Idiopathic Macular Telangiectasia Type 2 and its Relationship with Macular Pigment Optical Density?
Christopher Putnam*
University of Missouri-St Louis College of Optometry, St Louis, Missouri, USA
*Address for Correspondence: Christopher Putnam, University of Missouri-St Louis College of Optometry, St Louis, Missouri, One University Blvd, 417 Marillac Hall St Louis, MO 63121, USA, Tel: +210-360-0334; E-mail: [email protected] / [email protected]
Submitted: 21 November 2018; Approved: 20 December 2018; Published: 23 January 2019
Citation this article: Putnam C. Idiopathic Macular Telangiectasia Type 2 and its Relationship with Macular Pigment Optical Density. Int J Ophthal Vision Res. 2019;3(1): 001-002.
Copyright: © 2019 Putnam C. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Idiopathic macular telangiectasia type 2; Macular pigment; Spectral domain optical coherence tomography angiography
Download Fulltext PDF
Background: Idiopathic Macular Telangiectasia type 2 (IMT2) is a relatively uncommon clinical condition with an estimated prevalence of 1% within the general population. This condition can be challenging to precisely identify in early stages but advancements in clinical imaging to include fluorescein angiography and Optical Coherence Tomography Angiography (OCTA) can allow for timely diagnosis and prompt intervention that may leads to improved long-term clinical outcomes. Emerging literature has recognized the role of Macular Pigment (MP) in IMT2 in terms of Henle fiber layer deposition mechanisms and potential mitigation of inflammatory and oxidative stress. Primary care optometrists are in a unique position to facilitate early detection and manage through close evaluation and individualized lutein supplementation.
Case Report: A 56 year old Hispanic female presented to the public health optometric service reporting bilateral blurred vision that began approximately four years ago and has been progressively worsening over time. Entering distance uncorrected visual acuities were 20/150 in the right eye and 20/200 in the left eye with no improvement in pinhole acuities. Macular evaluation revealed a scattered drusen appearance with prominent pigmentary changes and atrophy in both eyes with subtle fibrotic changes in the left eye. High-resolution Optical Coherence Tomography (SD-OCT) and SD-OCT angiography (SD-OCTA) confirmed the diagnosis of IMT2 with choroidal neovascularization OS. The patient was referred to a retinal specialist for confirmation of the macular findings and initiation of anti-VEGF treatment for the neovascular changes OS
Advancements in primary care optometric imaging modalities have allowed for increased scope of care and enhanced management of central retinal conditions including macular telangiectasia. Emerging studies of MP anti-oxidant and anti-inflammatory properties may hold promise in the clinical, non-surgical management of macular telangiectasia. Further investigation into the role of MP spatial distribution effects on visual performance as well as MP deposition properties resulting from macular telangiectasia is warranted.
Introduction
Idiopathic Macular Telangiectasia type 2 (IMT2) is an acquired, bilateral maculopathy associated with a spectrum of clinical presentations related to inner retinal telangiectatic vascular anomalies [1]. As a result of this wide-ranging clinical presentation, cases of IMT2 often are underdiagnosed or misdiagnosed. Current retinal imaging modalities such as Spectral-Domain Optical Coherence Tomography (SD-OCT), multi-spectral fundus photography and Fluorescein Angiography (FA) are increasingly valuable to the understanding of the clinical pathology [2,3]. More recently, the emerging noninvasive technology known as Optical Coherence Tomography Angiography (OCTA) was shown to be particularly useful in the assessment and management of IMT2.
In 1982, Gass and Owakawa re-examined the classification of idiopathic macular telangiectasia and coining an alternate description termed idiopathic juxtafoveal retinal telangiectasis [4]. In 1993, Gass and Blodi performed a meta-analysis examining 140 such cases over a 28-year period and refined the clinical spectrum of these entities with subgroups and stages. The resulting classification structure resulted in 3 distinctive groups each with a presumed, independent etiology on the basis of fundoscopic findings, fluorescein angiographic features and clinical severity [5]. Furthermore, the advent of improved resolution, high-speed retinal angiography integrated with Optical Coherence Tomography (OCT) have provided an enhanced clinical understanding of the nature of the vascular abnormalities and the associated macular sequella. To some degree, these improved imaging techniques have led to findings paralleling histopathological observations described in the ophthalmic literature. Below is a review of the 3 subgroups.
Type 1: Aneurysmal telangiectasia
Clinical appearances of aneurysmal telangiectasia reported in the literature vary. However, the consistent retinal feature included microangiopathy with multiple capillary aneurysms both arterial and venous. These vascular abnormalities were evident in the superficial and deep retinal capillary circulations although larger aneuritic changes were confined to the superficial circulation. In addition, some patients had isolated non-perfusion or capillary ischemia with lipid deposition. The lipid deposition was more commonly recorded in association with numerous minor capillary abnormalities or focal, large aneurysms. Pathological findings also varied in location with areas of involvement not only in the juxtafoveolar and paramacular regions but also in the peripheral fundus. Some larger macroaneurysms were associated with hemorrhage. The central macular vascular abnormalities were associated with edema or cystic change in the retina identified with angiography and confirmed with OCT imaging. Preretinal neovascularization or sub-retinal neovacularization is uncommon. Related systemic diseases are not commonly associated with aneurysmal telangiectasia. More commonly, aneurysmal telangiectasia is a unilateral, exudative condition associated with micro and macroaneurysms in the absence of pigmentary changes, crystalline deposits or neovascularization. Vascular macular leakage is generally associated with intra-retinal cystic changes that are well-documented on OCT imaging.
