Effects of Glaucoma and Snoring on Cerebral Oxygenation in the Visual Cortex: a Study Using functional Near Infrared Spectroscopy (fNIRS)?
Laura M Ward1, Ross T Aitchison1, Gemma Hill1, Jayne Imrie1, Anita J Simmers1, Gordon Morrison1, David Mansfield2, Graeme J Kennedy1 and Uma Shahani1*
1Departments of Vision Sciences, & Engineering Glasgow Caledonian University, 70 Cowcaddens Road, Glasgow, G4 0BA, United Kingdom
2Inverclyde Royal Hospital, NHS Greater Glasgow & Clyde, Larkfield Road, Greenock, PA16 0XN, United Kingdom
*Address for Correspondence: Uma Shahani, Departments of Vision Sciences and Engineering Glasgow Caledonian University, 70 Cowcaddens Road, Glasgow, G4 0BA, United Kingdom, Tel: +141- 331 -3695; ORCiD: orcid.org/0000-0003-2597-9334; E-mail: [email protected]/[email protected]
Submitted: 16 October 2018; Approved: 23 October 2018; Published: 26 October 2018
Citation this article: Ward LM, Aitchison RT, Hill G, Imrie J, Shahani U, et al. Effects of Glaucoma and Snoring on Cerebral Oxygenation in the Visual Cortex: a Study Using functional Near Infrared Spectroscopy (fNIRS). Int J Ophthal Vision Res. 2018;2(2): 017-025.
Copyright: © 2018 Ward LM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: fNIRS; Glaucoma; Haemodynamic response; Oxyhaemoglobin; Deoxyhaemoglobin; Snorers; Visual cortex
Download Fulltext PDF
Purpose: The purpose of this study was to investigate the effects of snoring and glaucoma on the visual Haemodynamic Response (HDR) using functional Near Infrared Spectroscopy (fNIRS).
Methods: We recruited 8 glaucoma patients (aged 56-79), 6 habitual snorers (aged 26-61) and 10 healthy control participants (aged 21-78). Glaucoma patients were of varying subtypes and under care of ophthalmologists. Prior to testing visual acuity, blood pressure, heart rate and a medical history were taken. HDRs were recorded over the primary visual cortex (V1) using a reversing checkerboard paradigm.
Results & Discussion: All participants showed the characteristic increase of Oxyhaemoglobin concentration ([HbO]) and decrease of Deoxyhaemoglobin concentration ([HbR]) during visual stimulation (p < 0.001, η2 = 0.78). Despite this, there were significant group differences with a large effect size (η2 = 0.28). During visual stimulation normal participants had greater [HbO] compared to snorers and glaucoma patients (p < 0.01). Both glaucoma patients and snorers presented with comparable HDR for [HbO] and [HbR] in V1. Importantly, during visual stimulation, the increased [HbO] in glaucoma patients correlated well with their visual fields and self-reported activities of daily living (r = -0.98, r = -0.82, p < 0.05). Both glaucoma patients and snorers presented with an attenuated HDR in V1. Our results suggest a possible vascular link between these conditions.
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide [1], and affects approximately 2% of the UK population over 40 [2]. It is characterised by the eventual development of optic neuropathy. This is expressed as progressive Optic Nerve Head (ONH) damage with associated visual field loss. The exact pathophysiology of glaucoma is not yet fully understood, and although there are many established risk factors, the specific vascular dysregulation associated with glaucoma is not clear. Moore et al. [3] propose that the impairment of ocular blood flow could result in retinal ganglion cell death and changes in the ONH: both crucial elements in the pathophysiology of all glaucoma subtypes. It has been proposed that vascular dysregulation in the ONH may be due to vasospasm (abnormal vascular responsiveness [4]), independent of the effects of age [5]. This in turn, makes the eye more sensitive to fluctuations in Intraocular Pressure (IOP) and systemic blood pressure [6,7]. It is generally assumed that any vascular dysregulation in the ONH is localised to the blood vessels of the disc, however, there is a growing body of research suggesting deficits continue further up the visual pathway [8-14]. Evidence from healthy adults supports this: an artificial increase in IOP has been shown to result in decreased Visually Evoked Potential (VEP) amplitude [15]. It is increasingly recognised that there are a number of systemic risk factors associated with glaucoma, that include hypertension [8,16], other vascular risk factors16, ocular perfusion pressure [17], migraine [18] & diabetes [19]. Given the above literature, it is not surprising that there is an increasing body of evidence linking glaucoma to Obstructive Sleep Apnoea (OSA).
