfoliated Deciduous Tooth as the Source of Stem Cells: A Technique for Proliferation and Harvesting in vitro. Int J Stem Cell Res. 2017;3(1): 025-028.
Copyright: © 2017 Mandal S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Stem cells; Deciduous tooth; in vitro propagation; Chromosome analysis
Download Fulltext PDF
Since a long time, the field of stem cell biology has undergone a remarkable transformation with constant research on it and its various applications predicated to be coming into use for long term clinical cell based therapies. The present report describes extraction of mesenchymal cells from deciduous tooth and its propagation in vitro with a view to producing cells at a larger scale keeping in vitro acquisition of chromosomal aberration in mind. Pulp was extirpated from freshly exfoliated deciduous tooth and cultured within 30 minutes for colonization and harvesting of the stem cells from dental pulp. The cells had exhibited active growth. Chromosome analysis was considered for karyotyping and screening of acquired aberrations following harvesting of cultures in confluent stage and conventional cytogenetic technique. There was no evidence of abnormality in karyotype or in vitro acquisition of aberrations. The study was important to establish non-invasive collection of stem cells from biological waste (deciduous tooth), which could be monitored for chromosomal status and considered for testing of drugs/chemicals on stem cells in vitro. However, the study shall be carried out on larger sample size following passage culture for production at larger scale, which could be considered for clinical application for self or for people in need.
Introduction
Therapeutic application of stem cells has created an increasing interest in the study of undifferentiated cell types that constitute the ability to proliferate and differentiate into various tissues. When stem cells multiply, each new cell has the potential either to remain a stem cell or become another type of cell with a more specialized function. They have the capacity to renew themselves by cell division even after long periods of inactivity. This is the very reason why medicine in recent times have tried using these capacities for the treatment of various conditions especially degeneration conditions [1].
There are two types of stem cells - embryonic stem cells and adult stem cells. Embryonic stem cells are derived from embryos and adult stem cells are found throughout the body after development that multiply by cell division to replenish dying cells and regenerate damaged tissues. Topical research suggests that adult stem cells within bone marrow possess greater functional plasticity. After bone marrow transplantation, adult stem cells have been found in diverse non-hematopoietic tissues such as skeletal muscle [2], cardiac muscle [3], liver bile ducts [4,5] and vascular endothelium [6-9]. Adult stem cells also exist throughout the body in different other tissues such as brain, skin, retina, pancreas, peripheral blood, muscle, adipose tissue, and dental tissues [10].
Among the numerous stem cells that have been identified from dental tissues, those from the pulpal tissues include Dental Pulp Stem Cells (DPSC) and Stem Cells from Human Exfoliated Deciduous teeth (SHED). The dental pulp occupies the pulp chamber in the crown and root of the tooth, inside dentin, which has specialized connective tissue in the innermost part of the tooth along with blood vessels and nerves [11]. The cell-rich zone of pulp is principally composed of fibroblasts and undifferentiated mesenchymal cells with odontoblasts at the periphery of this tissue [12]. Fibroblasts are the most numerous cell types in the pulp. The odontoblast is responsible for the formation of dentine. The stem cells were shown to undergo proliferation and migrate to the site of injured odontoblasts and produce dentin.
In the present report, an attempt was made to harvest stem cells from human deciduous milk teeth. The target was to establish monolayer culture with stem cells and their propagation in vitro with a view to developing the technique for production of stem cells from SHED tissue for future therapeutic application for the owner of the stem cells or allogenic transplantation. The acquisition of chromosomal aberration as in vitro artifact was measured to ensure the suitability of its application in regenerative medicine.
Materials and Methods
Pulp tissue was collected from freshly extracted deciduous tooth which was due for exfoliation from a 5 year-old female child. The tooth was collected in collection medium and transported to the genetics lab within 30 minutes. Consent was taken from the patient’s guardian for its use in tissue culture for extraction and propagation of stem cells present in the tooth and publication of the result. Approval from Institutional Ethical Research Committee (IERC) was obtained for this work. The tissue was minced into small pieces for making single cell suspension as far as possible. Long-term tissue culture was set up in T25 flasks in replicate sets using cell suspension and small pieces of tissue-fragments in complete culture medium (AmnioMAX™-II Complete Medium, 3 CO2 Incubators, DJB Labcare Ltd, UK) at a temperature of 37oC and CO2 supply of 5%. The growth of the cultures was monitored regularly through inverted microscope (CKX41, Olympus, Japan) with supplementation or change of medium at 3-4 days interval.