Type 2: Perifoveal telangiectasia
Perifoveal telangiectasia tend to follow a bilateral presentation although there is a degree of asymmetry with regard to severity. Literature reports of concurrent diabetes mellitus and hypertension show an incidence rate of approximately 18.5% reported in the general population of the same age [6]. In contrast to the aneurysmal telangiectasia cases, the perifoveal telangiectasia cases are typically well-defined by their clinical and imaging manifestations. Early clinical changes consisted of a mild loss of transparency of the retina, usually in the temporal juxtafoveolar area but surrounding the fovea in more advanced cases. In these cases, the telangiectatic vessels were absent or barely evident clinically while retinal thickening was readily evident with OCT imaging. In patients with early clinical manifestations of perifoveal telangiectasia, fluorescein angiography shows mild, diffuse intraretinal leakage or staining while patients with more advanced stages of the disease had a prominent dilation of capillaries. In patients with more prominent telangiectasis, fluorescein leakage in the superficial circulation can also cascade over the deep capillary leakage. Additionally, superficial retinal circulations may reveal segmental or sectoral dilation overlying the dilated deep retinal telangiectatic vessels revealing capillary aneurysmal changes without lipid ischemia or preretinal neovascularization. Cystic spaces in the perifoveal area are uncommon. OCT imaging can show preservation of the inner limiting membrane that constituted the anterior aspect of the cystic changes. More advanced cases reveal deeper inner lamellar cysts with further loss of the outer retina and a correlating visual decline in the absence of prominent aneurysms, hemorrhage or lipid accumulation. Vitreoretinal interface crystalline deposits appeared at various stages of the disease as an inconsistent but characteristic finding which also included subretinal plaques of pigmentation and dilated right-angle retinal vessels. In some cases, the dilated vessels show retinal-retinal anastomosis within the retina or extending to communicate with new vessels beneath the retina or SRN. The development of SRN from deep retinal circulation may lead to neurosensory detachment, subretinal hemorrhage, fibrosis, and visual decline in advanced stages of the disease due to cystic changes in the retina. Perifoveal telangiectasia is a bilateral perifoveal disease that is not commonly associated with aneurysms or cystoid leakage. A non-proliferative stage is identified by exudative telangiectasia and foveal atrophy and the proliferative stage is characterized by SRM and fibrosis.
Type 3: Occlusive telangiectasia
Occlusive telangiectasia is highly uncommon classification of IMT2 characterized by ischemic foveal disease with flanking capillary dilation in response to retinal non-perfusion. In previous reviews, occlusive telangiectasia has been classified as perifoveal capillary non-perfusion disease manifesting as a result of systemic or cerebral familial syndrome.
A recent published study of perifoveal Müller cell depletion in IMT2 revealed an absence of macular pigment within the macula along with abnormally dilated macular capillaries were spatially correlated with regions of fluorescein leakage [7]. These telangiectatic vessels displayed additional pathologic features and no reactive Müller cells, a key factor in MP deposition, were identified. The areas deficient in Muller cells corresponded with regions of negligible macular pigment supporting the hypothesis that Muller glial cells are a critical component of IMT2. Retinal capillary telangiectasia is commonly the result of antecedent retinal vascular inflammatory or occlusive disease. In the macular region, diabetic retinopathy, hypertension, venous occlusion, inflammatory diseases and blood dyscrasias are the typical causative factors [8]. However, alternative forms of telangiectasia can develop in the macula and perifoveolar areas without a definitive etiology.
In a peer-reviewed comparison of IMT imaging techniques, en face SD-OCT C-scans and conventional B-scans both provided repeatable identification capabilities for a number of retinal findings. These retinal findings included inner crystalline deposits (15% of subjects), retinal capillary anomalies (100% of subjects), intra-retinal cysts (80% of subjects), hyper-reflective spots in the outer nuclear layer (100% of subjects) and external limiting membrane (80% of subjects), hyperplastic pigment plaques (30% of subjects), intra-retinal neovascularization (20% of subjects), photoreceptor loss (100% of subjects), and choroidal cavitations (30% of subjects). However, en face OCT C-scans provided more information than B-scans on intra-retinal neovascularization, photoreceptor loss and choroidal cavitations in addition to better visualization of the retinal vessels and telangiectasia than fluorescein angiography [9]. En face OCT showed promise as a noninvasive, reproducible technique that may lead to enhanced assessment and follow up for IMT2 associated retinal and choroidal changes. Recently, SD-OCTA has emerged in the literature as a reliable, reproducible, non-invasive method to accurately image early retinal changes associated with IMT2 [10]. Specifically, the OCTA capabilities to precisely record vascular abnormalities through the use of coherent motion imaging has provided a useful toll in detection of the choroidal neovascular changes associated profound vision loss second to IMT2 [11,12].
