The Mechanism of Hydrolysis Reaction of Adenosine Triphosphate Molecules for the Generation of Bio-Energy and its Properties in the Living Systems?
Pang X Feng*
University of Electronic Science and Technology of Chengdu, China
*Address for Correspondence: Pang Xiao Feng, University of Electronic Science and Technology of Chengdu, China; E-mail: pangxf2006@yaliyun.com
Submitted: 08 May 2017; Approved: 28 June 2017; Published: 29 June 2017
Citation this article: Feng PX. The Mechanism of Hydrolysis Reaction of Adenosine Triphosphate Molecules for the Generation of Bio-Energy and its Properties in the Living Systems. Int J Pharma Analy Acta. 2017;1(1): 001-008.
Copyright: © 2017 Feng PX, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Form; Living system; Bio-energy; Protein; ATP hydrolysis; Amide
Download Fulltext PDF
We here introduced and elucidated the mechanism of hydrolysis reaction of Adenosine Triphosphate (ATP) molecules for generation of bio-energy in the living Systems, in which the detained reaction processes of hydrolysis of ATP molecules and its properties, The center effects and functions of ATP molecules in the life activity, AIP enzyme and production of bio-energy in the hydrolysis of ATP molecules and The formation of ATP molecules and its relation with Δ-µH+ were described and explained. From these introductions and results we understand and knew that this mechanism of generation of bio-energy is its an important form, which exists widely in the life bodies of generation of bio-energy. Therefore, to elucidate and research this mechanism and its properties have quite important meanings in biology, biochemistry and biophysics.
Introduction
What is life or life activity? In the light of biophysicist’s points of view, so-called life or life activity is just the processes of mutual changes and coordination for the bio-material, bio-energy and bio-information in the live systems. Their synthetic movements and coordinative changes are just totally life activity. Therefore we can say that the bio-material is the foundation of life, the bio-energy is its center, the bio-information is the key of life activity, but the transformation and transfer of bio-information are always accompanied by the transport of bio-energy in living systems [1-2]. This means that the bio-energy played an important role in life activity, there are not life activity without bio-energy.
The bio-energy source for all life bodies on earth is the light of the sun, for example, the plants and photosynthesising bacteria use directly the energy of the sun. In the photosynthesis process the glucose molecules are formed from water and carbon dioxide, which can be denoted by
However, some organic reactions, such as anaerobic life of nitrite bacteria oxidizes the ammonia to the nitrites and the nitrate bacteria oxidize the nitrites to the nitrates, do not need the oxygen. They can also obtain the energy from these chemical reactions. The energies released in the chemical transformations in animal cells are converted, accumulated and used for the synthesis of new compounds in the states of non-equilibrium distributions of substances and ions inside and outside the cell.
As it is known, the bio-energy in life body comes mainly from that released from the hydrolysis reaction of Adenosine Triphosphate (ATP) molecules in mitochondria, which are the energy factories of the cell in the living systems. We here study mainly the mechanism of generation of bio-energy released in ATP hydrolysis and its features.
Obviously, these researches have quite important significances in life science and medicine because they are helpful to both reveal the mechanism of bio-energy transport in the life bodies, which is not clear up to now, and to elucidate the reasons and properties of the absorption of infrared light and electromagnetic waves by the animals and human beings, as well as to cure some diseases such as the stem rot.
The hydrolysis reaction of ATP molecule and its release of bio-energy
As it is known, Kal’kar, et al. [3-6] first proposed the idea of aerobic phosphorylation, which is carried out by the phosphorylation coupled to the respiration process in mitochondrion. Belitser, et al. [7-9] studied in detail the stoichometric ratios between the conjugated bound phosphate and the absorption of oxygen and gave further the ratio of the number of inorganic phosphate molecules relative to the number of oxygen atoms absorbed during the respiration, which is not less than two. Thus he thought that the transfer of electrons from the substrate to the oxygen is a possible source of bio-energy for the formation of two or more Adenosine Triphosphate (ATP) molecules per atom of absorbed oxygen. Therefore Belitser and Kal’kar’s researched results are foundations establishing modern theory of oxidative phosphorylation of ATP molecules in the cell. Plenty of men and women agreed their ideas and went on extensively and deeply investigations for this problem [10-16].
However, in such a case we must know clearly the mechanism and properties of the oxidation process, which involves the transfer of hydrogen atoms from the oxidized molecule to another molecule, in which there are always protons present in water and in the aqueous medium of the cell, thus we may only consider the transfer of electrons in this process. The necessary number of protons to form hydrogen atoms is taken from the aqueous medium. The oxidation reaction is usually preceded inside the cell under the action of special enzymes, in which two electrons are transferred from the food substance to some kind of initial acceptor, another enzymes transfer them further along the electron transfer chain to the second acceptor, etc. Thus a water molecule is formed in which each oxygen atom requires two electrons and two protons.