Sleep related breathing disorders have been widely recognised as being on a continuum of pathophysiological cardiovascular and respiratory responses, all of which may have important acute and chronic health implications [20]. Within this spectrum is OSA, which is characterised as repeated interruptions of breathing during sleep, caused by the collapse of the upper airway [21]. OSA has significant health consequences including chronic sleep deprivation [22], cognitive decline [23-26], migraine [27], and cardiorespiratory dysfunction with consequences such as hypertension [28,29], heart failure or disease [25,30,31] and stroke [30,32-34]. Yet, OSA is often under-diagnosed as it requires a full polysomnographic evaluation which is an overnight diagnostic tool in sleep medicine that incorporates a battery of tests [22]. Apnoeic episodes can cause fluctuations in cerebral blood flow both in wakefulness and in sleep [35]. Many habitual snorers may have undiagnosed OSA [36], yet research shows snoring has similar implications on general health and cerebral haemoydnamics as OSA [21,37-39]. Although there is evidence that suggests that snoring influences blood pressure through obesity, OSA and nocturnal hypoxia [40], there is an overwhelming body of literature that concludes that there is an increased risk of hypertension in snorers that is independent of age, weight or other lifestyle factors [41-44]. Snoring and nocturnal hypoxia are related to a wide number of ophthalmic complications most likely with a multifactorial origin [19]. While we aim to investigate the potential link between these two conditions, this relationship is particularly difficult to study as it may be confounded by other underlying risk factors such as hypertension and diabetes [45], that are systemic in nature.
There is mixed evidence regarding the relationship between glaucoma and OSA, both conditions with haemodynamic consequences. Whilst some studies have shown an increased prevalence of OSA in glaucoma patients [46-54], others have failed to support this finding [55-59]. In early 2015 two meta-analyses were published both reporting a statistically significant relationship with OSA as having an association with an increased prevalence of glaucoma. The first study included 12 research papers and reported the odds ratio of 1.65 [60] to be the measure of association between glaucoma and OSA. Therefore, the odds of glaucoma and OSA occurring together were higher than in the normal population. The second meta-analysis paper used a more systematic approach, categorising literature into either case-control or cross-sectional studies - including 9 of the original 12 studies used by Wu and Liu [60] and reported an odds ratio of 1.96 and 1.41 respectively by pooling data from 2.3 million participants [61]. Despite the difficulty of the many studies’ varying methodologies, inclusion criteria, types of patients (both glaucoma and OSA), there is undoubtedly strong evidence to suggest that a relationship exists between these two conditions.
There are two theories attempting to explain this association: a mechanical theory and a vascular theory. The mechanical theory centres on the link between pressures and glaucoma: OSA causes sleep disturbances and changes in sympathetic tone [44], metabolic dysfunction and systemic inflammation [29], which subsequently leads to ONH damage and potentially glaucoma [48,61,62]. Alternatively, the vascular theory postulates that during apnoeic events (temporary suspension of breathing) in OSA, the decrease in oxygen levels leads to progressive asphyxia exhausting the cerebrovascular reserve [38]. This in turn, results in damage to the ONH [46], retinal nerve fibre layer [17], and may have the potential to cause changes in brain activation and morphology [63]. The current study contributes to the vascular theory as well investigates the apparent link between glaucoma and snoring by examining the Haemodynamic Response (HDR) associated with each.