In a separate study, patients with retinal findings characteristic of IMT2 were interviewed regarding visual symptoms and corresponding age of onset in a large cross-sectional patient demographic. The most commonly reported clinical symptom was impaired reading ability (79%) followed by metamorphopsia (12%). The most frequently reported age of diagnosis was the seventh decade (76%) and 58% of the patients were symptomatic before the age of 60 years. The median delay between first reported symptoms and IMT2 diagnosis was ~7 years. Ten years after the first reported clinical symptoms, distance visual acuity of the better eye was better than 20/25 in 35% and ≤ 20/50 in only 17%. Increased awareness of IMT2 symptoms in conjunction with improved imaging retinal imaging of characteristic morphologic changes will likely lead to more timely, accurate diagnosis.
Macular Pigment (MP) is consists of of two principal elements: Lutein (L) and Zeaxanthin (Z) [13,14]. Within the human retina, MP is a membrane-bound compound predominantly found within the photoreceptor axons (outer plexiform layer and Henle layer within macular region) and the inner plexiform layer [15]. MP has also been documented at the level of the Retinal Pigmented Epithelium (RPE) [5] and photoreceptor outer segments [16]. MP is measured highest in the central retina peaking at the foveola and decreasing to optically undetectable levels at 8° of eccentricity [17]. Lutein is found at higher values within the peripheral retinal tissue as the ratio of L: Z inverts from 1:2.4 at the foveola to 1.8:1 in the parafovea to 2.7:1 in the peripheral retina [18,19]. The inversion of the L: Z ratio with eccentricity matches the corresponding rod:cone ratio demonstrated by Osterberg [20] and Curcio et al. [21]. Suggestive of structure-specific MP accumulation. Bone et al. posited that MP spatial distribution suggests a specific role in photoreceptor function [22]. Published work has posited three primary roles for MP: protection, neural efficiency and optical enhancement. In support of the protection role, MP has well-established potent antioxidant properties and acts as an efficient optical filter of short wavelength, high energy visible light. A protective association with age-related macular degeneration was postulated in the Eye disease case-control study [23] and explored further in the AREDS II study [24]. Current macular degeneration models implicate a combination of cumulative damage from Reactive Oxygen Intermediates (ROIs) created through metabolic processes catalyzed by high energy, short wavelength light and chronic inflammation [25]. Existing case reports involving IMT2 demonstrate a reduction in MP within the central 4° of eccentricity relative to demographic-matched controls [26] and preservation of MP at 5-7° of eccentricity [27]. The focal, centralized reduction of MP in patients with IMT2 represents a novel phenotypic retinal characteristic and lends evidence of compromised trafficking or deposition of MP as a feature of the disease process. Patients also showed a disproportionately greater reduction in zeaxanthin relative to lutein supporting the hypothesis that IMT2 may serve as an exemplar for MP deposition mechanisms within the retina. Zeimer et al. identified associations between changes in stages of macular telangiectasia and restrictions in visual function support the use of MP distribution classification as a severity scale for IMT2 [28]. Recent confocal reflectance studies have confirmed the earlier identified abnormalities in MP deposition and Müller cells pathology contributing to the underlying pathophysiology of IMT2 [29].
Case Report
A 56 year old Hispanic female presented to the public health optometric service reporting bilateral blurred vision that began approximately four years ago and has been progressively worsening over time. She denied history of trauma, surgery, pain, diplopia or loss of visual field in either eye. Her family medical and ocular history was unremarkable and she reported no current medical or ocular conditions. Her last medical and ophthalmic exam were unknown and she reported no known allergies or drug sensitivities. Entering distance uncorrected visual acuities were 20/150 in the right eye and 20/200 in the left eye with no improvement in pinhole acuities. Entrance testing showed equal, symmetric pupils with 3+ reaction to light in both eyes and full range of motion without restriction of both eyes during extraocular muscle motility testing. Confrontational visual field testing was full to finger counting with no evidence of peripheral field loss in either eye. Manifest refraction yielded a +1.50DS in the right eye and +2.50DS in the left eye with a best corrected visual acuity of 20/100 OD and 20/150 OS. Goldmann applanation tonometry was performed resulting in readings of 20mmHg in both the right and left eyes. Anterior segment slit exam findings showed unremarkable adnexa, lids and lashes with normal tearfilm findings in both eyes. The conjunctiva was recorded as 1+ symmetrical pinguecula nasal and temporal with unremarkable corneal findings in both eyes. The anterior chamber was deep and quiet with Van Herrick measurements of 3+ nasally and temporally in both eyes and the iris was flat with no signs of atrophy or neovascularization in either eye. The lens showed early nuclear sclerotic changes recorded as 2+ nuclear sclerosis.