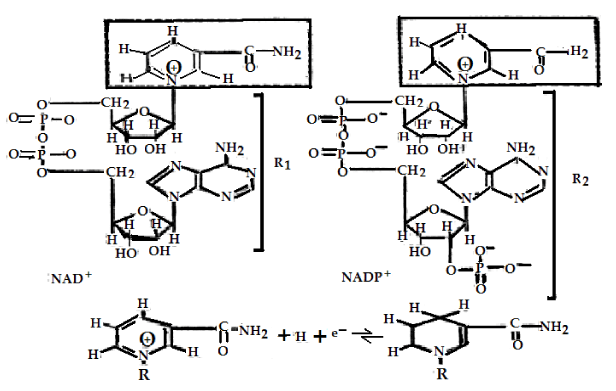
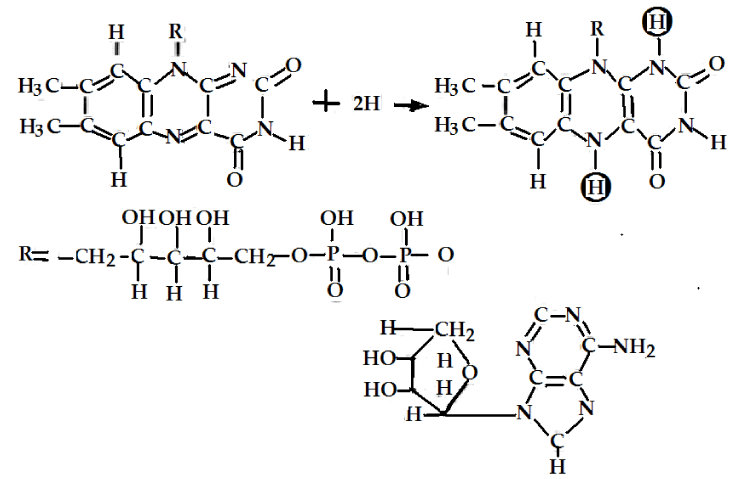
The main initial acceptors of electrons in cells [1-6] are the oxidized forms NAD+ and NADP+ of NAD (Nicotinamide Adenine Dinucleotide or Pyridine Nucleotide with two phosphate groups) molecules and NADP (Nicotinamide Adenine Dinucleotide Phosphate or pyridine Nucleotide with three phosphate groups, respectively, which are represented in figure 1, the changes of FAD (Flavin Adenine Dinucleotide or Flavoquinone) and FMN (Flavin Mono Nucleotide) are shown in figure 2. The above oxidized forms of these molecules serve for primary acceptors of electrons and hydrogen atoms through attaching two hydrogen atoms are expressed by
NADP+ + 2H+ + 2e- → NADP • H + H+ (1)
Where, NADP+ molecule becomes the reduced molecule NADP • H in this reaction [1-3,17-20]. The NAD+ molecule has also the same active center as the NADP+ molecule; it can be converted to the reduced molecule NADH through combining with two atoms of hydrogen, which is denoted by
NAD+ + 2H+ + 2e- → NADP+ + 2H+ (2)
Where, NAD+ and NADP+ are the coenzymes, which completes the reaction of dehydrogenation on compounds containing the group of atoms of H – C - OH through removing two hydrogen atoms.
In the presence of enzymes, such as the pyridine-dependent hydrogenases, and with the participations of NAD+ and NADP+ molecules, two hydrogen atoms are removed from this group of atoms in this case. Then one proton and two electrons can be converted to the reduced forms NADP • H or NAD • H by virtue of combining with NAD+ and NADP+ in this case, where the second proton can also be released. This mechanism can be also used to elucidate the oxidizing processes of the lactic acid (lactate) with the formation of pyruvic acid (pyruvate) and NAD • H, in which the reduced molecules NADP • H and their reactions occur when H+ + e- are added as electron donors (reducing agents) in other reactions. They are involved in a large number of biosynthetic processes, such as in the synthesis of fatty acids and cholesterol [10,14-16].
Therefore, the molecule NAD • H can serve as an electron donor in the process of oxidative phosphorylation, then the phosphorylation reaction can be represented by
H+ + NAD • H + 3H3PO4 + 3ADP + ½O2 → NAD+ +4H2O + 3ATP (3)
Where ADP called the adenosine diphosphate. The abbreviated form of this reaction can be written as
ADP + Pi → ATP + H2O (4)
This reaction can be simply denoted in figure 1. Thus three ATP molecules are formed from this reaction, in which the synthesis of ATP molecule are carried out in virtue of the transfer of two electrons from the NAD • H molecule along the electron transfer chain to the oxygen molecule in the mitochondria. In this way the energy of each electron is reduced by 1.14 eV. The reaction is called the phosphorylation of ADP molecules.
However, ATP molecules synthesized in this process can also reacts with water, which results in the energy release of about 0.43eV under normal physiological conditions with the help of special enzymes. The reaction can be represented by
ATP4- + H2O → ADPp + HPO42- + H+ + 0.43eV or ATP + H2O → ADP + Pi (5)
In this process ATP molecules are transformed as ADP molecules and the bio-energy of about -0.43eV is also released in this reaction. This is referred to as dephosphorylation reaction of ATP molecules [10-11,14-16].