functional Near Infrared Spectroscopy (fNIRS) can provide a measure of cortical processing of the associated HDR to neuronal firing, providing a non-invasive measure of imaging cortical processing. fNIRS is an optical neuroimaging technique that uses near infrared light to measure changes of blood oxygenation concentrations in the cortex, recording both Oxy- ([HbO]) and Deoxy-Haemoglobin ([HbR]) concentrations [64]. Previous research has used fNIRS to successfully characterise the HDR to visual, auditory and physiological stimuli, proving it to be a reliable neuroimaging technique [65-76]. This evidence has proven fNIRS to be a valuable neuroimaging tool for both normal and clinical populations to assess cerebral haemodynamics.
To our knowledge, this is the first study to use fNIRS to explore the haemodynamic relationship between glaucoma and snoring in terms of a task-related visual HDR. According to the vascular theory of OSA, apnoea reduces blood oxygenation, which in turn causes damage to the ONH. To test the hypothesis that apnoea also has detrimental effects on the primary Visual Cortex (V1), we completed a pilot study using fNIRS to measure the HDR in response to a reversing checkerboard stimulus in habitual snorers and in glaucoma patients.
Materials & Methods
Patients
We recruited participants with glaucoma or habitual snorers, with individual approximate age-matched controls. There were 8 glaucoma patients (range 56 - 78 years old, 3 females), 6 snorers (range 26 - 61 years old, 3 females) and 10 control participants (range 21 - 74 years old, 8 females). All participants were recruited from the Glasgow Caledonian University (GCU) Vision Centre patient database or from the GCU staff list. Participants who reported that they snored, and experienced frequent episodes of apnoea, were included in the snorers group and were considered potential OSA sufferers. Neither control nor glaucoma group participants reported such severe snoring and/or sleep related difficulties. None of the participants were current smokers, a number were on hypertensive medication and had other medical history; these details can be seen in table 1. A full medical history was taken and all participants completed a short general health questionnaire before beginning the tasks. This included a short health related Activities of Daily Living (ADL) questionnaire designed to assess how much illness interferes with patients’ daily living [77]. Resting blood pressure and heart rate were measured using a non-invasive blood pressure cuff applied to the left arm. This study was approved by the ethics Committee of Glasgow Caledonian University. Informed written consent was obtained from all participants prior to testing in accordance with the Declaration of Helsinki (Table 1).
Procedure
Visual assessment: All participants had measurements of visual acuity taken prior to testing. Glaucoma patients were selected from the GCU Eye Clinic database. All had undergone a recent eye examination within the GCU Eye Clinic and were under the care of an ophthalmologist for monitoring of their glaucoma. Measures of IOP, cup-to-disc ratio, and visual fields (Humphrey Visual Field Analyzer, Central 24-2 SITA FAST) were taken from the clinical record of their most recent eye examination (Table 1).
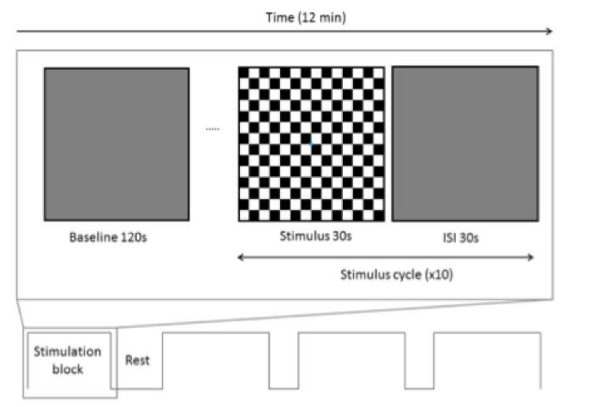
fNIRS protocol: A Frequency-Domain Multi-Distance (FD-MD) fNIRS system was used (OxiplexTSTM), allowing us to determine absolute quantities of cerebral haemoglobin chromophore concentration ([chromophore]). This instrumentation uses 2 wavelengths of light (690 nm, 830 nm), is frequency modulated (110 MHz) and uses near infrared light photon absorption, scattering and phase data to calculate change in [HbO] and [HbR]. To assess the HDR of V1 we recorded over O1 and O2 according to the EEG 10-20 International System of Electrode Placement [78]. A standard ISCEV visual stimulus was used [68,79] : full-field reversing checkerboard (100% contrast, 7.5 Hz temporal frequency, 30 minutes of arc check size). These parameters ensured all participants could comfortably perceive the stimulus and reliable data would be collected. Participants were seated in an upright position 1 meter away and were asked to fixate on a central blue dot displayed throughout the task. A pre-task baseline recording was collected in response to a grey screen of equal mean luminance to the checkerboard for 2 minutes. The reversing checkerboard or grey screen was intermittently displayed for 30 seconds each for 10 cycles. This instrumentation and protocol has been described in detail elsewhere [68,80,81]. Figure 1 shows the experimental set up.