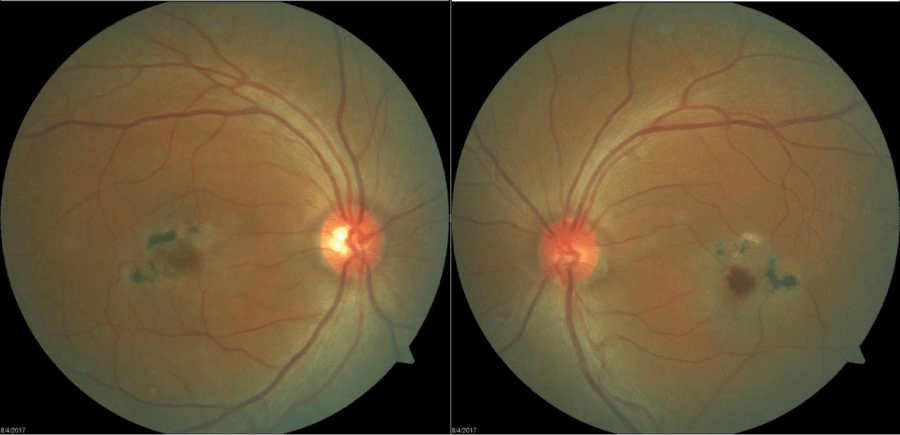
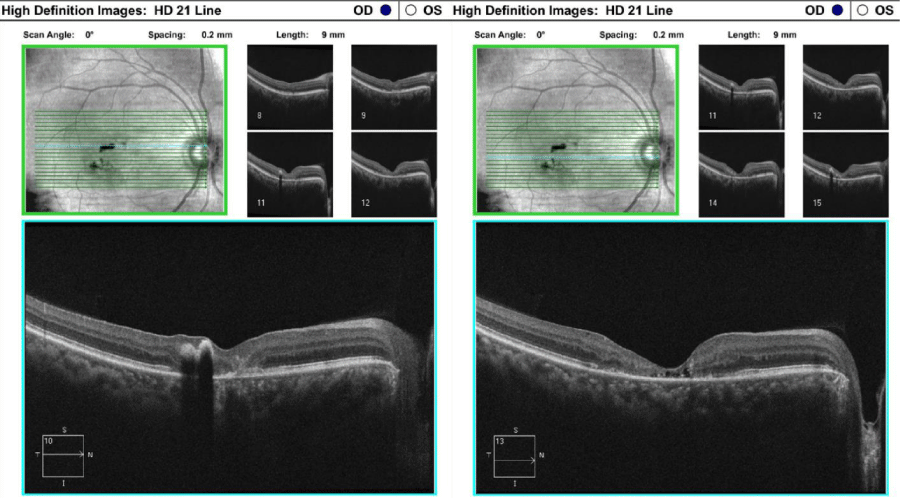
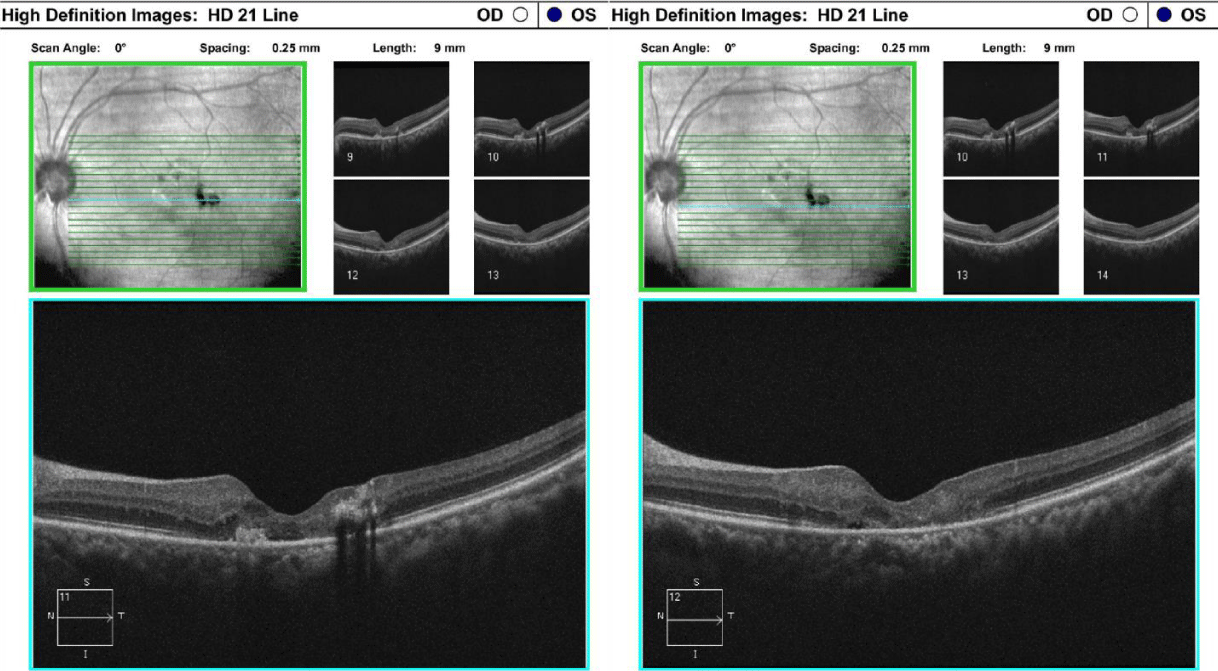
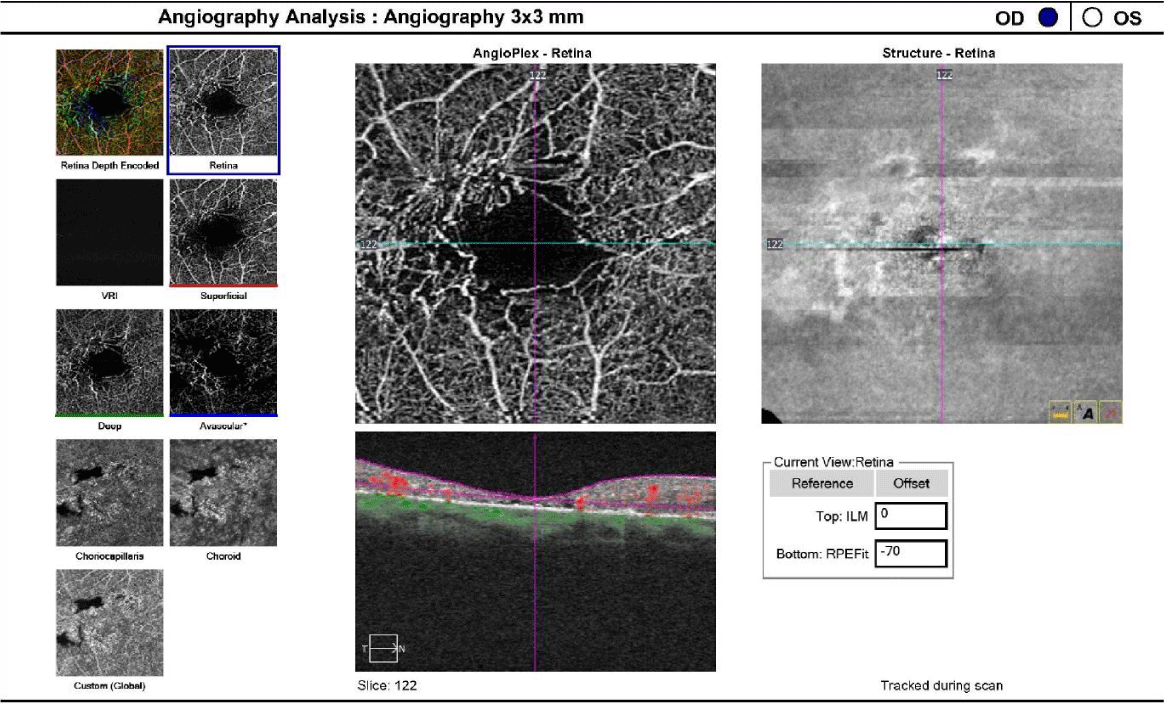
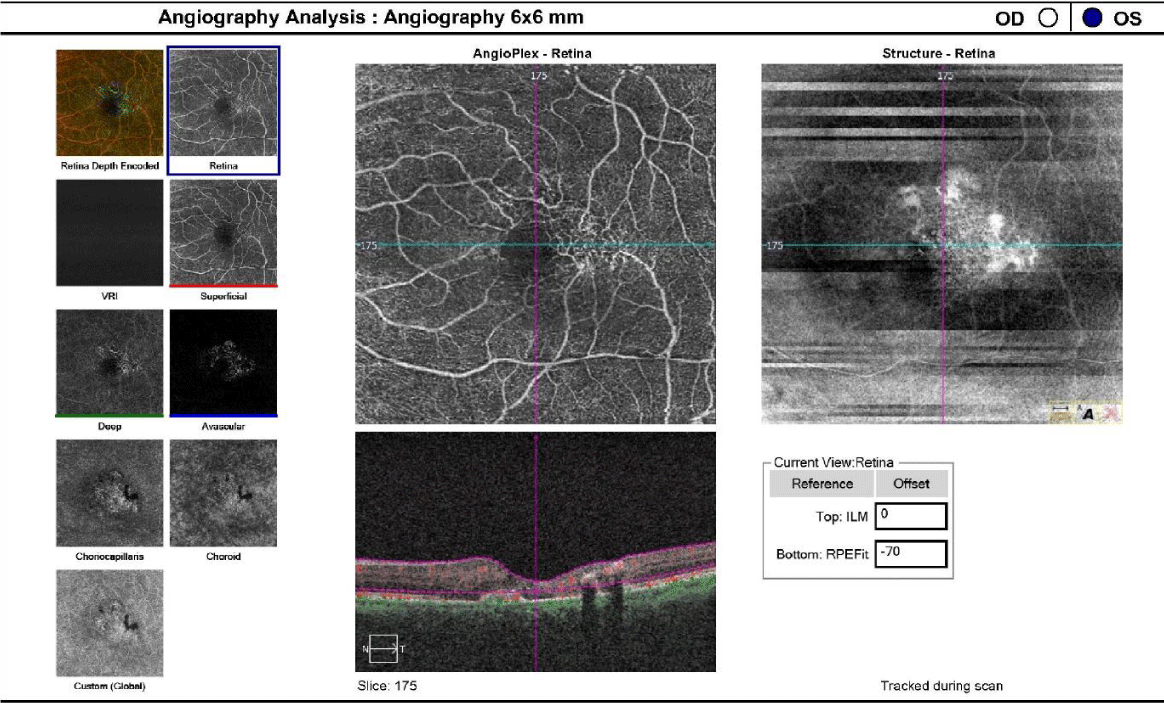
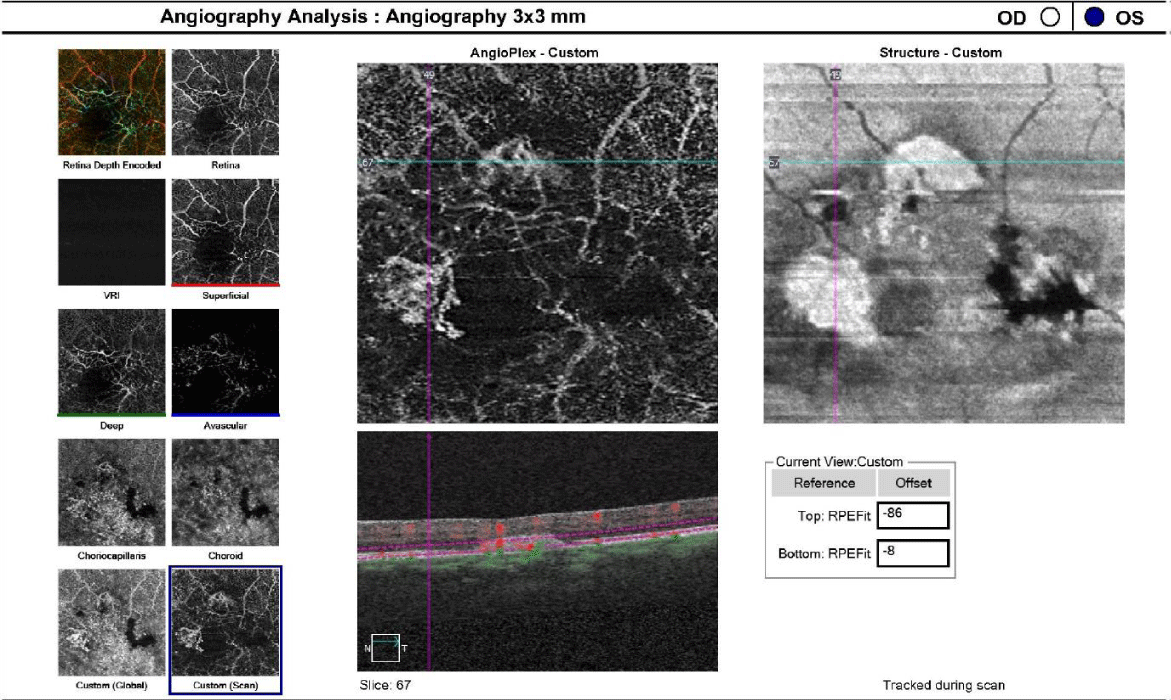
One drop of tropicamide 1% and phenylephrine 2.5% were instilled to each eye to allow a dilated retinal examination. The vitreous of each eye was grossly unremarkable with 1+ syneresis and no evidence of retinal traction in either eye. The optic nerve appeared well-perfused with distinct margins, 1+ peripapillary atrophy of the temporal disc margin and unremarkable neuroretinal rim with unremarkable nerve fiber findings in each eye. The cup-to-disc ratio was recorded as 0.50/0.50 right eye and 0.35/0.35 OS. Macular evaluation revealed a scattered drusen appearance with prominent pigmentary changes and atrophy in both eyes with subtle fibrotic changes in the left eye. Vessels appeared unremarkable in both eyes and peripheral retinal findings were unremarkable and recorded as flat, attached with negative holes, tears in both eyes. A baseline retinal photo of both eyes was taken (Figure 1). Due to the suspicious appearance of the fundus evaluation, the patient was also imaged using high-resolution Optical Coherence Tomography (SD-OCT) and SD-OCT angiography (SD-OCTA) in both eyes. The SD-OCT of the right eye indicated substantial choroidal and RPE disruption with sensory-retinal thinning and intra-retinal cystic formation (Figure 2) while the SD-OCT of the left eye indicated substantial choroidal disruption and sensory-retinal thinning similar to the right eye. However, foveal disruption nasal at the level of Bruch’s