The reaction in Equation (5) is called the hydrolysis reaction of ATP molecules, which is carried out by its phosphorylation and dephosphorylation. Thus the bio-energy of 0.43eV is released by means of this reaction process. This is just the mechanism of hydrolysis of ATP molecules. The bio-energy is widely used in plenty of biological processes and activities, such as the muscle contraction, neuroelectric pulse transfer along the neurolemma, DNA reduplication and work of calcium pump and sodium pump in cell membranes. Therefore, it is a main source of bio-energy in living systems. Just so, the mitochondrion is called as bio-energy factory.
The above results manifest clearly that the energy released in the above reaction are closely related to the growth and development of persons and animals. Thus, we can say that there is not life activity without the bio-energy.
On the other hand, we know from the above representations in the normal biological conditions the molecules in Equation (4), (5) are in various stages of ionization (ATP, ATP-, ATP2-, ATP3-,....), in which an increase in free energy ∆G in the reaction in Equation (4) and its decrease in reaction in Equation (5) depends all on their temperatures, concentrations of the ions Mg2+ and Ca2+ and the pH value of the medium. However, we can choose ∆G0 = -0.32 eV (~ 7.3 kcal / mole) in the normal conditions. If the appropriate corrections are made in the cases of the physiological pH values and suitable concentration of Mg2+ and Ca2+ inside the cell as well as the normal values for the concentrations of ATP and ADP molecules as well as the inorganic phosphate in the cytoplasm, then we can obtain the value of ~-0.54 eV (or ~-12.5 kcal / mole) for the free energy in the hydrolysis of ATP molecules [17]. Hence the free energy for the hydrolysis of ATP molecules is not a constant value. It may even not be the same at different sites of the same cell if these sites differ in the concentrations of ATP, ADP, Pi, Mg2+ and Ca2+.
Because the cells contain plenty of phosphorylated compounds then the hydrolysis of ATP molecules in the cytoplasm is always associated with the release of free energy, therefore the values of the standard free energy in the hydrolysis of ATP molecule are also different for some of these compounds, such as the free energies of phosphoenol pyruvate, 1, 3 – Diphospho - glycerate and creatine phosphate are -0.64, -0.51 and -0.44 eV, respectively.
The center effects and functions of ATP molecules in the life activity
It is different from general burning of materials, the oxygen’s are not directly used to transform the carbon as the carbon dioxide, but to produce the bio-energy and to form the energy current in virtue of the interaction with the bio-materials of proteins, sugar and fat molecules through plenty of middle chemical reactions and citric acid cycle mentioned above, its transformed ways and corresponding processes of energy current are shown in figure 3, which shows the ways of the energy current proposed by Kagawa [6]. Clearly, the energy current is generated by the transport of bio-energy released from the hydrolysis of ATP mentioned above, in which the carbons are oxidized to form the carbon dioxide by the interaction with the oxygen’s in the bio-tissues with water in life bodies. Finally the C02 produced are ruled out in vitro. However, the hydrogen atoms are transformed to the coenzymes of NAD and FAD under action of the dehydrogenase. In this case the hydrogen atoms in the reduction coenzymes of NADH2 and FADH2 formed are oxidized by the collectors formed by a series of enzymes in the electron transfer system to produce waters (H2O), where oxygen molecules O2, which come from the breath process and come further into the life body to expend in final stage of reaction for the electron transfer system, the latter are occurred on the surface of membrane of the cell or mitochondrion. The energy generated in the reaction of the oxidation- reduction in the electron transfer system is used to form ATP in virtue of the above reactions of phosphorylation and dephosphorylation, which are happened in the protein molecules, in which the coupling factor 1 (F1) with the function of enzyme are also occurred due to the action of electric-chemical energy produced on the surface of membrane. Because the oxygen’s are used in the above syntheses of ATP molecules and the electron transfer system, then we refer to it as the reaction of oxidative phosphorylation. This reaction occurs also in the photosynthesis in plants, but it is called the photon phospharylation, where the coupling factor and electron transfer occur on the membrane of the chlorophyll due to the fact that the energy of light is absorbed by it, but the F1 facts are replaced by ATPase (CF1) in this case, in which the energy need are obtained from the syntheses of ATP from ADP and Pi. Therefore, ATP molecules are almost the source of energy for all biological movements in the living systems involving the mechanical energy in the muscle contraction and work of calcium pump and sodium pump in cell membranes, the chemical and electric energy expended in the neuroelectric pulse transfer along the neurolemma and DNA reduplication. The energies transported by the energy current in figure 3 are used or expended finally in radiations of light, sound, heat and the emission of bio-photons the transmission of electricity in bio-tissues as well as the movements and syntheses of bio-matters, and so on. Therefore, we can say that there are not the activities without ATP molecule and the energy released from the hydrolysis of ATP. This manifests clearly that AIP molecules play the center functions in life activities [1-5].
