Laser Needling® and Natural origin, Highly Purified Polynucleotides (PN-HPT™) in Knee Osteoarthrosis: Benefits in Physiatry and Sports Medicine
Maria Conforti*
International Association Laser Therapy (IALT), Italy
*Address for Correspondence: Maria Conforti, Member, International Association Laser Therapy (IALT), 24125 Bergamo, Italy, ORCiD: 0000-0002-8025-3459; E-mail: conforti@mariaconforti.it
Submitted: 28 October 2020; Approved: 05 November 2020; Published: 06 November 2020
Citation this article: Conforti M. Laser Needling® and Natural origin, Highly Purified Polynucleotides (PN-HPT™) in Knee Osteoarthrosis: Benefits in Physiatry and Sports Medicine. Int J Sports Sci Med. 2020 Nov 06;4(2): 030-037. doi: 10.37871/ijssm.id53.
Copyright: © 2020 Conforti M. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: FP3, knee osteoarthritis; Laser Needling®; Polynucleotides highly purified technology™; PN-HPT™
Download Fulltext PDF
Background and Objectives: The purpose of the paper is to illustrate the real life clinical outcomes of a retrospective cohort of knee Osteoarthritis (OA) patients treated with a novel laser disease management program; at the same time, to define the most effective operative procedure. The new laser technique mimics the in-vitro benefits of Low-Level Laser Therapies (LLLT). The study compared the 3month efficacy and 6-month persistence of clinical and functional benefits after application of laser energy either externally with a standard High Power Laser Therapy (HPLT) laser device (AG1 device, FP3 version), or intra-articularly with the patented, low-energy AG8 intra articular fiber device (ultrasound-guided, same wavelengths, no handpiece). This innovative laser device reduces to one hundredth the applied energy density. The pain suppressing efficacy of the LLLT-like laser FP3 procedure is intended to act synergically with the strong biorestructuring and pain suppressing efficacy of natural origin polynucleotides (PN-HPT™ or Highly Purified Technology™) injected before the laser session. PN-HPT™ are widely used in knee OA management for their persistent viscosupplementation properties overlapping those of high molecular weight hyaluronic acid.
Trial design: retrospective comparison of:
✥Short term 3 month efficacy outcomes on pain and disability
✥6-month persistence of clinical improvements in two cohorts of patients with severe knee osteoarthritis. The active cohort knee OA patients (105 agonistic or recreative practitioners with persistent knee pain and disability resistant to conventional medical or physical therapies) were treated with an innovative intra articular low-energy AG8 physical therapy protocol (ambulatory “AG8 Protocol 3” combined with a preliminary PN-HPT™ knee injection); the control-cohort patients (109 patients with knee disease of similar severity) were treated with a standard, multi frequency HPLT ambulatory treatment protocol (FP3 device).
Outcome parameters: Western Ontario and McMaster Universities (WOMAC) assessments at baseline (T0) and after 2 weeks (T2) and 3 months (T3). Secondary parameters: Nociceptive and neuropathic pain; assessment: standard 10 cm Visual Analogue Scale (VAS) immediately before and at the end of each treatment session.
Results: Treatment with the AG8 protocol 3 / PN-HPT™ intra articular combination was associated with strongly significant short term (2 weeks) and medium-term (3 months) benefits vs. controls treated with a conventional FP3 extra-articular treatment protocol both for the WOMAC Total Score and WOMAC Pain and Function subscores. Benefits for the WOMAC Stiffness subscore were borderline non-significant. The subgroup analysis showed that the 2A (Grade-2 KL primary OA) and 2B (Grade-2 KL secondary (post-surgical) OA) mainly contributed to overall benefits.
Conclusion: The study showed the intra-articular Laser Needling® technique (ultrasound-guided AG8 laser device, Protocol 3 plus infiltration of a facilitating agent such as intra-articular PN-HPT™ gel) to be more effective on knee OA pain than the traditional extra-articular FP3 laser technique, with special reference to pain associated with primary OA.