Data analysis: Data were pre-processed in MATLAB as previously published68. Briefly, all data were normalised with respect to the pre-stimulus baseline and a moving average low-pass filter was applied. An average HDR to the checkerboard was calculated by averaging across all data responses to the experimental cycles. Lastly, a grand average was taken of the last 15 seconds of data per phase, representing the greatest stable change of the HDR [68,69,72,73,82-85].
Results
Visual Stimulation
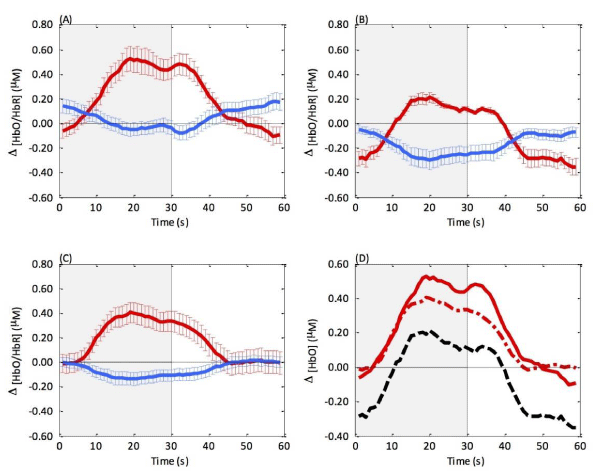
As no statistically significant differences were found between hemispheres (O1,O2) these data were averaged to create an overall V1 response. Additionally, to investigate potential differences at baseline, a 2-way ANOVA was performed on the pre-experimental data entering cerebral [chromophore] ([HbO], [HbR]) as a within-subject factor and group as a between-subject factor. This failed to show any group differences (F1,21 = 1.69, p > 0.05), indicating our samples were comparable before beginning the visual task and resting-state HDR were similar amongst participants. There was a clear response to the visual stimulation in all three groups. A two x 2 repeated measures ANOVA was performed with stimulation (checkerboard on, off) and cerebral [chromophore] ([HbO], [HbR]) as within-subject factors and groups entered as between-subject factors. There was a main effect of visual stimulation (F1,19 = 20.9, p < 0.001, η2 = 0.52), oxygenation (F1,19 = 7.47, p < 0.05, η2 = 0.28), and an interaction between the two (F1,18 = 58.2, p < 0.001, η2 = 0.75). When examining the overall HDR for each group this can be seen as a characteristic increase in [HbO] and decrease in [HbR] during the checkerboard stimulation compared to the baseline grey screen (Figure 2, A-C). Group differences were apparent with 3 group interactions all with large effect sizes: group and stimulation (F1,19 = 5.63, p < 0.05, η2 = 0.37), group and oxygenation (F1,19 = 11.57, p < 0.001, η2 = 0.55), and group, stimulation and oxygenation (F2,19 = 3.69, p < 0.05, η2 = 0.28). Post-hoc independent samples t-tests were used to compare the group differences with corrected confidence intervals of 99.99%. During the ‘on’ phase of visual stimulation, controls had a greater change of [HbO] compared to snorers (mean difference = 0.393, t12 = 3.45, p < 0.01, ds = 1.92), and glaucoma patients (mean difference = 0.350, t10 = 3.93, p < 0.01, ds = 1.91). These results represent the greatest stable change in the HDR in response to the reversing checkerboard and demonstrate compelling differences between the groups with large effect sizes. This can be seen in the overall HDR in figure 2 (D) wherein both glaucoma (dashed red line) and snorers (solid black line) demonstrate an attenuated [HbO] response to visual stimulation. Glaucoma and snorer groups were statistically comparable in terms of their HDR in V1 in both ‘on’ and ‘off’ phases for both [HbO] and [HbR] (p > 0.05). Please note these statistical results remained when the two glaucoma patients with concomitant AMD were removed from the analyses. In terms of [HbR] all groups were comparable during both phases of visual stimulation (p > 0.05) (Figure 2).