On the other hand, we know from the investigations that ATP and ADP molecules can form also a link between the high and low energy phosphate compounds, such as pyruvate kinase transfers the phosphate from phosphoenol pyruvate to ADP. Pyruvate and ATP are formed in virtue of a special enzyme in this reaction. Meanwhile, ATP molecules can transfer a phosphate group to the D-glucose and convert further it to glucose - 6 - phosphate with the help of the hexokinase. This indicates also that ATP molecule pays very important rules in life process and is a universal accumulator of bio-energy in the cells. These results indicate also that ATP molecule pays an important role in the life activity [3-5].
AIP enzyme and production of bio-energy in the hydrolysis of ATP molecule
As it is known that the energy which are used by bio-membranes to finish various activities, is acquired from the hydrolysis reaction of ATP molecules, but the synthesis of ATP molecules must be also gone simultaneously in this case. In these changed processes of the energy the ATP enzymes or ATP hydrolysis enzymes must be participated. This means that AIP enzymes can pay important functions in both acquirement and expenditure of bio-energy or speaking, it must participate into the reactions of both syntheses and decomposition of ATP molecules. Just so, Lardy and Avron extracted ATP enzymes from the membranes of mitochondrion and chlorophyll [21]. Then work of calcium pump and sodium pump in cell membranes in the activity of the cells must be started by virtue of the joins of Na+-, K+- and Ca2+-ATP enzymes, where K+-ATP enzymes carry K+ ions into inner membrane from external membrane, but Na+-ATP enzymes carry Na+ ions into inverse direction, Ca2+-ATP enzyme, which is in essence a actomyosin, carry continuously Ca2+ in the inner cells into the sarcoplasmic reticulum to activate the function of the muscle thus the latter is contracted in this case. Therefore, we can obtain that ATP molecules are direct source of bio-energy in living system.
In the expenditures of bio-energy the largest expenditures of energy with respect to the movement of muscle and transformation of the ions are the chemical functions in the synthetic reactions, which makes ATP decompose to AMP and inorganic pyrophosphate (PPi) under action of the ligase. Its main productions are the protein, nuclear acid and polysaccharide molecules, in which the ATP molecule expended for forming one chemical bond, are different, such as the peptide bond formed in protein molecules need expend 4 ATP molecules, but the dihydrogen phosphate ester bond and glycosidic bond formed in DNA need only expend 2 ATP molecules. This manifests that there are still one bond with high energy in PPi. This means that PPi decomposed as 2Pi through its hydrolysis under influence of the pyrophosphate enzyme in this case, in which the expenditures of energy is used to promote the syntheses of the reaction. In this case generated AMP becomes finally as ADP through expending one ATP molecule by the muscle kinase. However ATP molecules can be again formed in the processes of glycolysis and Oxidative phosphorylation.
Otherwise, the reaction of ATP enzyme occurs also in the following reaction of kinase (phosphorylase), which is represented by ATP +X → X - O - P + ADP, where generated phosphate ester (X - O - P) will happen the reaction: X - O - P +H2O → X - OH + Pi under affection of phosphate ester hydrolysis enzyme. These reactions can be simply written in Equation (5). Therefore, we can obtain that there are not the reaction of ATP enzymes without the ATP enzymes. Just so, the phosphate ester enzymes are certainly localized in the lysosomal in the cell, which can prevent the expenditures of ATP molecules without biological effects.
On the other hand it is known that coupling factor F1 has also the function of enzyme. So-called the coupling is just that the energy of synthesis of ATP is provided by freeing energy of electron transfer system. When the inner membrane of the mitochondrion is treated by strong mechanics, the proteins are freed. Although the electron transfer system exists still in the residual membrane vesicles, its energy cannot go on the coupling with the synthesis of ATP. However, the coupling can be recu the extracted protein and membrane vesicles are again combined, and then the factor is called the coupling factor. The membrane vesicle, which cannot synthesize ATP without coupling factor, is referred to as the deficient particles. If this coupling factor is purified we can obtain at least one protein with active ATP enzyme, which is called coupling factor 1 (F1), which projects on the surface of the membrane in the form of a protuberance. It is also a spherical protein with a diameter of about 9 nm and the weight of 38000 and are composed of 5 different sub-units, their sequences are α(53,200), β(50,800), γ(33,1000), δ(17, 300) and ε(5,570), where the numbers in the parentheses are their molecular weight. The ε sub-unit can restrain the activity of ATP enzyme, i.e., it can restrain the decomposition of synthesized ATP without any biological effects.
