Does Running Yield to Specific Cortisol and Testosterone Answer Yielding to an Own Phenotype? - A Review
Benedikt Gasser*
University of Berne, Zellmoosweg 33 6210 Sursee, Switzerland
*Address for Correspondence: Benedikt Gasser, University of Berne, Zellmoosweg 33 6210 Sursee, Switzerland, Tel: +417-881-707-11; E-mail: [email protected]
Submitted: 28 January 2018; Approved: 12 March 2018; Published: 15 March 2018
Citation this article: Gasser B. Does Running Yield to Specific Cortisol and Testosterone Answer Yielding to an Own Phenotype? - a Review. Int J Sports Sci Med. 2018;2(1): 016-024.
Copyright: © 2018 Gasser B. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: CRH; ACTH; Running; glucocorticoids; Mineralcorticoid; Androgen system
Download Fulltext PDF
This study aimed to summarize effects of running on the glucocorticoid, mineralocorticoid and androgen system. Interaction between running and the CRH-ACTH-Adrenal gland System with its interaction on steroid hormone level are not finally understood. It seems that the System mainly plays a role of intermediation. Some hints exist, that before running due to anticipation cortisol levels start to rise and peak during running. Normalization of concentration in healthy humans even after large exercises such as marathon running, 1000km running or crossing Alaska occurs within one week. In contrast to glucocorticoids androgens seem to be lower after running. For different androgens such as Testosterone or Dihydrotestosteron sulfate a decrease could be detected after exercising. For mineralcorticoid effects are heterogeneous, probably due to direct feedback via Renin-Angiotensin-Aldosterone System and therefore ACTH which parallel influences Cortisol secretion. Trying to decipher effects of training, it is to mention, that effects on steroid hormones are dependent on intensity – e.g. interval versus long-jog – as well as of absolute time of exercising. To sum up, especially the increased cortisol levels and decreased testosterone levels could yield to a manifestation of a different phenotype of endurance versus strength athlete. The ratio of Cortisol to Testosterone seems to be relevant for a phenotypic development. This ratio determines anabolic (high-ratio) versus catabolic (low-ratio) effects on different organ system e.g. skeletal muscle and can influence the development of a specific somatotype.
Introduction
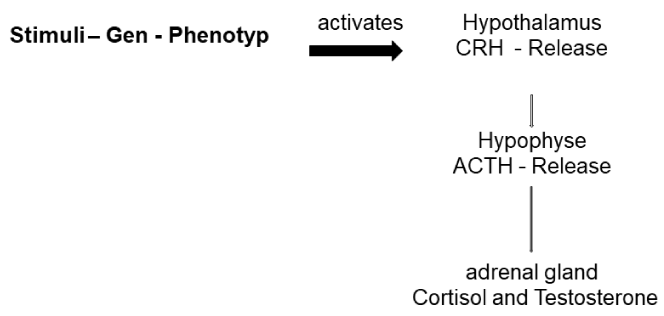
Exercise has a lot of healthy effects on different organ systems [1-3]. In the last decades it became evident, that for many illnesses such as some forms of cancer, diabetes type 2, dyslipidemia, metabolic syndrome, hypertonia, obesity, osteoarthritis, rheumatoid arthritis, myositis, osteoporosis or fibromyalgia physical activity is recommended where earlier counter instructions were made [1,2]. For many of these illnesses some form of low-grade inflammation are causative, while physical activity can inhibit inflammation cascades [1,2]. From an evolutionary point of view it is to mention that survival of Homo sapiens during evolution was dependent on the procurement of food, which in turn was dependent on physical activity [1,4]. Hence, gene selection (so-called “thrifty genes”) in the Late-Paleolithic era was influenced by physical activity [1,4]. Furthermore, convincing evidence shows that this ancient genome has remained essentially unchanged over the past 10´000 years and certainly not changed in the past 40-100 years [1,4]. These “thrifty genes” in combination with physical activity have various effects on different organ systems [1,3-5]. Thereby the Adrenocorticotropic Hormone (ACTH) System seems to play a key role in the feedback cascade [3,6]. Through different physical and psychical stimuli over a central adaption via hypothalamic Corticotropin Releasing Hormone secretion (CRH) to a stimulation of ACTH with secondary release especially of Cortisol mainly in the Zona fasciculata of adrenal gland [6,7]. Thereby interaction between mineralocorticoid and androgen system result, whereby these mechanisms are only partly controlled by ACTH [3]. This system played a major role in the evolution and is an essential element of sympathetic nervous system [4,6]. The survival of homo sapiens during evolution was dependent to gain nutrition which required the necessity to be physical active and required a constant activation and deactivation [4].
Evidence of effects of physical activity on glucocorticoid, mineralcorticoid and androgen system as well as its intermediator – CRH - ACTH System – through running has to be taxed as heterogeneous and fragmented. The sensitivity of the system astonishes e.g. the psychological dimension due to the fact that hints exist that Cortisol even before an exercise as consequence of anticipation of exhaustion start to rise [8]. Analyses show that Cortisol increases significantly two days before physical activity and showed an excess 24 h after [8]. A number of hints exist from different studies that different forms of training (moderate to intensive) yield to an increase of circulating Cortisol levels [8-10]. Trying to start on ACTH level, physical activity such as running yields to an increase of ACTH release respectively broader Hypothalamus-Pituitary-Adrenal (HPA) Axis is stimulated via CRH [13]. To sum up CRH release as consequence of physical activity stimulates ACTH, stimulates Cortisol in adrenal gland. To come to a first understanding, physical activity has lots of protective effects: CRH - ACTH system mediates Cortisol over different mechanism. Physical activity can protect from a number of illnesses especially those involving inflammation processes while inhibiting systemic inflammation factors (Glucocorticoids respectively Cortisol inhibit inflammation) and through releasing of anti-inflammatory cytokines (so called Myokines) from musculature such as IL-4, IL-6, IL-7, IL-15 promoting muscle hypertrophy [2,14,15]. These Cytokines, are produced and released by the exercising skeletal musculature and allow to have a secondary effect in other organ systems [16-18]. Thereby a bunch of intermediates’ can be mentioned such as IL-6, TNF-α, Leptin, Adiponectin, Ghrelin, Resistin, which are involved in the regulation of metabolic equilibrium and communicate metabolic states between organ systems from muscle to hypothalamus and liver and fat tissue, whereby AMPK System seems to be an important Integrator especially for endurance activity [2,5,19] (Figure 1). If stimuli are presented to hypothalamic an ACTH release occur which secondary affects adrenal gland and stimulates therefore stress cascade with glucocorticoid activation. Furthermore, physical activity seems to have protective effects not only via CRH - ACTH System but even more broad on cytokine and myokine level [2]. For skeletal muscle especially IL-6 can be mentioned, which seems to have a positive effect on health over inhibiting cytokines respectively inhibiting low-grade inflammation [20]. Mainly IL-6 was identified as so-called exercise Factor, which is mainly produced by contracting muscles and secreted to blood yielding to an induction of lipolysis as well as a suppression of TNF production and a stimulus of cortisol production [20]. Physical activity works protective against low-grade inflammation – especially over inhibiting of TNF and IL-6 production, whereby for these reasons training is recommended for illnesses, where earlier physical activity was contra induced [2,5,17,21]. Even in high-trained endurance athletes changes in IL-6 were reported as consequence of running [22]. A period of increased training intensity suppresses immune system inhibiting low-grade inflammation [22]. Especially role of skeletal muscle has to be mentioned which is a tissue with a share of about 40% of total body weight and is besides fat tissue a main player of energy balance and acts as puffer for the mentioned illnesses e.g. in the rheumatic field [6]. The organ needs over 30% of energy and is therefore a primary player of insulin stimulated glucose release which is relevant for Obesity and Diabetes, both illnesses are positively influenced by physical activity [1-3,6,23].