Attachment of the tissue cells was noticed within 24h of incubation with subsequent proliferation as monolayers of fibroblast cells. The growth of the colonies was quite satisfactory. One of the two cultures was terminated on 20th day for checking the karyotypic pattern and chromosomal aberration, if any, acquired in vitro. For harvesting of the cells, colonies were trypsinized followed by colchicine-hypotonic-fixation treatment [13]. Metaphase-chromosome preparation and G-banding was carried out following the standard technique [14]. A total of 25 cells were analyzed karyotypically following ISCN nomenclature [15]. Screening of 100 cells was performed for analysis of acquisition of chromosome aberration in vitro.
The second culture was maintained for 35 days and checked for chromosomal abnormalities. No passage culture was considered with this tissue; however, that could have been done since the colonies were active and growing exponentially. This exercise has been practiced mainly to check the feasibility of SHED as the source of stem cell propagation and therapeutic application.
Result and Discussion
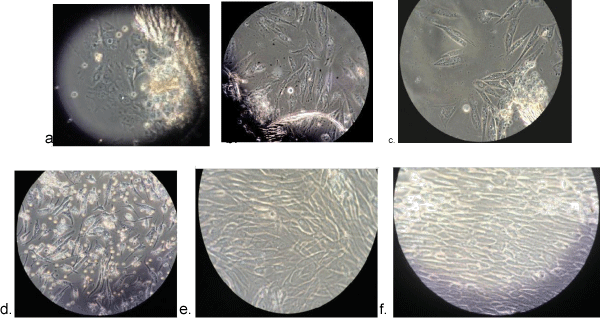
The cells extracted from SHED were growing actively as revealed in figures (Figure 1). Karyotypic classification of the metaphase chromosomes revealed 46, XX pattern. Screening of 100 cells didn’t reveal any numerical or structural abnormality of chromatid or chromosome pattern. Karyotyping of 25 cells didn’t present any inter- or intra-chromosomal rearrangement (Figure 2).
The present report establishes a protocol to successfully grow dental pulp stem cells from SHED safely and the cells can be further stored for future use in cell-based therapies. The search for more easily obtainable mesenchymal stem cells than those found in bone marrow has propelled interest in dental tissues. The pulp chamber conforms to the external form of the tooth crown and is placed centrally inside enamel and dentin [11]. The pulp is formed from cells of the dental papilla which appear as undifferentiated Mesenchymal Stem Cells (MSC) [12]. MSCs are reported to be present in human adult dental pulp (DPSC), exfoliated primary teeth (SHED), and periodontal ligament (Periodontal Ligament Stem Cells, PDLSC) by their capacity to generate clonogenic cell clusters in long-term culture [16]. MSCs have also been isolated from bone marrow, peripheral blood, placenta, adipose tissue, lung, and umbilical cord [17]. However, extraction of stem cells from deciduous teeth at the time of its exfoliation appears much easier than other tissues which may require invasion for collection. Also, the collection of stem cells from dental pulp seems a far convenient method along with the opportunity that each deciduous tooth allows a fresh collection option. Additionally, deciduous teeth are otherwise discarded as biological waste. MSCs are of stromal origin and may differentiate into a variety of tissues [16]. Therefore, the present tissue cells representing MSCs indicate a wide potential for proliferating and harvesting of stem cells for therapeutic application of various illness.
It has been reported that MSCs reside within the connective tissue of most organs [18]. MSC populations obtained from most tissues have gained attraction in clinical therapy due to their ability to differentiate, provide trophic hold, and modulate innate immune response [17], and also to differentiate into various cell types, including osteoblasts, chondroblasts, adipocytes, neuroectodermal cells, hepatocytes and so on [16]. MSC’s bioactive mediators and anti-inflammatory effects favor cell growth and tissue healing in the local microenvironment. MSCs are reported to changing the cytokine secretion of dendritic and T-cell subsets resulting in a shift from a pro-inflammatory environment to an anti-inflammatory stage [19,20]. Also a wide range of regulatory proteins present in MSC broaden its therapeutic efficacy; however, it may confound evaluation of their trans-differentiation efficacy [17].