The experiments indicated that the molecular weights of all ATP enzymes involving F1 and their sub-units are almost the same. This means that the configuration of the electron transfer on the membranes and corresponded mechanisms of generation of bio-energy possess all the same feature. For example, the experiments of electronic microscope confirm that the chloroplast, photosynthetic bacterias having the function of photosynthetic phosphorylation and the thermophilic bacterias have all ATP enzymes or F1 [3-6]. The F1 in the thermophilic bacterias is quite stable in the cases of both room, high and low temperatures, or under influences of the reagents, which can degenerate the features of protein molecules. Its α and β sub-units can combine with ATP and ADP molecules, but combined capability of α sub-unit with ATP and ADP is stronger than that of β sub-units because its position of combination is adjusted due to the change of the center of structure in the α sub-units arising from the variation of advanced structure. β sub-units has the combined capability with the dissociation constant of 0.5 Mol. / L, γ sub-unit is a gate for the H+ ion, δ and ε sub-units can promote the combination of F1 with F0 , ε sub-unit can restrain also the activity of ATP enzyme of F1. These are just the functions of five sub-units in the thermophilic bacterias.
Therefore, we know that the enzymes carrying out the above synthesis of ATP molecules from ADP molecules and inorganic phosphate in the coupling membranes of mitochondria are the same with those in the cytoplasmic membranes of bacteria, which are mainly composed of F1 with F0, the latter are joined each other by the small proteins F5 with F6 [3-6]. These proteins form also the F1 with F0 complex or the enzyme ATPase. In the coupling membrane of mitochondria and the cytoplasmic membrane of bacteria the complex F1 - F0 is positioned so that the enzyme F1 is the inside of the membrane. The enzyme F0 can extend from one side of the membrane to the other and has a channel which lets protons through. When two protons pass through the complex F1 - F0 in the coupling mitochondrial membrane one ATP molecule is synthesized inside the matrix by an ADP molecule and inorganic phosphate. This reaction is reversible. Under certain condition the enzyme transports the protons from the matrix to the outside using the energy of dissociation of ATP molecules, which may be observed in a solution containing isolated molecules of enzyme F1 and ATP. The largest two proteins in F1 take part in the synthesis and dissociation of ATP molecules, the other three proteins are apparently the inhibitors controlling these reactions.
If the enzyme F1 molecules are removed from mitochondria then the remaining F0 enzymes increase greatly the permeability of protons in the coupling membranes, which confirms that the enzyme F0 has a channel for the passage of protons which is constructed by the enzyme F1 However, the mechanism for the synthesis of ATP molecules by the enzyme ATPase is still not clear up to now.
The formation of ATP molecules and its relation with Δ-µH+
The mechanisms of formation of ATP molecules from ADP molecules under the action of ATP synthase in Equation (18) have been proposed [1,22] which are following three models:
(1) Boyer’s “mechanism of change of combination” for the action of ATP synthetase. Boyer thought that the structure of complex of ATP synthetase resembles with the sophisticated device of “water turbine”. When H + ions flow along their concentration gradient across the membrane of the mitochondria, in which the transshipment and return occur., then the drive “turbine” (base F0 factor) and ‘ rotation of connected ‘ rotor’ (handle) result in the variation of the conformation of ‘the “blade”(the head of F1 factor) combining the other end of the ‘rotor, this induces necessarily ADP and Pi to synthesize ATP molecules. Thus the latter is released in this case [1,22].
(2) The form of molecular structure of complex of ATP synthetase. As it is known, in the model of rotation of the rotor mentioned above, F1 factor is a key structure part or unit of ATP synthesis, in which each α sub-unit and β sub-unit have all one combined point with the nucleotide, namely, the combined points of nucleotide in β sub-unit have also the activity of catalysis of ATP synthesis or hydrolysis, where 3 α sub-unit and β sub-unit are cross wisely arranged around the same axis, which is similar a “orange disc”. In this case they form 6 polymer structure of the oblate spheroidal type with the high of 8 nm and the wide of l0 nm. γ sub-unit and ε sub-unit strongly incorporated together to form an axis or a rotor, which is inserted in the center of 6 dimer structure of the oblate spheroidal type of “3α3β” to make F1 factor and F0 factor mutual-couple. Thus they can interact sequently with 3 β sub-unit to vary and regulate the conformation of catalysis positions of 3 β sub-unit. ε sub-unit can suppress the ATP hydrolysis of enzyme and jam H + channel, which reduce further the function of the H+ leakage. The hydrophobic protein complex of F0 factors inserting in the inner-membrane contains three sub-units of a, b and c, in which many copying c sub-units are arranged in sequence as a ring structure of 12 polymers, but a sub-unit and b sub-unit are arranged out sides of c sub-unit with cyclic polymer structure, it and δ sub-unit, which constructed F1 factor, are combined together to form a F0 factor of the “stator”, which connected again with the F1 and F0. Thus the proton channels on the inner membrane in the mitochondrial have been constituted in this case, which can regulate the H+ fluid in the across membrane proton channel in virtue by the sub-units, which incorporates again to the oligonucleotides enzymes in the ring c sub-unit polymers. Meanwhile, it cans also cover the dynamic potential of the protons across the membrane to the torsional movement, which drives the rotation of “the rotor”. Then ADP and Pi together to synthesize ATP molecules in this case [1,22].