Glaucoma correlations
To investigate the relationship between optical measures of visual function and cerebral oxygenation, we computed a correlational analysis within the glaucoma group. Bias corrected and accelerated bootstrap confidence intervals using 5000 resamples and a 95% confidence interval [86] were used. All outcome measures were entered (fNIRS data, visual acuity, visual fields, blood pressure, heart rate, ADL and age). It has been suggested that the pattern standard deviation of the visual fields may underestimate the extent of glaucomatous damage [87]. We therefore used the mean defect as a global index. The IOPs were excluded from the statistical analysis because of normal daily fluctuations of IOP and also because several of the glaucoma patients used eye drops to lower their IOP (Table 1). There was a significant relationship between V1 [HbO] and the left eye visual field (r = -0.98, p < 0.001, CI = [-0.49 -1]). Also, the right visual field correlated significantly with patients’ reported ADL (r = -0.82, p < 0.05, CI = [-0.02 -0.99]). Note that these statistics remained when glaucoma patients with only one glaucomatous eye were removed. These correlations imply that those patients with worse mean defects on their visual fields had higher ADL scores, and smaller changes in [HbO] in response to the checkerboard stimulation, regardless of the inclusion/ exclusion of those glaucoma patients with concomitant AMD.
Discussion
To clarify the potential vascular relationship between glaucoma and OSA, we used fNIRS to quantify changes in [HbO] and [HbR] during a visual task. As this was a pilot study with inherent constraints, we recruited all subtypes of glaucoma patients and habitual snorers, but recruitment to these groups was mutually exclusive. Although all participants produced a reliable V1 HDR to the reversing checkerboard, with a distinctive increase of [HbO] and decrease of [HbR] during visual stimulation, there were statistically significant group differences with strong effect sizes. In response to visual stimulation, snorers had the smallest change in [HbO], followed by glaucoma patients who also showed an attenuated response in comparison to healthy participants (Figure 1D). These results show that 28% of variance in the HDR elicited by visual stimulation, is attributable to group characteristics (p < 0.05, η2 = 0.28), therefore the magnitude of these findings is considerable. Here, we report the novel finding that in response to visual stimulation, both glaucoma patients and habitual snorers present with an attenuated [HbO] response in comparison to healthy participants. This finding relates specifically to the systemic influences of each of these syndromes. Our glaucoma patients did not snore, and our habitual snorers did not have any glaucoma. Therefore, these groups were mutually exclusive. Accordingly, these results support the vascular theory hypothesis that apnoea may affect not just the ONH but further up in the visual pathway such as the visual cortex. There is significant support for this evidence of neuronal degeneration in glaucoma extending beyond the retina. In the primate model of glaucoma, neuronal loss was reported by Yucel et al. [88], in both M and P pathways of the Lateral Geniculate Nucleus (LGN) of the fellow eye, as well as the glaucomatous eye. This degeneration has been confirmed in humans using functional Magnetic Resonance Imaging (fMRI): not only do glaucoma patients present with LGN atrophy in comparison with healthy controls [11], but the degeneration correlates with their clinical severity as measured by visual fields [89]. High tension and primary open-angle glaucoma patients show a decrease in V1 cortical activation as measured by fMRI [90-94], whereas normal tension glaucoma patients do not [90]. Diffusion Tensor Imaging (DTI) has also highlighted the involvement of the entire visual pathway, with clinical glaucoma stages correlating with DTI parameters thought to reflect axonal damage to the optic radiations [95]. Lastly, a clinicopathological case of a male glaucoma patient supported this with visual pathway damage from the LGN to V1 correlating with visual field loss [10]. Regarding OSA, previous reports have shown no significant differences in V1 grey matter volume between patients and controls [63]; ours is the first study to report a reduction in functional activation in habitual snorers in comparison to health controls (Figure 1). These results complement previous literature in which fNIRS was used to record the HDR from frontal cortex of sleeping OSA patients, and where it was demonstrated that there was a reduced cortical response [67,96-99].