Abbreviations
AG8: Multifrequency, 4 wavelength, needle tipped Fiber Power 3 (FP3) intra articular laser device; AP-1: Activator Protein 1; BMI: Body Mass Index; FP3: Fiber Power 3; HKA: Hip Knee Angle; HPLT: High Power Laser Therapy; KL: Kellgren Lawrence grade; I.A.L.T.: International Association Laser Therapy; ICRP: International Committee on Radiological Protection; IL-1: Interleukin-1; LLL: Low-Level Laser; LLLT: Low Level Laser Therapy; LAMBA (λ): Wavelength; LASER: Light Amplification by Stimulated Emission of Radiation; MAPK: Mitogen Activated Protein Kinases; NF-kB: Nuclear Factor Kappa Light Chain Enhancer of Activated B Cells; NSAIDs: Non-Steroidal Anti Inflammatory Drugs; OA: Osteoarthritis; PNs: Polynucleotides; PN-HPT™: Polynucleotides Highly Purified Technology™; PRP: Platelet Rich Plasma; TNF-alpha: Tumor Necrosis Factor Alpha; VAS: Visual Analogue Scale; WADA: World Anti-Doping Agency; WOMAC: Western Ontario and McMaster Universities
Introduction
Globally, hip and knee Osteoarthritis (OA) ranked as the eleventh leading cause of disability among the 291 conditions investigated in the Global Burden of Disease 2010 study, with the Years Lived with Disability (YLDs) index almost doubling in less than 20 years, from 10.5 million YLDs in 1990 to 17.1 million in 2010 [1]. Nociceptive pain is the dominant and earliest OA symptom, and control of pain, the leading contributor to both disability and worsening quality of life, is the paramount problem facing all OA specialists. All OA symptoms pain, but also restricted range of motion and morning stiffness, crepitus, instability, and joint deformities - ultimately originate from disruption of the physiological balance between cartilage matrix degradation and repair [2,3].
Low Level Laser Therapy (LLLT), with power outputs in the range 0.001 to 0.1 watts or a few milliwatts per cm2, has long been an important tool in OA disease management for pain control and reduction of inflammation and edema [4-6]. HPLT (High Power Laser Therapy) laser devices have power outputs of some watts per cm2, are also active on inflammation and pro-inflammatory cytokines, and have also been used for OA symptom relief,[7] although with problems of energy dispersion and exaggerated rises of tissue temperature [5]. Fiber Power 3 (FP3) lasers, extensively used for pain relief in knee OA, are multi frequency HPLT devices that concomitantly leverage three wavelengths in the red and near-infrared 400 to 1,100 nm therapeutic range. This wavelength range allows an intense tissue penetration of light energy while minimizing untoward effects [8].
The innovative 4 wavelength, low-power, needle equipped AG8 intra articular (ultrasound-guided) fiber device that was used in this retrospective comparative study (Italian patent No. 102015000027296) uses the same wavelengths (laser and/or light emitting diode) of a standard FP3 device, but eliminates the FP3 handpiece. The AG8 fiber device is the outcome of more than 25 years of studies about the interaction between monochromatic Low Level Laser (LLL) and intra-articular tissues [8].
Recently, the combined use of LLL and intra articular Hyaluronic Acid (HA) injections was shown to prolong the functional joint longevity of worsening knee OA [9].Natural-origin polynucleotides, highly purified fragments of linear DNA also known with the acronym PN-HPT™ (PN-HPT™, Polynucleotides Highly Purified Technology), have repeatedly shown to be either an alternative or a complement to hyaluronic acid in knee OA and degenerative joint disease of other sites even in long term studies [10-15]. PN-HPT™ deserved to be also tested as an alternative to HA in LLL/HA protocols for pain and symptom relief in knee OA.
The combination of PN-HPT™ infiltrations (CONDROTIDE®) and laser treatments with the AG8 intra articular laser device, both echographically guided by a human operator, is patented under the brand Laser Needling® and has been refined since 2014. This paper, the first academic one about a Laser Needling® knee OA treatment protocol, illustrates the retrospective comparison of outcomes (3 month efficacy and 6 month persistence) in real-life knee OA patients treated with two laser OA management programs. Laser energy was applied at the same wavelength either externally, with a standard HPLT technique (multi-frequency FP3 laser), or intra-articularly with a low-energy FP3-like AG8 device (Laser Needling® technique) that allows to reduce to one hundredth the applied energy density. The purpose of the retrospective comparison was to compare the most frequently applied AG8 treatment protocol, which requires no more than 2 or sometimes 3 short treatment sessions (a few minutes) and is well defined in terms of number of sessions, laser applications per session and time spacing between sessions, with a standard FP3 treatment protocol, which may require long daily sessions (20-30 minutes) targeted to the pain trigger points.
Methods
Characteristics and features of the AG8 intra articular laser device:
• Remotely controlled (with microprocessor), multifrequency continuous emission, class IIb (93/42) FP3-like laser device.