________________________________________________________________________________________________________________________________________________________ (Cortisol) 1Serum Cortisol and Salivary Cortisol levels showed high significant correlation implying some irrelevance of method of measurement [11,12]. _________________________________________________________________________________________________________________________________________________________Trying to decipher effects of running on glucocorticoid, androgen and mineralcorticoid system findings from marathon can be mentioned. Karkoulias et al. [24] investigated immediately after a race and 1 week later in well-trained recreational runner’s steroid profiles. Cortisol (C) was increased one hour and interestingly Androgens (Androstenedione & Dehydroepiandrosteron-Sulphate (DHEAS)) seemed not to be involved. Total Testosterone (T) as well as free were significantly reduced one hour after the race [24]. The race yielded to an increase in C-levels and a decrease in T-levels, whereby one week later values were normalized [24]. Furthermore, Franca et al. [25] analyzed hormone concentrations during a Marathon. At the end of the Marathons T was significantly reduced and C increased.
Even on mineralocorticoid level it was detected that Aldosterone was significantly increased [26]. Ponjee et al. [27] conclude that Marathon running yields to an increase of C. In contrast to other findings also an increase of Testosterone was detected [27]. They also detected increase of DHEAS which acts as androgen [27].
Also, from mountain- respectively Ultra marathon some findings exist C, T, free T as well as the Ratios between free T and C were measured in athletes participating in a mountain marathon (3,860 Meter/ peak 5100 Meter/ arrival 3,400 m) [28]. It was measured on sea level, whereby Cortisol increased after Acclimatization and after the Ultra marathon; Testosterone was reduced after Acclimatization and after the race and came to normal range within 24h after the race [28].
There seems to be an effect of expectation yielding to an additional ACTH Stimulation with secondary increase of C-levels. These findings are conceivable with other findings of Oltras et al. [10], whereby effects of hormone levels in expectation of a race (about half-marathon distance) were analyzed. The ACTH Levels increased before the race and further after the race implying that physical and psychical activity are interacting [10]. Alterations in adrenal- sympathetic nerve function were indicated by increased levels of cortisol, epinephrine, and norepinephrine after Marathon in analyzed elite runners [29].
From ultra-marathon field investigations from 1000 km run from Sydney to Melbourne of highly trained athletes exist, whereby rest levels of Adrenalin, Noradrenalin, Dopamine were significantly increased, as well as C and ACTH-levels [20]. As a model of chronic physical stress, the ultra-marathon runner demonstrated a significantly altered baseline hormonal state as reflected in the primary mediators of the stress response, the catecholamines and the hypothalamic-pituitary-adrenal axis [20]. Their response to severe exercise is distinct from that of untrained individuals in which conjugated catecholamines decreased and ACTH increased, which may represent hormonal adaptation to prolonged stress [20].
Also, for solely females some investigations exist. In a comparison between incremental exercise and marathon running experienced female marathon runners volunteered to run to exhaustion according to an incremental treadmill protocol [30]. Four weeks after running a marathon ACTH and cortisol concentration immediately prior to the laboratory treadmill test, 3, 30 and 60 min later, as well as prior to the marathon, after 60 min and 120 min of running and 3, 30 min, and 24 h after completion of the run were analyzed [30]. Mean marathon running time was 3.22 h and for ACTH the baseline concentration was increased by 8.3- and 10.3-fold, respectively [30]. Cortisol concentration rose exponentially from a baseline and peaked at 2.2-fold 30 min after the run, when the maximal concentration also had been reached after the treadmill test, increasing 1.3-fold from baseline [30]. The maximal values for cortisol concentration after both exercises differed from each other, while the maxima of ACTH were similar [30]. ACTH concentration declined more slowly during the recovery after the marathon than after the treadmill and Cortisol concentration was below baseline 24 h later [30,21]. In comparison, with men studied earlier, female marathon runners showed lower baseline concentrations and larger increases in ACTH concentration after both types of exercise [30,21].
To sum up, running yields to a stimulation of ACTH with secondary release of Cortisol. Situations concerning androgens – e.g. Testosterone – is probably less clear whereby a decrease after running seem to occur [20,28,31-33].
Broad Interaction and Stimuli Release
Focusing broader on Hypothalamus Hormones Dessypris et al. [20] measured C, ACTH, Thyroid Stimulating Hormone (TSH) and Anti Diuretic Hormone (ADH) concentrations in runners starting at a marathon race. After the race middle concentrations of C, ACTH and ADH were significantly increased [20]. Interestingly TSH was not altered, implying a main mediator function of CRH-ACTH System [20]. The change of ADH probably result due to interaction with RAAS (Renin-Angiotensin-Aldosterone System) respectively ACTH. Addressing an even broader view for obese human findings indicate that cortisol respectively testosterone homeostasis is altered [3]. It was shown for adipose people, that C levels were normal or even low compared, but when corrected for mass per distribution volume increased [34]. Further ACTH response was reduced and Cortisol response was better in normally weighted than adipose humans [35].
Further distance of running has an effect of release of Cortisol and Testosterone [13]. For three groups of runners (100, 1500, 10000 meters respectively) ACTH and Cortisol were measured pre- versus post-race, whereby runners showed a significant increase in ACTH and Cortisol after the race and percentage increase in 1500 Meter and 10000 Meter was higher than in shorter distance [13].