We also show that ADL, a self-report measure of the patients’ perspective of how much their illness interferes with their social/ role activities, significantly correlated with visual field mean defect. This short but effective questionnaire has provided results similar to more extensive quality of life assessments for glaucoma patients [100,101]. Visual fields also correlated with [HbO] responses during visual stimulation. The results illustrated that those patients with worse fields presented with an attenuated [HbO] response to checkerboard stimulation, and felt subjectively that their vision was having a negative impact on their overall daily life. These findings directly support previous neuroimaging evidence of a strong correlation between reduced resting cerebral blood flow and loss of visual function [90-94]. The modified HDR in V1 in both resting state and functional tasks, indicates that the vascular dysregulation linked to glaucoma, has effects further up the visual pathway beyond retinal ganglion cells as previously discussed. We propose that visual field defects have a direct impact on V1 functioning, though this may be due to a number of reasons within the visual pathway such as retinal ganglion cell loss or vascular dysregulation of V1.
An interesting discussion point is Subject 17 who was referred to an ophthalmologist from the GCU Eye Clinic for suspected glaucoma and was tested prior to his ophthalmology appointment. He was subsequently assessed and diagnosed with narrow anterior chamber angles and asymmetric optic disc cupping, but with no evidence of glaucoma. Yet, compared to the other glaucoma patients, he had a similar cup-to-disk ration, IOP and visual fields (Table 1). When examining the HDR in V1, this subject also displayed a median response within the glaucoma group. This highlights the variability of the glaucoma syndrome along with the current diagnostic and monitoring techniques for disease progression. Here we propose fNIRS to be an objective tool that could assist in glaucoma diagnosis and monitoring. FDMD-fNIRS is portable, cost effective and could easily be used in an eye clinic or hospital setting to aid clinicians. In this way, fNIRS could be used to discriminate potential glaucoma sufferers based on their HDR. Additionally, during glaucoma screening and diagnosis, patients’ potential risk for developing OSA could be highlighted by NIRS for referral to a sleep clinic for further testing. Clearly future work would need to provide normative NIRS data for glaucoma patients who are not yet undergoing treatment. In this way we could ascertain whether this attenuated V1 HDR can be accounted for by reduced neuronal input to the visual cortex due to glaucomatous defects in the visual field.
Various limitations to this study warrant discussion. Firstly, our small sample size for each experimental group makes it difficult to reach any generalised conclusions to the wider populations. This weakness is not novel as it is clear that both of these syndromes do not have pathognomonic signs, with numerous overlapping risk factors and patients presenting with varied characteristics. However, as this was an exploratory study, with few exclusion criteria, we aimed to provide a representative sample of data on the haemodynamic consequences of both conditions. Future research would ideally involve diagnosed OSA patients using overnight polysomnography and NIRS, in which more generalised conclusions may be made. Here we present characteristic findings relating habitual snorers to glaucoma patients and healthy controls. Our data show for the first time that snorer’s and glaucoma patients’ haemodynamics are disturbed during a visual task. During checkerboard stimulation glaucoma patients and snorers were statistically comparable in terms of their HDR, with an attenuated [HbO] response compared to controls. Given the multiple systemic associations between glaucoma and OSA, this work contributes to the understanding of the haemodynamic consequences of these conditions, showing NIRS to be a suitable tool in assessing the HDR of patient populations. The difficulty in this field of research is to disentangle the numerous pathophysiological links between the two conditions. Indeed, we agree with Shi et al. [61], who highlight the necessity of careful control of systemic confounds when investigating glaucoma and OSA, and suggest OSA may merely be a marker of poor health, and not necessarily an independent risk factor for glaucoma.