membrane was highly suggestive of neovascular changes in the left eye (Figure 3). SD-OCTA analysis of the right eye confirmed the IMT2 features showing an increased foveal avascular zone with distinct sensory retinal thinning (Figure 4) and SD-OCTA analysis of the left eye confirms the macular telangiectasia features showing sensory retinal thinning with the well-demarcated disruption of Bruch’s membrane consistent with neovascular changes (Figure 5). The OCTA Custom analysis of the left eye clearly indicates neovascular changes at the level of Bruch’s membrane (Figure 6).
The patient was diagnosed with chorioretinal macular scarring in both eyes secondary to macular telangiectasia type II with suspicion of choroidal neovascularization OS. The patient was started on a high bioavailability 20 milligram lutein / 5 milligram zeaxanthin oral supplement to provide potential mitigation of retinal inflammation and lipid membrane peroxidation. Additionally, the patient was referred to a retinal specialist for confirmation of the macular findings and initiation of anti-VEGF treatment for the neovascular changes OS.
IMTII is a highly complex conditions with a diverse of accompanying clinical presentations ranging from Coat’s disease [30] to diabetic retinopathy [31] to age-related macular degeneration [32] to presumed unilateral cases [33]. Common to this diverse set of clinical conditions is the relationship with neovascular membrane development and an anomalous retinal vascular complex [34,35]. An emerging need for a non-invasive mitigation to progressive inflammatory damage with concurrent stabilization to retinal cytoarchitecture is crucial. MP has demonstrated