A further study explored changes in ACTH before and after a 4 h pedestrian race with measurements 10 min before and after the race [36]. After the race C-levels was 2.8 fold and ACTH-levels 3.5 fold increased [36]. A significant positive correlation between increase of ACTH and the absolved distance could be detected and between the increase of basal and post-race values of Cortisol and ACTH [36].
Effects of different exhaustive training programs on plasma levels of ACTH and Cortisol were elucidated. Runners were divided into a Sprint-Interval group, an endurance group or a combination [37]. Training was observed during 10 weeks and treadmill tests were conducted after 2, 4, 6, 8 and 10 weeks [37]. Significant exercise-induced increases in plasma ACTH and cortisol were observed for both pre- and post-training tests in all training groups. The Sprint-Interval group demonstrated significant post-training increases in ACTH and cortisol concentrations in response to maximal exercise implying that with Interval training responsiveness of ACTH system can be trained [37]. These findings could not be detected for other training methods pointing out relevance of Interval training. Immunological changes of hard strength- versus endurance training were part of further investigations, whereby Cortisol, ACTH, IL-6 increased 5 h after workout [38]. Physical activity increased ACTH, C as well as Leukocytes and T-cells but yielded to a decrease of B-cells [39]. A relationship between psychical activation and immune response via Interleukin and Cortisol could be shown [40]. Hints exist that not only CRH stimulates ACTH release but also ADH whereby it`s likely to suggest that an interaction with RAAS occurs [41]. As already mentioned, intensity seems to have an influence on ACTH release. Hints exist, that moderate physical activity (50-80% of VO2max) does not yield to a ACTH release but higher intensities of more than 90% of VO2max are necessary [42]. To conclude, hints exist that amount of Cortisol secretion is directly proportional to running intensity and running time [43].
Stimuli Already Centrally Released and Modulable Over Time
The pre-modulator CRH seems to be affected by running. The answer of CRH was analyzed while running on a treadmill by endurance athletes [44]. The mean plasma level of CRH increased 4.9 fold in men and 7 fold in women [44]. Concentration of Corticotropin increased significantly while anaerobic exercise tests. An answer on sub-maximal race without exhaustion could not detect significant changes [44].
Plasma concentrations of Aldosterone as well as ACTH were investigated while different tread mill intensities (50, 70 and 90% of VO2max) [45]. Men with little training and two groups of men with different training state (moderately trained, 15-25 miles/week, highly trained, more than 45 miles/week) were investigated concerning aldosterone homeostasis [45]. Acute physical activity was associated with increased Plasma aldosterone and ACTH activity, in all groups with intensities of 70 and 90% of VO2max [43]. There were now differences in trained and untrained [45]. The activity based increase of Plasma aldosterone concentration did not correlate with other regulatory concentrations [45].
The Hypothalamus-pituitary adrenal gland as well as the gonadal axis was analyzed in elderly long distance runners (68.9 +/- 4.2 years; training: 65 +/- 20 km/Week in the last 20 years) and in sedentary older subjects (year: 69.1 +/- 2.6 years) through an aerobe training while 20 Weeks (3 times per week/ 30-60 min running) [46]. Also, a Dexamethasone suppression test which induced an adrenal suppression, CRH and LHRH Stimulation as well as an exercise challenge (30 min Ergometer with 65% VO2max) was conducted [46]. Basal and post-DEX Plasma concentration of ACTH, Cortisol, LH, FSH and T differed not between runners and sedentary older subjects after Dexamethasone suppression test but interestingly, the basal free T was significantly lower in RUN versus SED [46].
The influence on hypothalamus was explored in a 6-week training (6 times a week) on Bike ergormeter while 31-33 min during 4 days each week with 90-96% (week 1-3) and 89-92% (week 4-6) at 4 mmol Lactate threshold on the first day and on day 21, with Interval training 3-5 x 3-5 min additionally every 2 days per week 117-127% and 115-110% [47]. A combined serum hormone level and pituitary test were made with artificial TRH, GNRH, CRH before and 6 weeks after training and 3 weeks after recreation [47]. TSH and STH did not change [47]. ACTH was increased after training and C-release was reduced after training period [47]. This had no influence of activity-induced Serum hormone levels (C, aldosterone, insulin, FSH, LH, TSH, ACTH, ADH and STH), which did not show any dependence from training except free T, which showed a significant decrease and post-exercise ACTH which showed an increase [47]. Cortisol sensitivity and ACTH Answer or Adaptation (FSH & LH answer) changed T-levels [47].
Arginine Vasopressin (AVP) and Atrial Natriuretic Peptide (ANP) are besides Aldosterone Hormones affecting fluid balance [48]. Endurance trained runners completed three separate test protocols with an increase of aldosterone after high-intensity training, steady-state training and longer endurance training [48]. Changes in fluid homeostasis was significantly different between ultra-marathon versus high-intensity and steady-state running, but only statistic significant increases pre- to post-run could be detected for ultra-marathon AVP, Cortisol, corticosterone, deoxycortisol and a significant post-run increase of Aldosterone after high-intensity training was detected [48].
Exercise is known to be a powerful stimulus for the endocrine system [24]. The hormonal response to exercise is dependent on several factors including the intensity, duration, mode of exercise (endurance versus resistance), and training status of the subject [24]. Serum cortisol and prolactin showed distinct rises 1 h after the race and returned to baseline 1 week later [24]. Androstenedione and dehydroepiandrosterone sulphate did not show any changes. Total testosterone as well as free testosterone dropped significantly 1 h after the race but returned to baseline 1 week later [24].
Cortisol, testosterone, free testosterone and the ratio between free testosterone and cortisol were monitored in six athletes participating in a marathon starting at 3,860 and finishing at 3,400 meters, having reached the top at 5,100 m altitude [28]. Blood was drawn at sea level before the departure for the mountain area, after a week of acclimatization, immediately after the marathon and after a 24-hour recovery period from the run [28]. Cortisol increased after acclimatization and especially after the marathon and decreased to normal values after recovery whereas Testosterone decreased after acclimatization, especially after the run and presented a partial recovery 24 h after the race [28].