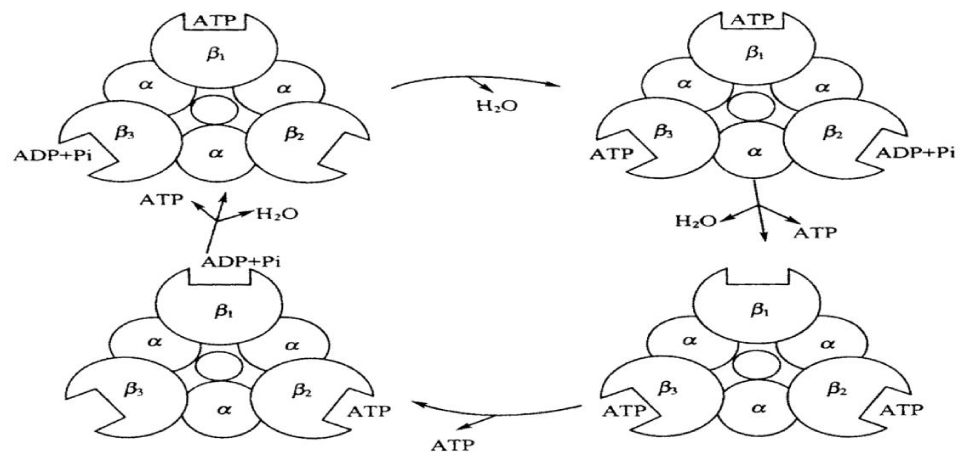
(3) The “rotation catalytic model of synthesis of ATP. In accordance with Boyer’s hypothesis in the mechanism of change of combination, ATP synthesis involved mainly the conformational change of 3 sub-units in F1 in the process of rotation of “the rotor” driven, then 3 β sub-units are semultanuosly in different conformational states. When each β sub-unit synthesizes one molecule of ATP using the catalysis method, which produce three different conformational states due to the difference of time of combination with the nucleotide, which are the close combined state (T state), loose state (L state) and open state (O state). Under the promotion of the proton flow, 3α3β 6 polymers will rotate 1200 relative to the rotor. In this case each β sub-unit occur correspondingly conformational variations, thus the affinities of ATP with ADP and Pi are changed, and then they will combine or disintegrate each other, which is shown in Figure 4.
Walker and Noji investigated successively one mechanism of this phenomenon, which were obtained from the results observed from different angles and different experiments based on Boyer’s assumption for the combined and changed mechanism in [22]. Finally they confirmed that Boyer’s assumption is correct. Just so, Boyer and Walker shared the 1997 Nobel Prize in chemistry.
We now study the relation of ATP molecules with the values of electric- chemical potential Δ-µH+ of H+ ions. The experimental researches indicated that ATP molecules can be synthesized only if the values of electric chemical potential Δ-µH+ of H+ ions on the membrane of mitochondria are suitable and enough. However, what is the value? In practice, the value can be obtained changes of the free energy generated in the reaction in Equation (5).
As it is known, the difference of electric-chemical potential between the inner and external membranes can be represented by
∆- μ + H+ = F∆ ϕ0-i + RTLN (H+)i / ( H+)0 (6)
Where ∆ϕi-0 is the difference of potential between inner (i) and external (0) the membranes, (H+) is the active degree of H+ϕ the above equation can be simply denoted as
Δ-µH+ = F∆ ϕ + 2.3 RT∆PH (7)
Because the result of variations of the free energy in the hydrolysis reaction and the reaction of shift of 2H+ are same in the equilibrium state and according to definitions of electric-chemical potential and the result of [ATP] = [ADP] in the equilibrium of the synthesis and decomposition of ATP molecules Yasuo Kagawa found out the difference of electric-potential between the inner and external membrane of mitochondrion, which is of
∆ϕ = 60∆PH + 210 mV (8)
For the membrane of chloroplast, which is treated by the alkali and acid, we can think the ∆ϕ = 0 because the lipid of the chloroplast is the galactolipid, its permeability of the anion is very large [4,18-20]. Then we can obtain from Equation (21) that the difference of pH value between the inner and external membranes in the mitochondrion is ∆PH = 3.5. This means that ∆PH = 3.5 is a quire necessary and suitable condition for the synthesis of ATP molecules. If it increased to 7, then ATP molecules cannot be synthesized from ADP and Pi. The above results and Equation (20) indicated clearly that the synthesis of ATP molecules must require the certain values of Δ-µH+ the membrane possesses. Obviously the difference of electric-chemical potential Δ-µH+ is formed due to the difference of concentration [H+] of H+ and the difference of electric potential, ∆ϕ, between the inner and external membranes of the mitochondrion. Then we can affirm that the difference of electric-chemical potential formed can be restrained and eliminated by the inhibitors of electron transfer.