the ability to reduce singlet oxygen [36], moderate ROIs [37], inhibit cell membrane perioxidation [38] and reduce lipofuscin formation [39]. The presence of MP within the photoreceptor outer segments and retinal pigmented epithelium offers further support of the ROI and singlet oxygen reducing properties of MP [40,41]. Work by Beatty et al. confirmed the inhibition of light-induced oxidative damage within retinal tissue [42]. Their study showed that metabolic oxidative products including singlet oxygen, free peroxyl radicals, and ROIs are attenuated in the presence of MP. The free radical scavenging abilities of MP attenuates the progression of damage to lipophilic structures including cellular membranes and axonal structures [43]. MP shows a high affinity to lipid containing structures and, along with their efficiency in peroxyl radical mitigation, may serve a critical role in cell membrane protection and oxidative damage [44]. Central to the benefits of increased MPOD is the de novo nature of MP and the well-documented response to oral supplementation [45,46]. However, study of MP distribution response to oral supplementation of L and Z in patients with IMT2, have shown MP increases only in locations were MP was present at baseline [47]. According to Eposti et al. a majority of IMT2 patients demonstrate a normal total MP value across the central 21° with highly abnormal paracentral distribution within the central 16° of foveal eccentricity [48]. This finding further highlights the hypothesis that impaired L and Z transport and deposition underlies the pathogenesis of IMT2. Further research into MP deposition mechanisms in IMT2 patients is warranted.