Further hints exist from runners, cyclists, and skiers participating in a 161-km ultra-endurance race on a snow-packed course in the Alaskan wilderness [49]. ACTH increased significantly with no difference among runners, cyclists, and skiers pre-race versus post-race [49]. Cortisol increased significantly pre-race to post-race and post-race cortisol was significantly higher in runners versus skiers [49]. Norepinephrine increased significantly pre-race to post-race with no difference among divisions, Epinephrine did not change significantly during the race [49]. These data suggest activation of both the sympathetic-adrenal-medullary and hypothalamic-pituitary-adrenocortical axes from an ultra-endurance race in the cold and reveal the degree of stress hormone responses to this exhausting bout of exercise [49]. Concerning chronic effects (4 weeks) – sensitivity of system increased through training – (probably meant higher cortisol, testosterone answer through training).
Negative relationships between circulating testosterone and certain stress hormones (i.e., cortisol and prolactin) in humans were several time reported [50,51]. These relationships have subsequently been used in hypotheses explaining the subclinical resting testosterone levels often found in some endurance-trained males [51]. Endurance-trained males volunteered to run at 100% of their ventilatory threshold on a treadmill until volitional fatigue [51]. Blood samples were taken at pre-exercise baseline (B0); volitional fatigue (F0); 30 min (F30), 60 min (F60), and 90 min (F90) into recovery; and at 24 h post-baseline (P24 h). At F0 [mean running time = 84.8 (3.8) min], exercise induced significant changes from B0 in total testosterone, cortisol and prolactin [9]. All three of these hormones were still significantly elevated at F30; but at F60 only cortisol and prolactin were greater than their respective B0 values [51]. Free testosterone displayed no significant changes from B0 at F0, F30, or the F60 time point [51]. At F90, neither cortisol nor prolactin was significantly different from their B0 values, but total and free testosterone were reduced significantly from B0 [51]. Cortisol, total testosterone and free testosterone at P24 h were significantly lower than their respective B0 levels [51]. Significant negative relationships existed between peak cortisol response (at time F30) versus total testosterone (at F90 and at P24 h) [51]. In conclusion, the present findings give credence to the hypothesis suggesting a linkage between the low resting testosterone found in endurance-trained runners and stress hormones, with respect to cortisol [51].
Taking an internal medicine perspective the evoking of Stress yields to an activation of HPA Axis over the CRH-ACTH System, whereby in improper work of sympathetic and parasympathetic nerve function illness processes are suggested [52]. Physical activity is a potent stimuli of HPA axis whereby intensity as well as time of running respectively physical activity is relevant [53] Hints exist that training does yield to a permanent increase of Cortisol within physiological borders [54].
Based on the above mentioned on CRH-ACTH System through running the secondary release of C can be used as marker of overtraining respectively to detect potential overreaching for other stress-related responses. In principle e.g. with BORG-Scale (6-20) fatigue can easily be measured without difficulty, whereby high validity of BORG-Scale (6-20) was shown even for large population [55,56]. Garcin et al. 2002 used BORG-Scale in young runners (middle distances) and could show, that an overtraining can easily being detected [55,15]. Furthermore, especially measurement of salivary cortisol has proven to be an easy way in order to detect an overtraining [57,58].
The pathophysiology of the condition is still unknown. Hypothalamic-pituitary function was studied by determining the hormonal responses to insulin-induced hypoglycemia in five asymptomatic male marathon runners during a 4-month period in which they ran 42-, 56-, and 92-km races and in four overtrained male athletes [4]. The response of the asymptomatic runners was not different when tested 1 month before and within 48 h after the 42- and 92-km races [59]. The plasma cortisol, ACTH, GH, and PRL responses to insulin-induced hypoglycemia in the four overtrained athletes were lower than their responses after the rest and lower than the responses of the asymptomatic runners [59,4]. From a normal answer of this and other hormones on TRH and LHRH was concluded that overtraining has a hypothalamic and not pituitary origin [60].
Interestingly, for chronic fatigue syndrome Hydrocortisone was used with success [61,62]. Interestingly, already Pawlow implied that with a stronger stimuli answer first increase to decrease after the peak (inverted U-shape) [63-65]. Even the involving of T and C has probably an influence on phenotype [4]. Hints can be found, that highly trained athletes show increased basal levels of Cortisol and ACTH [66]. The above mentioned is in accordance with the cytokine-hypothesis of overtraining [23]. Cytokine-hypothesis sees the local muscle damage induced through to intensive training which is a chronic process [23,64]. An initially local inflammation process can become chronic and have systemic effects e.g. via Interleukins [23,64]. Especially for Interleukin 6, which was taxed as exercise factor, intensity dependent release was described [57,59]. In combination with the mentioned of Cortisol and Testosterone metabolism skeletal musculature with a weight of 15 kg and biggest of human body – this can probably act as puffer for exercise stimuli. This suggestion as puffer organ can be combined with the thought that somatotype has substantial influence on performance capacity [67]. This implies that better trained persons have better organic conditions and can in consequence better persist huge stimuli. The possibilities stimuli are perceived and adaptions take place are large [2]. Even small effects are described such as reduced oxygen pressure in higher altitudes proning phenotype and implying high sensitivity of skeletal muscle to external stimuli [68-70]. For athletes’ in overtraining ACTH levels in rest were the same to control but cortisol was deeper [67].
The relationship of Testosterone and Cortisol
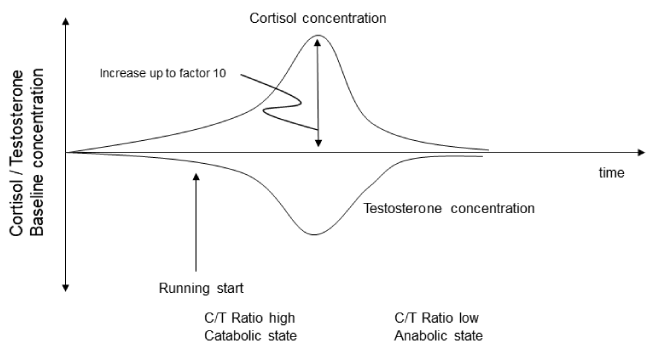
Focusing away from effects of physical activity on Cortisol also androgens respectively Testosterone can be mentioned. The answer seems to be different e.g. Testosterone decreased by -46% 5-days after a military field training [71]. The combination of these two hormones yield to the Testosterone / Cortisone Ratio (T/C). The relationship is often used as indicator of anabolic/catabolic balance and was also used for psychophysics function [72-74]. Also in woman increased testosterone levels were detected in endurance trained versus inactive persons [75] (Figure 2).
