Egyptian Case of Parry-Romberg Syndrome
Nada Elsaid*, Ahmed Saied and Mohamed Abd El-Salam
Department of Neurology, University of Mansoura, Egypt
*Address for Correspondence: Nada Elsaid, Faculty of Medicine, Department of Neurology, University of Mansoura, Egypt, Tel: +002-010-058-912-35;
E-mail: [email protected]
Submitted: 02 February 2018; Approved: 26 May 2018; Published: 30 May 2018
Citation this article: Elsaid N, Saied A, El-Salam MA. Egyptian Case of Parry-Romberg Syndrome. American J Rare Dis Diagn Ther. 2018;1(1): 001-003.
Copyright: © 2018 Elsaid N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Hemifacial atrophy; Parry-Romberg syndrome; Neck atrophy; Scleroderma
Download Fulltext PDF
Hemifacial atrophy aka Parry-Romberg syndrome is an idiopathic neurodegenerative disease characterized by insidious onset and gradually progressive course of atrophy of one side of the face. Several causes were proposed for its pathogenesis but malformation of cerebral sympathetic nervous system disturbing the fat metabolism has been proposed as a primary cause. The relation between Parry-Romberg Syndrome and localized scleroderma is debatable. Several associated conditions have been reported; alopecia and pigmentation of the involved skin, ocular disorders in 10- 35% of cases; neurologic disorders as focal epilepsy, headache and paroxysmal trigeminal neuralgia. The objective of this work is to discuss the general characteristics, etiology, physiopathology, differential diagnosis and treatment of progressive hemifacial atrophy through the presentation of a clinical case.
Introduction
Hemifacial atrophy aka Parry-Romberg syndrome is an idiopathic poorly understood neurodegenerative disease named after the 2 physicians who first reported this syndrome [1,2].
It is characterized by insidious onset and slowly progressive course of atrophy of one side of the facial structures including skin, muscles, and bones [3]. The extent of the atrophy is usually limited to unilateral facial affection, and the ipsilateral involvement of body is rare. Bilateral facial involvement was described in 5% to 10% of cases [4]. Typically, the onset of this disease starts insidiously within the first or the second decade of life with slowly progressive course over years and, then, it stabilizes. Earlier onset is associated with bigger consequences [4]. Atrophy that starts late in the 2nd decade of life is less remarkable because facial growth is almost complete. These outstanding cosmetic troubles impact the patients’ self-identity and psyche [5,6].
The other common features of this syndrome are the enophthalmos, the deviation of the angle of the mouth and nose to the diseased side. Several associated conditions have been reported; alopecia and pigmentation of the involved skin, ocular disorders in 10-35% of cases; neurologic symptoms as focal epilepsy, headache and paroxysmal trigeminal neuralgia. Oral manifestations may include hemiatrophy of the lip and tongue, short mandibular body and/or ramus of the mandible, delayed tooth eruption, and malformed tooth roots [3,7].
Case Report
In 2016, an 11 years old male patient was admitted to our department for assessment of progressive right facial atrophy of 4 months duration.
History
The patient and his relatives started to recognize facial asymmetry since 2 months. It was not associated with weakness of facial muscles (patient was able to raise his eyebrows, close his eyelids, blow his check), not associated with weakness of the muscles of mastication (patient was able to open and close the mandible, and able to perform side to side mandibular movements). The condition was not associated with sensory manifestations along the trigeminal distribution or any manifestations suggesting other cranial nerve affection. There were no symptoms suggestive of motor or sensory system affection involving upper and lower limbs. No history of seizures or loss of consciousness.
Past medical history, developmental history and review of his systems were unremarkable.