• Coherent light beams contemporarily emitted in three wavelengths out of the four ones generated by the device, all in the maximally biostimulating range (590-960 nm). All wavelengths, previously tested with a standard HPLT FP3 device, were not associated with any local untoward effect.
• Energy application: either by traditional handpiece (power up to 12 watts) or, more innovatively, with the help of a quartz optical fiber (400 µm) and a single-use intra-articular 21G needle.
The modulated energy emission generates a steadily excited electromagnetic field with the same bio stimulating effects, at the target joint tissues below the skin and muscle planes, which are elicited by LLLT [8]. The 21G needles of variable length eliminate all phenomena of light refraction and reflection in superficial tissue layers, thus minimizing tissue overheating and the loss of efficacy due to energy dispersion. This allows energy densities that are much lower than those required with standard FP3 devices; the light scattering at the tip of the needle is only associated with some local vasodilatation and does not perturbate the steady bio stimulating effect of the electromagnetic field [8].
All intra-articular AG8 protocols mimic the outcomes and evidences of LLLT in-vitro studies, reduce the in-vivo energy exposure of tissues to one hundredth of that associated with standard HPLT techniques, and reduce the length and number of laser sessions. Moreover, all AG8 protocols are in full agreement with the guidelines of the International Commission on Radiological Protection (ICRP, European Union Official Gazette, L 013, 17th January 2014) and analogous regulatory documents [16] concerned with minimization of body exposure to ionizing and non-ionizing electromagnetic radiation. Indications and contra-indications of AG8 protocols are the same of any physical therapy; at the same time, AG8 protocols are also an infiltrative therapy with the contra indications of all infiltrative therapies.
Study design
Five AG8 intra articular protocols have so far been studied, with variable dosimetry of the three active wavelengths of the multifrequency AG8 laser device as a function of the target tissue volumes and the musculoskeletal conditions to be treated. When taking volumes to be treated and energies required into consideration, the “AG8 Protocol 3” is ideal for the treatment of painful disorders of the knee and shoulders. The “AG8 Protocol 3”, combined with a preliminary PN-HPT™ knee injection, was the ambulatory protocol applied to 105 agonistic or recreative practitioners of sports activities like tennis, running and bicycle with persistent knee pain and disability resistant to conventional medical or physical therapies. Controls were a cohort of 109 FP3 treated knee OA patients with disease of similar severity. The two prospective cohorts of ambulatory subjects were collected over 36 months (March 2015 to March 2018), with an individual short term “efficacy follow up” of 3 months and a “persistence follow up” of any symptom improvement of 6 months.
Table 1 illustrates the cohort demographics of the patients of the AG8 Protocol 3 / PN-HPT™ treatment program, classified as non-responders to previous knee OA pain-control standard strategies. A two week wash out period was required before admission to the AG8 Protocol 3 / PN-HPT™ program. No other medical or physical therapy was administered concomitantly to the combined treatment program; antipyretics for common respiratory disorders arising between control visits. The mean age of the 55 female and 54 male subjects of the FP3 control cohort was 47,89 ± 12,37 (p = 0.065 vs. AG8 Protocol 3 / PN-HPT™ cohort).
Patient Selection and Categorization and Study Procedures
All enrolled patients with degenerative OA had tibiofemoral lesions and refused surgery, including prosthetic implantation.
A. Inclusion criteria: Patients with compromised efficiency in sports activities due to persistent pain and disability despite previous medical or physical therapies; up to Kellgren and Lawrence radiographic Grade-3 OA (joint space narrowing ≤ 2 mm, moderate multiple osteophytes, some initial sclerosis and possible deformity of bone ends) [17].
B. Exclusion criteria: Patients less than 25 or more than 75 years old; Kellgren and Lawrence radiographic Grade-4 OA; patellofemoral chondropathy and OA with patellar instability and vertical gonalgia; very severe varus and valgus knee (hip-knee-ankle angle, HKA >20°);[18] rheumatologic disorders (psoriatic arthritis, etc.); patients in treatment with corticosteroids or anticoagulants, thrombocytopenia or coagulopathies; local or systemic infections; usual contra indications to physical therapies such as tumors, pregnancy and psychiatric disorders. Previous surgery for lesions of the anterior cruciate ligament or traumatic meniscal injuries did not prevent screened patients from inclusion.