The intensity of training influences Cortisol and T respectively the ratio T/C [73]. The hydration state can further modify and regulate the anabolic/catabolic balance [73]. Influence of running upon T, C and T/C Ratio, when started in a hypohydrated state was analyzed [73]. Runners completed four 10-min treadmill runs whereby hydration state differed (euhydrated or hypohydrated), furthermore intensity was variable between (70% or 85% VO2max) [73]. Body weight, urine osmolality and specific weight were measured before and 20 min post-exercise with T, C and Ratio T/C [73]. Except for heart rate while 70% VO2max exercises, heart rate and lactate were about equal between euhydration and hypohydration in given intensity [73]. Furthermore hypohydration had no measurable influence on T-concentration [73]. Although intensity had no influence upon T, Cortisol concentration were higher while hypohydration as compared to euhydration before and 20 min post-exercise [73]. Furthermore, T/C was significantly lower 20 min Post-exercise with 70 % VO2max when individuals were hypohydrated before (T/C = 0.055) versus euhydrated (T/C = 0.072) [41]. These results imply that depending from hydration status T/C is changed and therefore anabolic/catabolic balance [73]. A further study analyzed testosterone/cortisol (T/C) ratio in long-distance runners during a relay competition and during the three days following the competition [76]. Two teams of four relay sub-elite runners (VO2max = 67.0 ml x min(-1) x kg(-1) in males and 56.8 ml x min(-1) x kg(-1) in females) took part in a six-hour relay race [76]. During the race, cortisol levels reached approximately 1.5-fold basal levels [76]. These levels remained high till late evening, (higher than morning values, when normal resting levels are 4 to 6-folds lower) [76]. Surprisingly, wakening levels during the following days were lower than resting levels [76]. Testosterone did not vary in females; then, male values only are reported [76]. During the race they decreased gradually and remained low till night [76]. During the following three days, testosterone levels were higher than resting day levels. The T/C ratio amplifies these variations: low during the race till retiring, (currently associated with a catabolic tendency) and reversely high during the following three days (associated with a high anabolic tendency) [76].
As expected, a catabolic tendency occurs during a long distance run (increase in cortisol level followed by a drop in testosterone level) [76]. More surprising is the high anabolic tendency noted during the recovery period (low cortisol and high testosterone levels) [76].
Ten endurance athletes’, 3-settings, Interval Training (INT), Tempo Race (TEMP) and body weight Circuit Training (CIR), during several days [11]. Blood and salivary pre- and 0, 15, 30 and 60 min post-exercise whereby peak post-exercise salivary cortisol was significantly higher as pre-exercise in all trials [11]. After INT, salivary cortisol remained increased after 60 min post-exercise. Salivary gland T increased in all trials significantly [11]. Peak C and T levels increased in the same time in plasma and salivary [11]. Further, effects of 4 weeks moderate aerobic exercise on outcome measures of saliva stress hormones in healthy adult volunteers were elucidated [1]. C increased significantly from -48 h and displayed a baseline overshoot with +24 h being significantly lower than -48 and +0 h, fT decreased significantly from -48 h to +0 h and remained lower at +24 h and +48 h [1]. After 4 weeks of exercise, there were significant increases in cortisol and free testosterone levels, along with significant decrease in the ratios between testosterone and cortisol levels (T/C) [50].
Changes of hormonal and immune factors were analyzed while a 5000 m race [77]. Salivary samples were analyzed for C, T whereby T/C ratios were significantly reduced pre versus post-race [77].
Some further hints exist from other endurance sports such as rowing and cycling. While 9 Months of Olympic Games elite rowers were analyzed with T/C Ratios and it was implied that T/C ratios are reduced during rowing training [60]. Findings seem to be valid not only for rowing but also for cycling. Post training, resting testosterone levels decreased from and resting cortisol levels increased from pre training to post training indicating a catabolic state [16].
This yields to the question if reduced training changes T/C ratios. Changes in well-trained runners seem to be within physiological borders [54]. In runners with good level (VO2max 60 ml/kg/min) reduction of Training (80 km to 25 km) yielded only to a decrease of Cortisol within upper area of physiological borders to lower areas [54]. Well-trained runners have a T-level in the lower range of physiological borders and Cortisol in increased range yielding to a decreased T/C ratio [54]. Undoubtedly overtraining influences ability of a body to respond adequately upon stress [78]. As reaction on stimuli body cannot relax and lower Cortisol levels were detected [78].
Changes in endurance capacity are not only associated with hormonal but also with structural and biochemical changes especially of mitochondria [79]. Earlier studies concerning endurance training in women show for untrained young woman running three times a week on aerobic threshold that ACTH was increased 5 min after physical activity and less elevated 30 min later [80]. Maximal lactate and BORG-Scale were unchanged as indicator for stable exertion [80]. These results implicate, that endurance training stimulates hormonal response via ACTH with significantly lower concentrations of ACTH [80].
FT was compared between high-intensity Interval training and Steady State Endurance Training (SSE) [81]. Interval was while 90 sec with 100-110% maximal oxygen uptake (VO2max) and 90-sec active break at 40% of VO2max while 42-47 min [20]. The SSE Session consisted of a 45-min course in 60-65% VO2max, total work output was the same for both exercises [81]. A 45-min supine rest control session (CON) was additionally conducted. All three sessions took place on different days [81]. Pre-session and 12-h post-session (12POST) Blood samples were collected and used FT, SHBG, LH, 3- α-androstanediol glucuronide (3-α Diol G) and cortisol [81]. IE and SSE yielded to an increase of FT with larger extent after IE [81]. 5α-reductase marker 3-α Diol G answer with 12POST IE was significantly increased while FT was significantly reduced; whereby such a change did not occur with SSE [81]. These results imply that FT through Androgen sensitive tissue is more responsible on IE than SSE [81]. IE yielded to a larger increase than Steady-state Training [81]. The decrease of catecholamine excretion during night could be a result of a reduced sympathetic activity, whereby it´s unclear if this has its origin in central nerves system [82]. Results could imply that this is due to a reduction of regeneration potential being a predisposition e.g. for overtraining [82].
In eight middle distance runners and nine long distance runners an increase in intensity and training volume was conducted [82]. Seven runners participated in both studies [82,35]. The average training volume was 85.9 km and increased to 176.6 km/Week while intensity was increased (ITV) [82].
A Plateau in endurance performance and a decrease in max performance were detected probably due to an overtraining [82]. The nightly catecholamine exertion was reduced (47-53%), in contrast to exercise-related plasma catecholamine answer, which was increased [82]. Rest and activity dependent Cortisol and Aldosterone levels were reduced [82]. Interestingly, even animals seem to have the ability to control hormone homeostasis as it was shown that mice can influence hormone concentrations through using of a walk wheel inducing a modulating effect on phenotype [83]. To sum up, endurance seems to yield to an increase in Cortisol levels while Testosterone levels seem to decrease allowing speculating that continuous endurance activity yields to a new phenotype clearly sensitive upon training regime.