- Peter Charbel Issa, Mark C Gillies, Emily Y Chew, Alan C Bird, Tjebo FC Heeren, Tunde Peto, et al. Macular telangiectasia type 2. Prog Retin Eye Res. 2013; 34: 49-77. https://goo.gl/cLSW5P
- Hee MR, Izatt JA, Swanson EA, Huang D, Schuman JS, Lin CP, et al. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995; 113: 325-332. https://goo.gl/12vrGH
- Voo I, Mavrofrides EC, Puliafito CA. Clinical applications of optical coherence tomography for the diagnosis and management of macular diseases. Ophthal Clin North Am. 2004; 17: 21-31. https://goo.gl/xRzJTgs
- Gass JD, Owakawa RT. Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthal. 1982; 100: 769-780.
- Gass JD, Blodi BA. Idiopathic juxtafoveolar retinal telangiectasis: update of classification and follow-up study. Ophthalmology. 1993; 100: 1536-1546. https://goo.gl/2EjL6g
- Powner MB, Gillies MC, Zhu M, Vevis K, Hunyor AP, Fruttiger M. Loss of Muller's cells and photoreceptors in macular telangiectasia type 2. Ophthalmology. 2013; 120: 2344-2352. https://goo.gl/fYKtvL
- Davidorf FH, Pressman MD, Chambers RB. Juxtafoveal telangiectasis: a name change. Retina. 2004; 24: 474-478. https://goo.gl/XdUs41
- Eliassi Rad B, Green WR. Histopathologic study of presumed parafoveal telangiectasis. Retina. 1999; 19: 332-335. https://goo.gl/cqQz7W
- Trabucchi G, Brancato R, Pierro L, Introini U, Sannace C. Idiopathic juxtafoveolar retinal telangiectasis and pigment epithelial hyperplasia: an optical coherence tomographic study. Arch Ophthalmol. 1999; 117: 405-406. https://goo.gl/uXnwr3
- Nalcı H, Şermet F, Demirel S, Ozmert E. Optic coherence angiography findings in type-2 macular telangiectasia. Turk J Ophthalmol. 2017; 47: 279-284. https://goo.gl/9Qz5tZ
- Toto L, Di Antonio L, Mastropasqua R, Mattei PA, Carpineto P, Borrelli E, et al. Multimodal imaging of macular telangiectasia type 2: focus on vascular changes using optical coherence tomography angiography. Investigative Invest Ophthalmol Vis Sci. 2016; 57: OCT268- OCT276. https://goo.gl/vbCsNk
- Villegas VM. Kovach JL. Optical coherence tomography angiography of macular telangiectasia type 2 with associated subretinal neovascular membrane. Case Rep Ophthalmol Med. 2017; 2017: 8186134. https://goo.gl/p1ez5s
- Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision Res. 1985; 25: 1531-1535. https://goo.gl/awiimn
- Handelman GJ, Dratz EA, Reay CC, van Kuijk JG. Carotenoids in the human macula and whole retina. Invest Ophthalmol Vis Sci. 1988; 29: 850-855. https://goo.gl/7FKdj6
- Whitehead AJ, Mares JA, Danis RP. Macular pigment: a review of current knowledge. Arch Ophthalmol. 2006; 124: 1038-1045. https://goo.gl/LGB6Nb
- Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001; 72: 215-223. https://goo.gl/nZ7rrs
- Pease PL, Adams AJ, Nuccio E. Optical density of human macular pigment. Vision Res. 1987; 27: 705-710. https://goo.gl/FwztZf
- Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthal Vis Sci. 1988; 29: 843-849. https://goo.gl/kX3vai
- Rapp LM, Maple SS, Choi JH. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest Ophthalmol Vis Sci. 2000; 41: 1200-1209. https://goo.gl/SseBSN
- Osterberg G. Topography of the layer of rods and cones in the human retina, Acta Ophthal. 1935; 6: 1. https://goo.gl/YxiT5i
- Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990; 292: 497-523. https://goo.gl/Ktbo6q
- Bone RA, Landrum JT, Friedes LM, Gomez CM, Kilburn MD, Menendez E, et al. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res. 1997; 64: 211-218. https://goo.gl/aiqmSm
- Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994; 272: 1413-1420. https://goo.gl/RD1gsy
- Chew EY, Clemons TE, SanGiovanni JP, Danis RP, Ferris FL, Elman MJ, et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthal. 2014; 132: 142-149. https://goo.gl/J4s4nJ
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008; 14: 194-198. https://goo.gl/VNYh3h
- Charbel Issa P, van der Veen RL, Stijfs A, Holz FG, Scholl HP, Berendschot TT. Quantification of reduced macular pigment optical density in the central retina in macular telangiectasia type 2. Exp Eye Res. 2009; 89: 25-31. https://goo.gl/2AcsKd