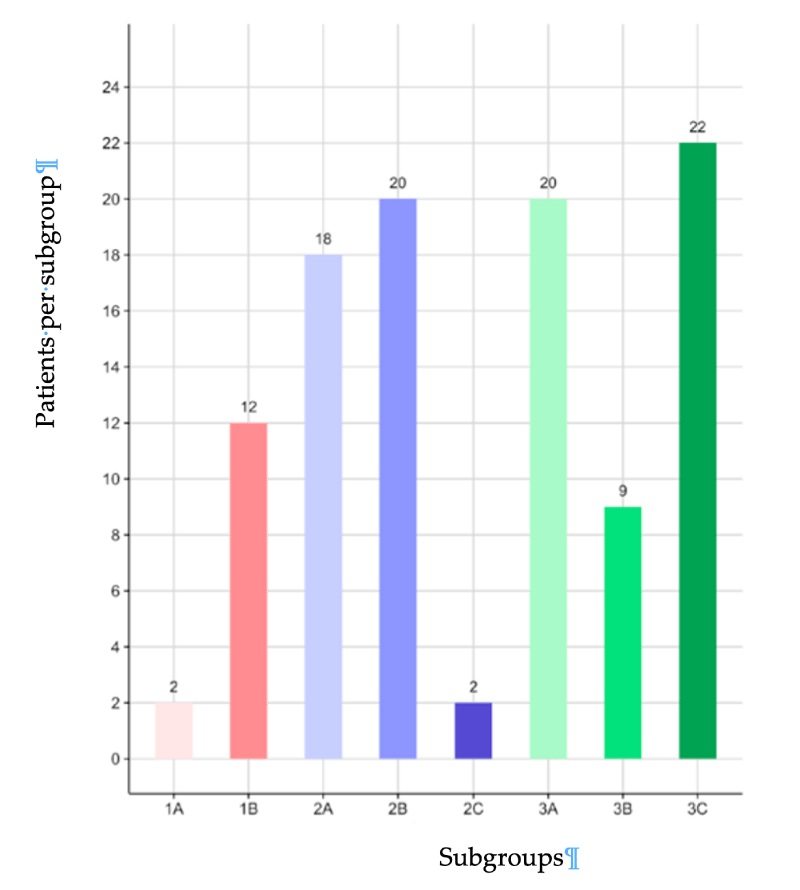
The retrospectively enrolled patients were divided into 9 subgroups according to the etiology of knee pain-age-related and degenerative, past selective or total meniscectomy and/or ligament repair, tibial plateau fracture and osteosynthesis, axial defects (HKA < 20°) with overweight: [18-20].
• 1st subgroup [1A ]: Grade-1 Kellgren-Lawrence (KL) primary OA
• 2nd subgroup [1B ]: Grade-1 KL secondary (post-surgical)OA
• 3rd subgroup [1C ]: Grade-1 KL OA due to axial defects less than 20° and overweight (still practicing non-agonist activity, no significant problems in everyday social life)
• 4th subgroup [2A ]: Grade-2 KL primary OA
• 5th subgroup [2B ]: Grade-2 KL secondary (post-surgical)OA
• 6th subgroup [2C ]: Grade-1 KL OA due to axial defects less than 20° and overweight (no sports activity but still active in everyday social life)
• 7th subgroup [3A ]: Grade-3 KL primary OA
• 8th subgroup [3B ]: Grade-3 KL secondary (post-surgical) OA
• 9th subgroup [2C ]: Grade-3 KL OA due to axial defects less than 20° and overweight (disruptive problems in everyday life)
The number of patients in each subgroup is shown in Figure 1. The age distributions in subgroups were close to the age distribution of the overall cohort, with the expected lower mean ages in post-surgical subgroups 1B and 2B (40.2 ± 12.0 and 47.4 ± 11.2, respectively) due to enrolled patients as young as 25 in both subgroups. Bilaterally treated patients (4 cases) were considered as independent assessments and counted twice.
All enrolled patients gave their informed consent. No change of the predefined, standard total energy per session or the number of sessions were allowed under the AG8 Protocol 3; conversely, a second irradiation in the same session with the same total energy was permitted if needed, possibly modifying the angle of needle insertion. The procedure was performed with standard commercial 21G needles and 40-µm optical fibers with plastic guide and sterile insertion through the usual portals of approach to the knee joint (Figure 2). The echo guided procedure with a 3-13 MHz linear probe was preferred in obese subjects or in case of abundant joint fluid. The same protocol was applied to the FP3 treated patients. The settings of the conventional HPLT extra-articular FP3 laser device were double those adopted in the AG8 Protocol 3 / PN-HPT™ program.