The above results show that the form of ∆ϕ and Δ-µH+ or ∆PH and corresponding the synthesis of ATP molecules using ∆ϕ are important function of the bio-membrane. Just so, we can say that the bio-energy comes from the bio-membrane, or speaking the membranes of the mitochondrion is the source of the bio-energy released by hydrolysis of ATP. The ∆ϕ formed on the bio-membrane can also be used in plenty of biological processes, such as the resting potential with 70 - 90 mV on the neurocytes is formed through the differences of distribution and concentration of Na+ and K+ between the inner and external membranes due to the actions of Na+ -ATPase and K+-ATPase.
As it is known, the synthesis of ATP molecules and generation of energy in the hydrolysis reaction of ATP are closely related to the transfers of electrons and H+ in the transfer systems. In the transfer system the transfer of electrons is started from the dehydrogenation of NADH2, etc. It goes on in the forms of reactions of H→H+ + e-, and Fe3+ + e-Fe2+. In these reactions According to the properties of association of reactions of oxidation and reduction participated by flavin, cytochrome and nonheme iron, we know that their transfers can be carried out along the direction from lower reaction system to higher reaction system. The collection system of these materials of oxidation and reduction is called the respiration chain or electron transfer system, in which the Fe, which exists in the heme of hemoglobin and the ferritin, plays a main and key rule. The main variations of oxidation of NADH can be described as:
The flavin → nonheme iron → CoQ cytochrome b → cytochrome c1 → cytochrome c → cytochrome a, a3 and → O2. Its concrete processes of the transfer can be also denoted by: the substrate NAD FMN 2(FeS) Q 2 [b C C1 a a3] O2.
In this scheme the components of the respiratory chain are placed in the order of potentials. Apart from cytochromes the respiratory chain is made up of flavin mononucleotide molecules, iron-sulphur proteins, ubiquinone molecules and some protein molecules. The NAD+ molecules form the main link between the citric cycle and the electron transfer chain, in virtue of this chain a pair of electrons are transferred to the oxygen atom. Thus the electrons occur as pairs in the NAD • H2 molecule. The chain is terminated by the transfer of two electrons to the oxygen atom. Inside of the chain the electrons are transferred one and two at a time [14-16].
The energy of electron transfer in this system is acquired from the activity of H+-ATPase driven by Δ-µH+ on the membrane. In the transport of H+, the H+ is come from H in the substrate, which is freed from the reaction of oxidation-reduction: AH2 + 2Fe3+ → A+ 2Fe2+ +2H+ [4-5,18]. Therefore, the transport system of H+ ion and electron transfer system are anisotropically arranged on the membrane to form together a ring to transport H+. This is just the mechanism of ring transport of H+. This model is constructed by the compounds on the surface of fat body, which is formed by adding the ascorbic acid (AH2) into the iron compounds of two valence iron, in which the difference of electric-chemical potential of H+ appears, which promotes the transport of H+ in the proportion of 2H+/ATP under the action of H+/ATPase. Its concrete process of transport is as follows. The pyridine dehydrogenase transfers two H atoms from food (substrate S•2 H) to NAD+, which are shifted again into the inner of the matrix to combine with OH- and to produce further water. The reaction can be represented by S·2H+NAD+ ↔ S+NAD•H+H++H++OH+→H2O, where S is the oxidized substrate. In this case the direct transport of a pair of H atoms from the NAD • H molecule to the Flavin Mono Nucleoitide (FMN) molecule occurs by the action of flavin-dependent dehydrogenase enzymes. Thus the reduced molecules FMN•H2 are formed by the reaction: NAD • H+FMN+H+ ↔ NAD+FMN•H2 in the inner side of the conjugated membrane. In this reaction, NAD • H molecule transports its one H+ and two electrons to the FMN molecule, the second H+ comes from the surround environment.
Therefore, we can obtain from the above investigations that the synthesis of ATP molecules are always accompanied by the electron transfer and the transport of H+ ions under actions of ATPase and Δ-µH+ on the membrane of the mitochondria. This implies that there are not the syntheses of ATP molecules without these processes and reactions in the living system. So, the syntheses of ATP molecule are a very complicated biological process.