Examination
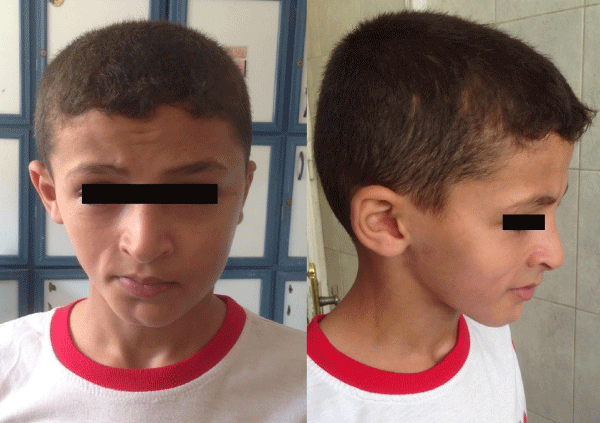
During the physical examination, it was noted that this patient presented facial asymmetry with a marked atrophy of the right side of the face, with deviation of lips and nose towards the right side, with an evident demarcation line between both sides (coup de sabre) in the right side of mandibular mentum region, extending upward to involve lips and nose producing marked depression. It was associated with wasting in the right side of the neck with no weakness. Mild right eye enophthalmy was noted (figure1). No associated hyperpigmentation on the affected skin or alopecia. Intraorally inspections showed no tongue atrophy or deviation. No atrophy noted in upper or lower limbs. Neurological examination was otherwise normal.
Work up
Radiological assessment via plain X-rays and Computed Tomography (CT) head scans showed no abnormalities. Anti-dsDNA, anti-single-stranded DNA (anti-ssDNA), Anticentromere (ACA) and Antinuclear (ANA) were negative. Rheumatoid Factor (RF) was also negative. Nerve conduction study of the facial nerve showed evidence of right facial nerve neuropathic affection with evidence of regeneration.
Discussion
Progressive hemifacial atrophy, as described in the case above, is a rare idiopathic degenerative condition affect not only the esthetic, but also the functionality of the attained hemiface.
Several causes were proposed for its pathogenesis but malformation of cerebral sympathetic nervous system disturbing the fat metabolism has been proposed as a primary cause [8,9]. The atrophic disease process was also proposed to follow the pattern of trigeminal nerve innervation [10]. The presence of autoantibodies as anti-double stranded DNA antibody (anti-dsDNA) in some cases suggested that this syndrome may be a localized form of scleroderma [11-13]. No available evidence of a mendelian inheritance [13]. Trauma, viral infections, endocrine disturbances are believed to be also associated to the pathogenesis of the disease [14]. The current consensus consider PRS as a spectrum of localized scleroderma (Morphoea) called linear scleroderma [15,16].
Clinically, the skin can be dry and hyperpigmented. Some patients present a demarcation line between normal and abnormal skin, resembling a big linear scar, known as “coup de sabre,” as could be noticed in our patient [4]. En coup the sabre is considered as a clinical form (linear Morphoea) not just a visual effect observed in PRS [17]. Ipsilateral exposition of teeth occurs when lips are involved. Mouth and nose are deviated to the affected side, deviating also facial and dental midlines. Ocular involvement is frequent, and the most common manifestation is the enophthalmus, due to fat loss around the orbit, as was observed with a mild degree in the present case. Occasionally, there may be some neurological disorders, such as paroxysmal trigeminal neuralgia, facial paresthesia, headache, intracranial aneurysm and contralateral focal seizures [4]. These complications were not found in our case.
Differential diagnoses include hemifacial microsomia aka Goldenhar syndrome or first and second branchial arch syndrome, but these are congenital non-progressive conditions. Other differential diagnosis is partial lipodystrophy (Barraquer-Simon Syndrome), but it is usually affect the adipose tissue bilaterally [14].
Rasmussen encephalitis is similar to Parry-Romberg syndrome as regard the age of onset, unilateral manifestation and occurrence of focal seizures [18].
Parry-Romberg Syndrome is self limited condition with no definitive treatment. However, generally the course of the Morphea disease is unpredictable and severe functional and cosmetic disability may result. Few prognostic studies have been conducted, but findings to date suggest that the disease tends to run a chronic or intermittent-recurrent course and frequently causes sequelae. At the more severe end of the spectrum, the disease can progress over years and cause significant atrophy, growth retardation, irreversible structural deformities, joint contractures and severe functional, cosmetic and psychological disabilities [17,19,20]. Affected patients should have multi-speciality approach provided by physicians, dentists, phonoaudiologists, and psychaitrists. The treatment is usually based on replacement of the lost adipose tissue after cessation of disease progression. In addition to esthetic improvement, neurological disorders symptomatic treatment is indicated [21].
- Parry H. Collections from the unpublished medical writings of the late Caleb hillier parry. Vol. I. Underwoods. London. 1825; 478. https://goo.gl/szwh5S
- Henoch E, Romberg M. Klinische Ergebnisse. A. Forstner; Berlin. 1846: 75-81.