Baseline and follow-up assessments -Western Ontario and McMaster Universities (WOMAC) assessments (WOMAC version 3.1 for the knee), were carried out at admission (baseline, T0) and after 2 weeks (T2) and 3 months (T3). A standard 10-cm Visual Analogue Scale (VAS) were used to assess nociceptive pain, respectively, immediately before and at the end of each session. A telephone VAS interview was carried out after 6 months to assess persistence of pain benefits, if any.
Laser Needling® sessions to counteract pain, muscle contracture, and edema were followed by at least 8 weeks (4 sessions per week) of domiciliary self training over the 3 month follow up, starting from the second week, to help the recovery of the knee joint function and muscle strength with the help of standard passive and isometric concentric and eccentric active muscle reinforcement techniques.
Statistical analysis
All analyses were performed with the STATA/SE 12.1 software (StataCorp LLC, 4905 Lakeway Drive, College Station, Texas 77845-4512, USA). The efficacy parameters assessed were:
WOMAC total score and WOMAC pain, joint stiffness, and function subscores; assessment: total score and subscores after 2 weeks (T2) vs. baseline (T0).
VAS pain scores: Scores after 1 and 2 weeks (T1 and T2) and after 3 months (T3) vs. baseline (T0).
Differences between semi-quantitative scores were analyzed with the one tail non parametric Mann Whitney U-test with a p < 0.05 significance threshold.
Results
Overall comparisons
Table 2 summarizes the outcomes of the short term, 2-week retrospective comparisons. Differences vs. baseline were strongly significant both for WOMAC Total Score and WOMAC Pain and Function subscores and borderline non-significant for the WOMAC Stiffness subscore. As regards the main investigated symptom, pain, significant improvements of the VAS efficacy scores were recorded, in agreement with WOMAC Pain subscores, after 2 weeks; VAS nociceptive pain scores improvements persisted after 6 months (data not shown).
The patients allocated to the Laser Needling® technique with the AG8 laser device (Protocol 3) facilitated by the intra-articular injection of PN-HPT™ verbally reported improvements in pain already a few hours after the first treatment session.
Subgroup comparisons
Short-term (WOMAC Pain subscore) and medium-term (VAS score) in the 2A subgroup showed statistically significant differences between the AG8 Protocol 3 / PN-HPT™ and FP3 treatment protocols (Table 3). The AG8 / PN-HPT™ treatment protocols showed pain benefits also in the 2B subgroup (Grade-2 KL secondary (post-surgical) OA) (Table 4).
Safety and drop outs
Summarizing, no intra-articular side effects; minor laser induced local hyperemia was frequent, but transient in no more than a few hours. Three patients, subjectively satisfied of the first treatment session despite the feeble 2-point VAS reduction, refused the second Laser Needling® session after 7-10 days.
Discussion
The overall results of the study may be summarized as follows: in combination with an intra articular PN-HPT™ gel injection as facilitating agent, the intra-articular Laser Needling® technique, centered on the LLL-like properties of the low energy, ultrasound-guided FP3-like AG8 intra-articular laser device (treatment protocol 3), is more effective, on the pain associated with knee OA, than the extra articular High Power Laser Therapy (HPLT) with a AG1 laser device, FP3 version. This is especially true for the pain associated with primary OA more than for secondary OA. This higher efficacy, which the exploratory VAS interviews seemed to confirm to extend over a 6 month follow up (data unreported), develops over time despite a concomitant reduction to one hundredth of the applied energy density compared with the AG1 technique. Several participants could resume their amateur sports activity after a few weeks; the protocol might also be especially useful in young subjects or subjects with concomitant disorders. The outcomes of this first Laser Needling® study seem to suggest strong efficacy on pain after 2 weeks, a possibly somewhat more selective efficacy on function (statistical significance only in the 2A or Grade-2 KL primary OA subgroup), and poor efficacy on joint stiffness. However, this is also the case for the extra articular FP3 traditional treatment option. Overall, outcomes of both laser strategies are poor on knee OA pain associated with overweight or varus-valgus deformities.