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004; 432: 345-352. https://goo.gl/yUpRco
- Hoppeler H, Baum O, Matthias Mueller M, Glenn Lurman G. Molekulare Mechanismen der Anpassungsfähigkeit der Skelettmuskulatur. Schweizerische Zeitschrift fur Sportmedizin und Sporttraumatologie. 2011; 59: 6-13. https://goo.gl/NZGJaa
- Zintl F. Ausdauertraining: Grundlagen, Methoden, Trainingssteuerung. (4. Aufl.) Wien, Zurich: BLV; 1997. https://goo.gl/oG4Mvk
- Chakravarthy MV, Booth FW. Eating, exercise, and "thrifty" genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol. 2004; 96: 3-10. https://goo.gl/K7C97v
- Steffny H. Optimales Lauftraining. Sudwestverlag, Munchen. 2010. https://goo.gl/3aCyJs
- Silbernagel S. Taschenatlas der Physiologie, 6. Auflage, Thieme, Berlin. 2000; 228-231.
- Schmikli SL, Brink MS, De Vries WR, Backx FJ. Can we detect non-functional overreaching in young elite soccer players and middle-long distance runners using field performance tests? Br J Sports Med. 2011; 45: 631-636. https://goo.gl/NyDUTo
- Anderson T, Lane AR, Hackney AC. Cortisol and testosterone dynamics following exhaustive endurance exercise. Eur J Appl Physiol. 2016; 116: 1503-1509. https://goo.gl/Zc4jad
- Hill EE, Zack E, Battaglini C, Viru M, Viru A, Hackney AC. Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest. 2008; 31: 587-591. https://goo.gl/oKQpse
- Oltras CM, Mora F, Vives F. Beta-endorphin and ACTH in plasma: effects of physical and psychological stress. Life Sci. 1987; 40: 1683-1686. https://goo.gl/pYykZJ
- Tanner AV, Nielsen BV, Allgrove J. Salivary and plasma cortisol and testosterone responses to interval and tempo runs and a bodyweight-only circuit session in endurance-trained men. J Sports Sci. 2014; 32: 680-689. https://goo.gl/q5FstM
- Powell J, DiLeoT, Roberge R, Coca A, Kim JH. Salivary and serum cortisol levels during recovery from intense exercise and prolonged, moderate exercise. Biol Sport. 2015; 32: 91-95. https://goo.gl/8tcwWc
- Petraglia F, Barletta C, Facchinetti F, Spinazzola F, Monzani A, Scavo D, et al. Response of circulating adrenocorticotropin, beta-endorphin, beta-lipotropin and cortisol to athletic competition. Acta Endocrinol (Copenh). 1988; 118: 332-336. https://goo.gl/kSmqU9
- Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: Is skeletal muscle an endocrine organ? Exerc Sport Sci. Rev.2005; 33: 114-119. https://goo.gl/eTLNqL
- Lundberg IE, Nader GA. Molecular effects of exercise in patients with inflammatory rheumatic disease. Nat Clin Pract Rheumatol. 2008; 4: 597-604. https://goo.gl/k7x8Vj
- Hoogeveen AR, Zonderland ML. Relationships between testosterone, cortisol, and performance in professional cyclists. Int J Sports Med. 1996; 17: 423-428. https://goo.gl/A4ZbYQ
- Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm. 2008; 2008: 109502. https://goo.gl/7kHdBw
- Stefanyk LE, Dyck DJ. The interaction between adipokines, diet and exercise on muscle insulin sensitivity. Curr Opin Clin Nutr Metab Care. 2010; 13: 255-259.https://goo.gl/6DpS5s
- Steinberg GR, Watt MJ, Febbraio MA. Cytokine regulation of AMPK signalling. Front Biosci (Landmark Ed). 2009; 14: 1902-1916. https://goo.gl/g852Ru
- Pestell RG, Hurley DM, Vandongen R. Biochemical and hormonal changes during a 1000 km ultramarathon. Clin Exp Pharmacol Physiol. 1989; 16: 353-361. https://goo.gl/TNcPR7
- Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006; 16: 3-63. https://goo.gl/Zqw8mJ
- Robson Ansley PJ, Blannin A, Gleeson M. Elevated plasma interleukin-6 levels in trained male triathletes following an acute period of intense interval training. Eur J Appl Physiol. 2007; 99: 353-360. https://goo.gl/AVfesa
- Smith LL. Cytokine hypothesis of overtraining: a physiological adaptation to excessive stress? Med Sci Sports Exerc. 2000; 32: 317-331. https://goo.gl/SCK5n2
- Karkoulias K, Habeos I, Charokopos N, Tsiamita M, Mazarakis A, Pouli A, et al. Hormonal responses to marathon running in non-elite athletes. Eur J Intern Med. 2008; 19: 598-601. https://goo.gl/6AfvHB
- França SC, Barros Neto TL, Agresta MC, Lotufo RF, Kater CE. Divergent responses of serum testosterone and cortisol in athlete men after a marathon race. Arq Bras Endocrinol Metabol. 2006; 50: 1082-1087. https://goo.gl/nGE3ck
- Niessner A, Ziegler S, Slany J, Billensteiner E, Woloszczuk W, Geyer G. Increases in plasma levels of atrial and brain natriuretic peptides after running a marathon: are their effects partly counterbalanced by adrenocortical steroids? Eur J Endocrinol. 2003; 149: 555-559. https://goo.gl/kh65JE
- Ponjee GA, De Rooy HA, Vader HL. Androgen turnover during marathon running. Med Sci Sports Exerc. 1994; 26: 1274-1277. https://goo.gl/HyPSTz
- Marinelli M, Roi GS, Giacometti M, Bonini P, Banfi G. Cortisol, testosterone, and free testosterone in athletes performing a marathon at 4,000 m altitude. Horm Res. 1994; 41: 225-229. https://goo.gl/i2x5AN
- Maron MB, Horvath SM, Wilkerson JE. Acute blood biochemical alterations in response to marathon running. Eur J Appl Physiol Occup Physiol. 1975; 34: 173-181. https://goo.gl/ZK1epK
- Heitkamp HC, Huber W, Scheib K. beta-Endorphin and adrenocorticotrophin after incremental exercise and marathon running--female responses. Eur J Appl Physiol Occup Physiol. 1996; 72: 417-424. https://goo.gl/QEZkSM
- Dessypris A, Wagar G, Fyhrquist F, Makinen T, Welin MG, Lamberg BA. Marathon run: effects on blood cortisol - ACTH, iodothyronines - TSH and vasopressin. Acta Endocrinol (Copenh). 1980; 95: 151-157. https://goo.gl/gaXWCC
- Heitkamp HC, Schmid K, Scheib K. Beta-endorphin and adrenocorticotropic hormone production during marathon and incremental exercise. Eur J Appl Physiol Occup Physiol. 1993; 66: 269-274. https://goo.gl/jTAH4h
- Philipp E, Wilckens T, Friess E, Platte P, Pirke KM. Cholecystokinin, gastrin and stress hormone responses in marathon runners. Peptides. 1992; 13:125-8. https://goo.gl/unKdtV
- Roelfsema F, Pereira AM, Veldhuis JD. Impact of adiposity and fat distribution on the dynamics of adrenocorticotropin and cortisol rhythms. Curr Obes Rep. 2014; 3: 387-395. https://goo.gl/Mmdqd9