Therefore, the present attempt of extraction of MSCs from SHED would not only facilitate non-invasive collection of stem cells, that too from a biological waste, but also help in proliferation of these cells in vitro for therapeutic application. in vitro propagation would further facilitate creation and maintenance of secondary cultures by passaging of the primary cultures for long-term and chromosomal analysis at periodic-intervals would indicate its usefulness for clinical therapy. The cells can also be directed for specific cell/tissue-type in vitro itself before its application in regenerative medicine. Additionally, the cells in vitro can be considered for clinical research and also for testing genotoxocity of newly formulated drugs, and thus help in identification of signature molecules as potential drug targets. The present exercise has limitation on sample size; however, it has validated the primary technique required for extraction and colonization of stem cells from exfoliated tissue. Nevertheless, the technique should be employed on larger sample size for validation of production of these stem cells at a larger scale.
Acknowledgement
The authors acknowledge laboratory facility provided by MGM New Bombay Hospital, Navi Mumbai, India.
- Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001; 107: 1395-1402. https://goo.gl/dAxCzU
- Ferrari G, Angelis D, Coletta M, Paolucci E, Stornaiuolo A, Cossu G et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998; 279: 1528-1530. https://goo.gl/FikD7i
- Bittner RE, Schofer C, Weipoltshammer K, Ivanova S, Streubel B, Hauser E, et al. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat Embryol. 1999; 199: 391-396. https://goo.gl/4ouyxL
- Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000; 6: 1229-1234. https://goo.gl/FMPrNL
- Transfusion Medicine Reviews. 2001; 15: 247. https://goo.gl/mim5s6
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase NA, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999; 284: 1168-1170. https://goo.gl/5zRLhp
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997; 275: 964-966. https://goo.gl/bdSFHt
- Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest. 2000; 106: 571–578. https://goo.gl/CB96pn
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al Ischemia-and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999; 5: 434-438. https://goo.gl/3FM4cK
- Shi Q, Rafii S, Hong-De Wu M, Wijelath ES, Yu C, Ishida A, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998; 92: 362-367. https://goo.gl/WWjntU
- Sedgley CM, Botero TM. Dental stem cells and their sources. Dent Clin North Am. 2012; 56: 549-561. https://goo.gl/bta61W
- Berkovitz BK, Holland GR, Moxham BJ. Oral anatomy, histology and embryology. Elsevier. 2016. https://goo.gl/BGtUek
- Kumar GS. Orban's oral histology & embryology. Elsevier Health Sciences. 2014. https://goo.gl/3TJrQp
- Ganguly BB, Kadam NN. 2014. Prenatal diagnosis of fetus with short limbs caused by three abnormal chromosomes inherited from both parents. Int J Hum Genet. 14: 83-90. https://goo.gl/iFKeem
- Mandal Shouvik, Kadam Nitin N, Ram Sabita M, Ganguly BB, Shenoy Vanitha U. Ectodermal Dysplasia and Anodontia associated with Ring Chromosome 18. J Contemporary Dentistry. 2016; 6: 220-224. https://goo.gl/BU79Gc
- ISCN. An International System for Human Cytogenomic Nomenclature. Eds: McGowan-Jordan J, Simons A, Schmid M. S. Karger AG, Basel (Switzerland). 2016. https://goo.gl/BGf9bw
- Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005; 8: 191-199. https://goo.gl/FsBHLB
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem cells. 2007; 25: 2896-2902. https://goo.gl/UisPZA
- Young HE, Mancini ML, Wright RP, Smith JC, Black AC Jr, Reagan CR, et al. Mesenchymal stem cells reside within the connective tissues of many organs. Dev Dyn. 1995; 202: 137-144. https://goo.gl/4r6mNB
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005; 105: 1815-1822. https://goo.gl/JjPKFr
- Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen‐induced arthritis. Arthritis Rheum. 2007; 56: 1175-1186. https://goo.gl/BmSRd8

Sign up for Article Alerts