Promotion of tissue regeneration, reduction of inflammation, and relief of pain by all LLL devices including AG8 are not associated with any ablative or thermal mechanism, but with the cytochemical changes that follow the light absorption by cells (photochemical effects)[4]. Increased nitric oxide release and tissue perfusion, and suppression of pro-inflammatory cytokines, mediators and related pathways like Tumor Necrosis Factor Alpha (TNF-alpha), Interleukin-1 (IL-1) and the related Mitogen Activated Protein Kinases (MAPK) pathway are prominent candidates among outcomes of LLL treatments [21-23]. The same might be true for transcription factors NF-kB and AP-1, activators of pro-inflammatory genes in chondrocytes [21-23]. This intracellular effects may well explain the favorable outcomes of LLL therapy in the control of knee OA pain [24-26].
Based on this background of elective anti-inflammatory activity, the Laser Needling® procedure candidates for a distinctive role in the earliest phases of OA development, with special reference to pain, when anatomical damages are still minor and interference with the biochemical mechanisms leading to pain may be highly fruitful.
The PN-HPT™ based (CONDROTIDE®) gel formulation injected as indispensable complement to the Laser Needling® procedure, is a transparent, colorless, viscoelastic solution of highly hydrophilic PN-HPT™ (concentration: 40 mg in 2 mL in single-use, apyrogenic, 2 mL glass prefilled syringes). PN-HPT™ have long-term persistent moisturizing, viscoelastic and viscosupplementation properties comparable to high-molecular weight hyaluronic acid [12]. All the content of one prefilled syringe is usually injected; as HA, it helps to diffuse by diffraction the monochromatic and concentrated radiant energy all over the knee joint cartilage and inflamed synovium.
Yet the PN-HPT™ contribution might well extend beyond this latter passive action. By steadily supplying nucleotides and other precursors to chondrocytes, PN-HPT™ maximize the trophic and protective activities on knee chondrocytes and cartilage, in fact more than HA [27,28]. Pain control after intra-articular injection of polynucleotides is also more vigorous and rapid compared with HA. [12-14]. PN-HPT™ might also conceivably slow the progression of joint damage. Combined with the AG8 intra-articular laser protocol, the trophic activity on chondrocytes developed by PN-HPT™ conceivably synergize with the photochemical effects of the intra articular LLL device. Of course, elucidating the interesting, and possibly quite likely, issue of synergy will need further, targeted studies.
There are other procedural question marks that only future studies will be able to solve. The ideal portal to access to the knee joint cavity is a still undefined issue; other unclear issues are the ideal number of intra articular energy administrations per session and the real persistence of pain control over time. The outcomes of the VAS interviews after 6 months, although suggestive and supported by previous studies with LLL devices, [24] can be considered nothing more than explorative and preliminary and in need of properly designed investigation. Studies in other indications, eg. shoulder disorders and overburden disorders of muscles and tendons, are also warranted. The same can be said for combined administration with other biological techniques like PRP (Platelet Rich Plasma) and stem cell infusions.
As regards the long term acceptance of the Laser Needling® technique in knee OA management, it needs no more than one or two treatment sessions of a few minutes, compared with repeated, even daily,long session of traditional laser devices targeted to pain trigger points, and many patients asked to repeat the procedure over the next 15 months. Some of the other Laser Needling® benefits:
• As an intra articular technique unaffected by skin pigments, phototypes, the skin color, and tattoos have no relevance.
• The laser radiation can be easily targeted to the more important area to be treated, even if deep or under bone tissue, by modifying the needle inclination.
• The rapid and persistent anti-inflammatory effect of Laser Needling® often avoids the need for corticosteroid injections, with their adverse effects on cartilage as well as reporting to the World Anti-Doping Agency (WADA). For the same reason, Laser Needling® should be in the future considered first choice in knee OA patients with concomitant diabetes of hypertension. The same is true for young individuals with borderline lesions of meniscus and joint cartilage before surgery.
• Eliminating the need for corticosteroid injections eliminates steroid doping problems in individual practicing sports at more than amateur level.
• No risks of allergy or drug intolerance: PN-HPT™ have no potential for that [12-15].
• Energies involved are always well below the International Committee on Radiological protection (ICRP) thresholds.
Conclusion
The intra-articular Laser Needling® technique (multi-channel AG8 laser device, Protocol 3) in combination with intra articular PN-HPT™ gel injection (CONDROTIDE®, Mastelli Srl, Sanremo, Italy) is a novel procedure, centered on the LLL-like properties of the low-energy FP3-like AG8 device, mainly targeted to OA pain control. The study showed the new intra-articular laser technique to be more effective on knee OA pain than the traditional extra-articular FP3 laser technique, with special reference to pain associated with primary OA.