- Helb HM, Charbel Issa P, VAN DER Veen RL, Berendschot TT, Scholl HP, Holz FG. Abnormal macular pigment distribution in type 2 idiopathic macular telangiectasia. Retina. 2008; 28: 808-816. https://goo.gl/Cd7Nwf
- Zeimer, MB, Padge B, Heimes B, Pauleikhoff D. Idiopathic macular telangiectasia type 2: distribution of macular pigment and functional investigations. Retina. 2010; 30: 586-595. https://goo.gl/NZCh2W
- Issa PC, Berendschot TT, Staurenghi G, Holz FG, Scholl HP. Confocal blue reflectance imaging in type 2 idiopathic macular telangiectasia. Invest Ophthalmol Vis Sci. 2008; 49: 1172-1177. https://goo.gl/Fvh5W8
- Lee ST, Friedman SM, Rubin ML. Cystoid macular edema secondary to juxtafoveolar telangiectasis in Coats’ disease. Ophthalmic Surg. 1991; 22: 218-221. https://goo.gl/hN6LEM
- Chew EY, Murphy RP, Newsome DA, Fine SL. Parafoveal telangiectasis and diabetic retinopathy. Arch Ophthal. 1986; 104: 71-75. https://goo.gl/wsxw1w
- Yannuzzi LA, Negrao S, Iida T, Carvalho C, Rodriguez Coleman H, Slakter J, et al. Retinal angiomatous proliferation in age related macular degeneration. Retina. 2001; 21: 416-434. https://goo.gl/9YGU4h
- Zarei M, Mazloumi M, Karkhaneh R, Roohipoor R. Idiopathic macular telangiectasia type 2: A six-year study with multimodal imaging of a presumed unilateral case. Journal of Current Ophthalmology. 2018; 30: 368-373. https://goo.gl/7cqQDj
- Engelbrecht NE, Aaberg TM Jr, Sung J, Lewis ML. Neovascular membranes associated with idiopathic juxtafoveolar telangiectasis. Arch Ophthalmol. 2002; 120: 320-324. https://goo.gl/hpGMwY
- Lafaut BA, Aisenbrey S, Vanden Broecke C, Bartz-Schmidt KU. Clinic pathological correlation of deep retinal vascular anomalous complex in age related macular degeneration. Br J Ophthalmol. 2000; 84: 1269-1274. https://goo.gl/kHrfR7
- Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003; 23: 171-201. https://goo.gl/ZRbL2Y
- Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989; 274; 532-538. https://goo.gl/oSMCxu
- Lim BP, Nagao A, Terao J, Tanaka K, Suzuki T, Takama K. Antioxidant activity of xanthophylls on peroxyl radical-mediated phospholipid peroxidation. Biochim Biophys Acta. 1992; 1126; 178-184. https://goo.gl/vD4Q12
- Sundelin SP, Nilsson SE. Lipofuscin-formation in retinal pigment epithelial cells is reduced by antioxidants. Free Radic Biol Med. 2001; 31: 217-225. https://goo.gl/neJ5dV
- Sommerburg OG, Siems WG, Hurst JS, Lewis JW, Kliger DS, van Kuijk FJ. Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr Eye Res. 1999; 19: 491-495. https://goo.gl/CkXe93
- Rapp LM, Maple SS, Choi JH. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest Ophthalmol Vis Sci. 2000; 41: 1200-1209. https://goo.gl/Bj1GCe
- Beatty S, Boulton M, Henson D. Macular pigment and age-related macular degeneration. British Journal Ophthalmology. 1999; 83: 867-877. https://goo.gl/TVnwYE
- Landrum JT. Reactive Oxygen and Nitrogen Species in Biological Systems: Reactions and Regulation by Carotenoids. Carotenoids and Human Health. 2013: 57-101. https://goo.gl/XdQScu
- Stahl W, Sies H. Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta. 2005; 1740: 101-107. https://goo.gl/QsS4cb
- Rodriguez‐Carmona M. Kvansakul J, Harlow JA, Köpcke W, Schalch W, Barbur JL. The effects of supplementation with lutein and/or zeaxanthin on human macular pigment density and colour vision. Ophthalmic Physiol Opt. 2006; 26: 137-147. https://goo.gl/5xcJdS
- Bone RA, Landrum JT, Cao Y, Howard AN, Alvarez-Calderon F. Macular pigment response to a supplement containing meso-zeaxanthin, lutein and zeaxanthin. Nutr Metab (Lond). 2007; 4: 45-53. https://goo.gl/PYvx3B
- Zeimer MB, Krömer I, Spital G, Lommatzsch A, Pauleikhoff D. Macular telangiectasia: patterns of distribution of macular pigment and response to supplementation. Retina. 2010; 30: 1282-1293. https://goo.gl/X5kLa7
- Degli Esposti S, Egan C, Bunce C, Moreland JD, Bird AC, Robson AG. Macular pigment parameters in patients with macular telangiectasia and normal subjects: Implications of a novel analysis. Invest Ophthal Vis Sci. 2012; 53: 6568-6575. https://goo.gl/RRL8SR

Sign up for Article Alerts