Sambongi, et al. [23] studied the influences of mechanical rotation of the c subunit oligomer in ATP synthase (F0F1), which is the smallest motor enzyme known, on the energy of ATP hydrolysis, where F0F1, found in mitochondria, synthesizes adenosine 5’-triphosphate (ATP) coupling with an electrochemical proton (or H+) gradient and also reversibly hydrolyzes ATP to form the gradient. An actin filament connected to a c subunit oligomer of F0 was able to rotate by using the energy of ATP hydrolysis. The rotary torque produced by the c subunit oligomer reached about 40 pico – Newton - nanometers, which is similar to that generated by the generated by the gamma subunit in the F1 motor. These results suggest that the gamma and c subunits rotate together during ATP hydrolysis and synthesis. Thus, coupled rotation may be essential for energy coupling between proton transport through F0 and ATP hydrolysis or synthesis in F1. The proton- transporting ATP synthase, F0F1, consists of a catalytic sector, F1 or F1–adenosine triphosphatase (ATPase) (a3b3g1d1e1), and a proton pathway, F0 (a1b2c12). The crystal structure of the bovine a3b3g complex indicates that the a and b subunits are arranged alternately around the NH2- and COOH- terminal a helices of the gamma subunit. The isolated F1 hydrolyzes ATP, followed by gamma subunit rotation, which is driven by conformational changes of the catalytic subunits. The gamma subunit rotation in F1 has been observed by biochemical experiments and has been suggested directly that it relates to the counterclockwise rotation of an actin filament connected to the gamma subunit. The gamma subunit rotation in F1 should be transmitted to the membrane sector, F0, in order to complete ATP hydrolysis–dependent proton transport. The detailed underlying mechanism of the energy transmission between F0 and the gamma subunit remains unknown as yet. If the c subunit oligomer rotates counterclockwise (the same direction as gamma) in the membrane, then the ATP hydrolysis–dependent gamma subunit rotation could be connected mechanically to the F0 sector. In this regard, c subunit rotation has been proposed. They designed several experimental systems to examine this possibility. The gamma and electron complex is shown to be a rotor and the a, b, d complex is proposed to be a stator in F0F1. Therefore, we fixed F1 a (or b) subunits on a glass surface to demonstrate the rotation of an actin filament connected to the F0 c subunit, or conversely, the c subunits were fixed and the rotation of a or b was examined. ATP-dependent rotation was only successfully observed with the system described below. ,em>Escherichia coli
F0F1 was immobilized on a cover slip linked to the NH2- terminus of each a subunit. A c subunit Glu 2 was replaced by cysteine and then biotinylated to bind streptavidin and a fluorescently labeled actin filament. The gamma subunit cysteine residues were replaced with alanine in order to avoid direct binding of the actin filament to this subunit. Thus, cysteine is present only in the c subunit of the presumed rotor complex.Tsunoda, et al. [21,24] studied also the rotation of the c subunit oligomer in fully functional F1Fo ATP synthase. They thought that previous studies had established that the central gamma and epsilon subunits of the F1 part rotate relative to a stator of alpha and beta and delta subunits during catalysis. They showed that the ring of c subunits in the F0 part moves along with the gamma and epsilon subunits. This was demonstrated by linking the three rotor subunits with disulfide bridges between cysteine residues introduced genetically at the interfaces between the gamma, epsilon, and c subunits. Essentially complete cross-linking of the gamma, epsilon, and c subunits was achieved by using CuCl to induce the oxidation. However, this fixing of the three subunits together had no significant effect on ATP hydrolysis, proton translocation, or ATP synthesis, and each of these functions retained inhibitor sensitivity. These results unequivocally place the c subunit oligomer in the rotor part of this molecular machine.
The above investigations are very helpful to understand the properties of synthesis of ATP molecules and the influences of mechanical rotation of the c subunit oligomer in ATP synthase (F0F1) on the energy released by ATP hydrolysis.
- Pang Xiao Feng. Biophysics. Press of University of Electronic Science and Technology of China. Chengdu: 2007.
- Davydov AS. Biology and quantum mechanics. Pergamon Press: Oxford; 12. https://goo.gl/rNfLtb
- Kagawa Y. Biological and Bio-energy (Chinese) interpreted by Lan shu Cheng and Xi Xie. Science publisher; Beijing: 1986.
- Belitser VA, Tsybakova ET. On the mechanism of respiration-coupled phosphorylation. Biokhimiya. 1939; 4: 516.
- Kovac L. Biochemical mutants: An approach to mitochondrial energy coupling. Biochim, Biophys. Acta.1974; 346: 101. https://goo.gl/LsmbjG
- Kagawa Y. Science: 1974; 44: 417.
- Kagawa Y. Methods in membrane biology, edited. Kora E, Plenum Press: 1974. https://goo.gl/Fx7p9Y
- Kagawa Y. Reconstitution of the energy transformer, gate and channel subunit reassembly, crystalline ATPase and ATP synthesis. Biochim. Biophys. Acta. 1978; 505: 45-93. https://goo.gl/rxMmr3
- Tsunoda SP, Aggeler R, Yoshida M, Capaldi RA. Large conformational changes of the ɛ subunit in the bacterial F1F0 ATP synthase provide a ratchet action to regulate this rotary motor enzyme. Proc Natl Acad Sci USA. 30. 2001; 173: 898-902. https://goo.gl/jN9c5q
- Huo ZH. Cell biology, advanced education Press, Beijing; 1995. https://goo.gl/LLqE3f
- Sambongi Y, Iko Y, Tanabe M, Omote H, Atsuko IK, Ueda I, et al. Mechanical rotation of the c subunit oligomer in ATP synthase (F0F1): direct observation. Science. 1999; 286: 1722-1723. https://goo.gl/L2qwZA
- Wang GZ. Biological chemistry, Sichuan Press of science and technology. Chengdu. 1992.


Sign up for Article Alerts