- Azarbayjani MA, Vaezepor F, Rasaee MJ, Tojaril F, Pournemati P, Jourkesh M, et al. Daily timing of salivary cortisol responses and aerobic performance in lean and obese active females. Bratisl Lek Listy. 2011; 112: 213-217. https://goo.gl/GvA9He
- Estorch M, Fuente T, Serra-Grima R, Flotats A, Berna L, Sanz D, et al. The effect of a race 4 hours in duration on the production of beta-endorphin and adrenocorticotropic hormone. Med Clin (Barc). 1998; 111: 770-773. https://goo.gl/cKBbVV
- Kraemer WJ, Fleck SJ, Callister R, Shealy M, Dudley GA, Maresh CM, et al. Training responses of plasma beta-endorphin, adrenocorticotropin, and cortisol. Med Sci Sports Exerc. 1989; 21: 146-153. https://goo.gl/cakwga
- Risoy BA, Raastad T, Hallen J, Lappegard KT, Baverfjord K, Kravdal A, et al. Delayed leukocytosis after hard strength and endurance exercise: Aspects of regulatory mechanisms. BMC Physiol. 2003; 3: 14. https://goo.gl/94XK7s
- Park HY Nam SS, Tanaka H Lee DJ. Hemodynamic, hematological, and hormonal responses to submaximal exercise in normobaric hypoxia in pubescent girls. Pediatr Exerc Sci. 2016; 28: 417-422. https://goo.gl/juiExM
- Wolkow A, Aisbett B, Reynolds J, Ferguson SA, Main LC. Acute Psychophysiological relationships between mood, inflammatory and cortisol changes in response to simulated physical firefighting work and sleep restriction. Appl Psychophysiol Biofeedback. 2016; 41: 165-180. https://goo.gl/ZhGAMX
- Smoak B, Deuster P, Rabin D, Chrousos G. Corticotropin-releasing hormone is not the sole factor mediating exercise-induced adrenocorticotropin release in humans. J Clin Endocrinol Metab. 1991; 73: 302-306. https://goo.gl/NExmv5
- Rahkila P, Hakala E, Alen M, Salminen K, Laatikainen T. Beta-endorphin and corticotropin release is dependent on a threshold intensity of running exercise in male endurance athletes. Life sci. 1988; 43: 551-558. https://goo.gl/iVspVE
- Bonen A. Effects of exercise on excretion rates of urinary free cortisol. J Appl Physiol. 1976; 40: 155-158. https://goo.gl/eMfySz
- Rahkila P, Hakala E, Salminen K, Laatikainen T. Response of plasma endorphins to running exercises in male and female endurance athletes. Med Sci Sports Exerc. 1987; 19: 451-455. https://goo.gl/AHKnnV
- Luger A, Deuster PA, Debolt JE, Loriaux DL, Chrousos GP. Acute exercise stimulates the renin-angiotensin-aldosterone axis: adaptive changes in runners. Horm Res. 1988; 30: 5-9. https://goo.gl/GJQcH1
- Struder HK, Hollmann W, Platen P, Rost R, Weicker H, Kirchhof O, et al. Neuroendocrine system and mental function in sedentary and endurance-trained elderly males. Int J Sports Med. 1999; 20: 159-166. https://goo.gl/gP3ghR
- Lehmann M1, Knizia K, Gastmann U, Petersen KG, Khalaf AN, Bauer S, et al. Influence of 6-week, 6 days per week, training on pituitary function in recreational athletes. Br J Sports Med. 1993; 27: 186-192. https://goo.gl/vKBZDD
- Hew-Butler T, Noakes TD, Soldin SJ, Verbalis JG. Acute changes in endocrine and fluid balance markers during high-intensity, steady-state, and prolonged endurance running: unexpected increases in oxytocin and brain natriuretic peptide during exercise. Eur J Endocrinol. 2008; 159: 729-737. https://goo.gl/5kvNJu
- Stuempfle KJ, Nindl BC, Kamimori GH. Stress hormone responses to an ultraendurance race in the cold. Wilderness Environ Med. 2010; 21: 22-27. https://goo.gl/imX3CT
- Alghadir AH, Gabr SA, Aly FA. The effects of four weeks aerobic training on saliva cortisol and testosterone in young healthy persons. J Phys Ther Sci. 2015; 27: 2029-2033. https://goo.gl/YJd3FR
- Daly W, Seegers CA, Rubin DA, Dobridge JD, Hackney AC. Relationship between stress hormones and testosterone with prolonged endurance exercise. Eur J Appl Physiol. 2005; 93: 375-380. https://goo.gl/d35Xc6
- Egloff N, Egle UT, von Kanel R. Therapie zentralisierter Schmerzstörungen. Praxis. 2009; 98: 271-283. https://goo.gl/1cKt5s
- Duclos M, Tabarin A. Exercise and the Hypothalamo-Pituitary-Adrenal Axis. Front Horm Res. 2016; 47: 12-26. https://goo.gl/nwp8zt
- Houmard JA, Costill DL, Mitchell JB, Park SH, Fink WJ, Burns JM. Testosterone, cortisol, and creatine kinase levels in male distance runners during reduced training. Int J Sports Med. 1990; 11: 41-45. https://goo.gl/iCWBC8
- Garcin M, Fleury A, Billat V. The ratio HLa: RPE as a tool to appreciate overreaching in young high-level middle-distance runners. Int J Sports Med. 2002; 23: 16-21. https://goo.gl/BLKP78
- Scherr J, Wolfarth B, Christle JW, Pressler A, Wagenpfeil S, Halle M. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol. 2013; 113: 147-155. https://goo.gl/xb6sFV
- McLean BD, Coutts AJ, Kelly V, McGuigan MR, Cormack SJ. Neuromuscular, endocrine, and perceptual fatigue responses during different length between-match microcycles in professional rugby league players. Int J Sports Physiol Perform. 2010; 5: 367-383. https://goo.gl/EZb43J
- Papacosta E, Nassis GP. Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise science. J Sci Med Sport. 2011; 14: 424-434. https://goo.gl/dWaubn
- Barron JL, Noakes TD, Levy W, Smith C, Millar RP. Hypothalamic dysfunction in overtrained athletes. J Clin Endocrinol Metab. 1985; 60: 803-806. https://goo.gl/V2gAT5
- Vervoorn C, Quist AM, Vermulst LJ, Erich WB, de Vries WR, Thijssen JH. The behaviour of the plasma free testosterone/cortisol ratio during a season of elite rowing training. Int J Sports Med. 1991; 12: 257-263. https://goo.gl/dLBsqX
- McKenzie R, O'Fallon A, Dale J, Demitrack M, Sharma G, Deloria M, et al. Low-dose hydrocortisone for treatment of chronic fatigue syndrome: a randomized controlled trial. JAMA. 1998; 280: 1061-1066. https://goo.gl/D9DbEZ
- Cleare AJ, Heap E, Malhi GS, Wessely S, O’Keane V, Miell JP. Low-dose hydrocortisone for chronic fatigue syndrome: a randomized crossover trial. Lancet. 1999; 353: 455-458. https://goo.gl/tW97uY
- Mateeff D. Muskelermüdung und allgemeine Ermüdung; I. Teil. Theorie & Praxis der Körperkultur. 1957; 6: 718-727.