Acknowledgement
Mastelli S.r.l, Sanremo, Italy, is the producer of polynucleotides HPT™ (Highly Purified Technology) and (CONDROTIDE®), the proprietary formulation of viscoelastic and pro-trophic polynucleotides for intra articular injection that was used in this retrospective study. Dr. Maria Conforti wishes to acknowledge the support by Mastelli S.r.l. with publication of the study results.
- Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014 Jul;73(7):1323-30. doi: 10.1136/annrheumdis-2013-204763. Epub 2014 Feb 19. PMID: 24553908.
- Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010 Nov;6(11):625-35. doi: 10.1038/nrrheum.2010.159. Epub 2010 Oct 5. PMID: 20924410.
- Boehme KA, Rolauffs B. Onset and Progression of Human Osteoarthritis-Can Growth Factors, Inflammatory Cytokines, or Differential miRNA Expression Concomitantly Induce Proliferation, ECM Degradation, and Inflammation in Articular Cartilage? Int J Mol Sci. 2018 Aug 3;19(8):2282. doi: 10.3390/ijms19082282. PMID: 30081513; PMCID: PMC6121276.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Lasers Med Sci. 2014 Spring;5(2):58-62. PMID: 25653800; PMCID: PMC4291815.
- Ohshiro T. New classification for single-system light treatment. Laser Ther. 2011;20(1):11-5. doi: 10.5978/islsm.20.11. PMID: 24155507; PMCID: PMC3806081.
- Karu T. The science of low-power laser therapy. Amsterdam, The Netherlands: Gordon and Breach Science Publishers. 1998
- Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337-361. doi: 10.3934/biophy.2017.3.337. Epub 2017 May 19. PMID: 28748217; PMCID: PMC5523874.
- Conforti M, Fachinetti GP. High power laser therapy treatment compared to simple segmental physical rehabilitation in whiplash injuries (1° and 2° grade of the Quebec Task Force classification) involving muscles and ligaments. Muscles Ligaments Tendons J. 2013 Jul 9;3(2):106-11. doi: 10.11138/mltj/2013.3.2.106. PMID: 23888293; PMCID: PMC3711700.
- Ip D, Fu NY. Can combined use of low-level lasers and hyaluronic acid injections prolong the longevity of degenerative knee joints? Clin Interv Aging. 2015 Aug 5;10:1255-8. doi: 10.2147/CIA.S86907. PMID: 26346122; PMCID: PMC4531024.
- Saggini R, Di Stefano A, Cavezza T, Saladino G, Bellomo RG. Intrarticular treatment of osteoartropaty knee with polynucleotides: a pilot study with medium-term follow-up. J Biol Regul Homeost Agents. 2013 Apr-Jun;27(2):543-9. PMID: 23830403.
- Migliore A, Graziano E, Martin LSM, Sorbino Q, Raichi M, Boni G. Four year management of hip osteoarthritis with intra articular polynucleotides: A real-life cohort retrospective study. In press on Curr Ther Res.
- Giarratana LS, Marelli BM, Crapanzano C, De Martinis SE, Gala L, Ferraro M, Marelli N, Albisetti W. A randomized double-blind clinical trial on the treatment of knee osteoarthritis: the efficacy of polynucleotides compared to standard hyaluronian viscosupplementation. Knee. 2014 Jun;21(3):661-8. doi: 10.1016/j.knee.2014.02.010. Epub 2014 Feb 24. PMID: 24703391.
- Vanelli R, Costa P, Rossi SM, Benazzo F. Efficacy of intra-articular polynucleotides in the treatment of knee osteoarthritis: a randomized, double-blind clinical trial. Knee Surg Sports Traumatol Arthrosc. 2010 Jul;18(7):901-7. doi: 10.1007/s00167-009-1039-y. Epub 2010 Jan 29. PMID: 20111953.
- Dallari D, Sabbioni G, Del Piccolo N, Carubbi C, Veronesi F, Torricelli P, Fini M. Efficacy of Intra-Articular Polynucleotides Associated With Hyaluronic Acid Versus Hyaluronic Acid Alone in the Treatment of Knee Osteoarthritis: A Randomized, Double-Blind, Controlled Clinical Trial. Clin J Sport Med. 2020 Jan;30(1):1-7. doi: 10.1097/JSM.0000000000000569. PMID: 31855906.
- Saggini R, Di Stefano A, Cavezza T, Saladino G, Bellomo RG. Intrarticular treatment of osteoartropaty knee with polynucleotides: a pilot study with medium-term follow-up. J Biol Regul Homeost Agents. 2013 Apr-Jun;27(2):543-9. PMID: 23830403.