- Deshingkar SA, Barpande SR, Bhavthankar JD, Humbe JG. Progressive hemifacial atrophy (Parry-Romberg Syndrome). Contemp Clin Dent. 2012; 3: S78-81. https://goo.gl/hbr23c
- Pinheiro TP, Silva CC, Silveira CS, Botelho PC, Pinheiro Md, Pinheiro Jde J. Progressive Hemifacial Atrophy--case report. Med Oral Patol Oral Cir Bucal. 2006; 11: E112-E114. https://goo.gl/zhfmEy
- Jurkiewicz MJ, Nahai F. The use of free revascularized grafts in the amelioration of hemifacial atrophy. Plast Reconstr Surg. 1985; 76: 44-55. https://goo.gl/mNHHWn
- Lakhani PK, David TJ. Progressive hemifacial atrophy with scleroderma and ipsilateral limb wasting (Parry Romberg Syndrome). J R Soc Med. 1984; 77: 138-139. https://goo.gl/QdBgVT
- Whyman RA, Doyle TC, Harding WJ, Ferguson MM. An unusual case of hemifacial atrophy. Oral Surg Oral Med Oral Pathol. 1992; 73: 564-569. https://goo.gl/pZUAsN
- Finesilver B, Rosow N. Total hemiatrophy. JAMA. 1938; 5: 366-368. https://goo.gl/Y85fzb
- T D Foster. The effects of hemifacial atrophy of dental growth. British Dental Journal. 1979; 146: 148-150. https://goo.gl/F1XAUr
- Anderson J, Molony D, Haan E, David J. Familial Parry-Romberg Disease. Int J Pediatr Otorhinolaryngol. 2005; 69: 705-708. https://goo.gl/dJuza1
- Kayanuma K, Oguchi K. A case of progressive hemifacial atrophy associated with immunological abnormalities. Rinsho Shinkeigaku. 1994; 34: 1058-1060. https://goo.gl/Pt7BoR
- Garcia-de la Torre I, Castello-Sendra J, Esgleyes-Ribot T, Martinez-Bonilla G, Guerrerosantos J, Fritzler MJ. Autoantibodies in Parry-Romberg syndrome: a serologic study of 14 patients. J Rheumatol. 1995; 22: 73-77. https://goo.gl/CjbzWV
- Gonul M, Dogan B, Izci Y, Varol G. Parry-Romberg syndrome in association with anti-dsDNA antibodies: a case report. J Eur Acad Dermatol Venereol. 2005; 19: 740-742. https://goo.gl/s9HCs4
- Pensler JM, Murphy GF, Mulliken JB. Clinical and ultra-structural studies of Romberg´s hemifacial atrophy. Plast Reconstr Surg. 1990; 85: 669-676. https://goo.gl/WfSUUB
- Peterson LS, Nelson AM, Su WP. Classification of morphea (localized scleroderma). Mayo Clin Proc. 1995; 70: 1068-1076. https://goo.gl/rmzDzY
- Laxer R, Zulian F. Localized scleroderma. Current opinion in Rheumatology. 2006; 18: 606-613. https://goo.gl/hDLVNc
- Aranegui B, Jimenez-Reyes J. Morphea in Childhood: An Update. Actas Dermosifiliogr. 2018; 109: 312-322. https://goo.gl/k1y174
- Straube A, Padovan CS, Seelos K. [Parry-Romberg syndrome and Rasmussen syndrome: only an incidental similarity?]. Nervenarzt. 2001; 72: 641-646. https://goo.gl/QHxkf9
- Weibel L, Sampaio MC, Visentin MT, Howell KJ, Woo P, Harper JI. Evaluation of methotrexate and corticosteroids for the treatment of localized scleroderma (morphoea) in children. Br J Dermatol. 2006; 155: 1013-1020. https://goo.gl/FUs2Po
- Saxton-Daniels S, Jacobe H. An evaluation of long-term outcomes in adults with pediatric-onset morphea. Arch Dermatol. 2010; 146: 1044-1045. https://goo.gl/tA1BtN
- Roddi R, Riggio E, Gilbert PM, Hovius SE, Vaandrager JM, van der Meulen JC. Clinical evaluation of techniques used in surgical treatment of progressive hemifacial atrophy. J Craniomaxillofac Surg. 1994; 22: 23-32. https://goo.gl/d8WDRD

Sign up for Article Alerts