- Vogel, R. Übertraining: Begriffsklärungen, ätiologische Hypothesen, aktuelle Trends und methodische Limiten. Schweizerische Zeitschrift für Sportmedizin und Sporttraumatologie. 2001; 49: 154-162. https://goo.gl/cKXqu1
- Wassiljewa WWW. Einige Bemerkungen zu den Mechanismen der Ermüdung und des Übertrainings. Theorie & Praxis der Körperkultur. 1955; 4: 207-215.
- Luger A, Deuster PA, Gold PW, Loriaux DL, Chrousos GP. Hormonal responses to the stress of exercise. Adv Exp Med Biol. 1988; 245: 273-280. https://goo.gl/ugjxvv
- Kandel M, Baeyens JP, Clarys P. Somatotype, training and performance in Ironman athletes. Eur J Sport Sci. 2014; 14: 301-308. https://goo.gl/9JF43r
- Schaeffer PJ, Villarin JJ, Lindstedt SL. Chronic cold exposure increases skeletal muscle oxidative structure and function in Monodelphis domestica, a marsupial lacking brown adipose tissue. Physiol Biochem Zool. 2003; 76: 877-887. https://goo.gl/KQJV6n
- Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, et al. The metabolic role of IL-6 produced during exercise: is IL-6 an exercise factor? Proc Nutr Soc. 2004; 63: 263-267. https://goo.gl/WCWZ2i
- Terrados N. Altitude training and muscular metabolism. Int J Sports Med 1992; 13: 206-209. https://goo.gl/19vF7o
- Vaara JP, Kalliomaa R, Hynninen P, Kyrolainen H. Physical fitness and hormonal profile during an 11-week paratroop training period. J Strength Cond Res. 2015; 29: 163-167. https://goo.gl/r4qTzE
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999; 22: 422-426. https://goo.gl/HXSQXk
- Maresh CM, Whittlesey MJ, Armstrong LE, Yamamoto LM, Judelson DA, Fish KE, et al. Effect of hydration state on testosterone and cortisol responses to training-intensity exercise in collegiate runners. Int J Sports Med. 2006; 27: 765-770. https://goo.gl/G32fe6
- Talbott, S. The Cortisol Connection. Why Stress makes you fat and ruins your health - and what you can do about it. Hunter House; 2007. https://goo.gl/MXgtRt
- Lindholm C, Hirschberg AL, Carlström K, von Schoultz B. Hormone anabolic/catabolic balance in female endurance athletes. Gynecol Obstet Invest. 1993; 36: 176-180. https://goo.gl/Wy3cH2
- Lac G, Berthon P. Changes in cortisol and testosterone levels and T/C ratio during an endurance competition and recovery. J Sports Med Phys Fitness. 2000; 40: 139-144. https://goo.gl/DTTq7o
- Li CY, Hsu GS, Suzuki K, Ko MH, Fang SH. Salivary immuno factors, cortisol and testosterone responses in athletes of a competitive 5,000 m race. Chin J Physiol. 2015; 58: 263-269. https://goo.gl/p26jb3
- Gleeson M. Biochemical and immunological markers of over-training. J Sports Sci Med. 2002; 1: 31-41. https://goo.gl/deE2KW
- Puntschart A, Claassen H, Jostarndt K, Hoppeler H, Billeter R. mRNAs of enzymes involved in energy metabolism and mtDNA are increased in endurance-trained athletes. Am J Physiol. 1995; 269: 619-625. https://goo.gl/oR842U
- Heitkamp HC, Schulz H, Rocker K, Dickhuth HH. Endurance training in females: changes in beta-endorphin and ACTH. Int J Sports Med. 1998; 19: 260-264. https://goo.gl/84JCxy
- Hackney AC, Hosick KP, Myer A, Rubin DA, Battaglini CL. Testosterone responses to intensive interval versus steady-state endurance exercise. J Endocrinol Invest. 2012; 35: 947-950. https://goo.gl/r1zLYb
- Lehmann M, Gastmann U, Petersen KG, Bachl N, Seidel A, Khalaf AN, et al. Training-overtraining: performance, and hormone levels, after a defined increase in training volume versus intensity in experienced middle- and long-distance runners. Br J Sports Med. 1992; 26: 233-242. https://goo.gl/w8ZD4K
- Garland T Jr, Zhao M, Saltzman W. Hormones and the evolution of complex traits: Insights from artificial selection on behavior. Integr Comp Biol. 2016; 56: 207-224. https://goo.gl/M1CFFU
- Kenefick RW, Maresh CM, Armstrong LE, Castellani JW, Whittlesey M, Hoffman JR, et al. Plasma testosterone and cortisol responses to training-intensity exercise in mild and hot environments. Int J Sports Med. 1998; 19: 177-181. https://goo.gl/NyYDfh

Sign up for Article Alerts