- Urban D, Javorniczky J. Laser and Optical Radiation Guidelines. Non ionizing Radiation Protection: Summary of Research and Policy Options. Wood AW & Karipidis K. Ed. Wiley; 2017
- KELLGREN JH, LAWRENCE JS. Radiological assessment of osteo arthrosis. Ann Rheum Dis. 1957 Dec;16(4):494-502. doi: 10.1136/ard.16.4.494. PMID: 13498604; PMCID: PMC1006995.
- Thienpont E, Schwab PE, Cornu O, Bellemans J , Victor J. Bone morphotypes of the varus and valgus knee. Arch Orthop Trauma Surg. 2017;137:393-400. doi.org/10.1007/s00402-017-2626-x
- Jørgensen U, Sonne-Holm S, Lauridsen F, Rosenklint A. Long-term follow-up of meniscectomy in athletes. A prospective longitudinal study. J Bone Joint Surg Br. 1987 Jan;69(1):80-3. doi: 10.1302/0301-620X.69B1.3818740. PMID: 3818740.
- Sheehy L, Felson D, Zhang Y, Niu J, Lam YM, Segal N, Lynch J, Cooke TD. Does measurement of the anatomic axis consistently predict hip-knee-ankle angle (HKA) for knee alignment studies in osteoarthritis? Analysis of long limb radiographs from the multicenter osteoarthritis (MOST) study. Osteoarthritis Cartilage. 2011 Jan;19(1):58-64. doi: 10.1016/j.joca.2010.09.011. Epub 2010 Oct 13. PMID: 20950695; PMCID: PMC3038654.
- Karu TI, Pyatibrat LV, Afanasyeva NI. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg Med. 2005 Apr;36(4):307-14. doi: 10.1002/lsm.20148. PMID: 15739174.
- Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011 Jan;7(1):33-42. doi: 10.1038/nrrheum.2010.196. Epub 2010 Nov 30. PMID: 21119608.
- Alves AC, Vieira R, Leal-Junior E, dos Santos S, Ligeiro AP, Albertini R, Junior J, de Carvalho P. Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res Ther. 2013;15(5):R116. doi: 10.1186/ar4296. PMID: 24028507; PMCID: PMC3979014.
- Alfredo PP, Bjordal JM, Junior WS, Lopes-Martins RÁB, Stausholm MB, Casarotto RA, Marques AP, Joensen J. Long-term results of a randomized, controlled, double-blind study of low-level laser therapy before exercises in knee osteoarthritis: laser and exercises in knee osteoarthritis. Clin Rehabil. 2018 Feb;32(2):173-178. doi: 10.1177/0269215517723162. Epub 2017 Aug 4. PMID: 28776408.
- Huang Z, Chen J, Ma J, Shen B, Pei F, Kraus VB. Effectiveness of low-level laser therapy in patients with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015 Sep;23(9):1437-1444. doi: 10.1016/j.joca.2015.04.005. Epub 2015 Apr 23. PMID: 25914044; PMCID: PMC4814167.
- Rayegani SM, Raeissadat SA, Heidari S, Moradi-Joo M. Safety and Effectiveness of Low-Level Laser Therapy in Patients With Knee Osteoarthritis: A Systematic Review and Meta-analysis. J Lasers Med Sci. 2017 Summer;8(Suppl 1):S12-S19. doi: 10.15171/jlms.2017.s3. Epub 2017 Aug 29. PMID: 29071029; PMCID: PMC5642172.
- Guizzardi S, Uggeri J, Belletti S, Cattarini G. Hyaluronate increases polynucleotides effect on human cultured fibroblasts. J Cosmet Dermatol Sci Appl. 2013;3:124-28. doi: 10.4236/jcdsa.2013.31019.
- Luisa Gennero, Tetyana Denysenko, Gian Franco Calisti, Andrea Vercelli, Carlo Maria Vercelli, Stefano Amedeo, Silvia Mioletti, Enrico Parino, Manuela Montanaro, Antonio Melcarne, Carola Juenemann, Enrico De Vivo, Alessandro Longo, Giovanni Cavallo, Rocco De Siena. Protective effects of polydeoxyribonucleotides on cartilage degradation in experimental cultures. Cell Biochemistry and Function. 2012 Sep 24;31:214-27. doi.org/10.1002/cbf.2875


Sign up for Article Alerts