To conclude, the HDR recorded from V1 to visual stimulation is attenuated regardless of the pathophysiology of our snorers and glaucoma patients. Our results show the potential of NIRS to assess changes in the HDR in identifying potential ‘at risk’ glaucoma patients. Likewise for habitual snorers before they develop OSA. Further data on untreated glaucoma patients is crucial, and it may be that the V1 HDRs indicate early glaucomatous changes. This, in conjunction with existing diagnostic techniques and fNIRS measures of V1, would be advantageous in the diagnosis of glaucoma. We have presented novel results that show the potential of fNIRS as a method that provides an objective measure of absolute cerebral oxygenation in clinical populations.
Acknowledgements
Our deepest gratitude is extended to all the participants and patients that volunteered their time to take part in this study.
Author Contribution Statement
LW, RA, GH, JI, DM, GK and US made a substantial contribution to the concept and design, acquisition of data or analysis and interpretation of data. LW and US drafted the article or revised it critically for important intellectual content.
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012; 31: 152-181. https://goo.gl/1FMxme
- Glaucoma Costing Report: Implementing NICE guidance. London. 2009. https://goo.gl/p2dLx3
- Moore D, Harris A, Wudunn D, Kheradiya N, Siesky B. Dysfunctional regulation of ocular blood flow: A risk factor for glaucoma. Clin Ophthalmol. 2008; 2: 849-861. https://goo.gl/U8dxBK
- O’Brien C. Vasospasm and glaucoma. Br J Ophthalmol. 1998; 82: 855-856. https://goo.gl/uaSotv
- Boehm AG, Koeller AU, Pillunat LE. The effect of age on optic nerve head blood flow. Invest Ophthalmol Vis Sci. 2005; 46: 1291-1295. https://goo.gl/SsceUi
- Flammer J, Pache M, Resink T. Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Prog Retin Eye Res. 2001; 20: 319-349. https://goo.gl/QG17pE
- Hayreh SS. Blood flow in the optic nerve head and factors that may influence it. Prog Retin Eye Res. 2001; 20: 595-624. https://goo.gl/tAyJN3
- Gugleta K, Fuchsjager-Mayrl G, Orgul S. Is neurovascular coupling of relevance in glaucoma? Surv Ophthalmol. 2007; 52: 139-143. https://goo.gl/iZ77Cz
- Gupta N, Yucel YH. Brain changes in glaucoma. Eur J Ophthalmol. 2003; 13: 32-35. https://goo.gl/wDPaSd
- Gupta N, Ang LC, Noël de Tilly L, Bidaisee L, Yucel YH. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol. 2006; 90: 674-678. https://goo.gl/zyFnkE
- Gupta N, Greenberg G, de Tilly LN, Gray B, Polemidiotis M, Yucel YH. Atrophy of the lateral geniculate nucleus in human glaucoma detected by magnetic resonance imaging. Br J Ophthalmol. 2009; 93: 56-60. https://goo.gl/pTFxBB
- Zeitz O, Mayer J, Hufnagel D, Praga R, Wagenfeld L, Galambos P, et al. Neuronal activity influences hemodynamics in the paraoptic short posterior ciliary arteries: A comparison between healthy and glaucomatous subjects. Investig Ophthalmol Vis Sci. 2009; 50: 5846-5850. https://goo.gl/Bnkm2Y
- Nucci C, Martucci A, Cesareo M, Mancino R, Russo R, Bagetta G, et al. Brain involvement in glaucoma: advanced neuroimaging for understanding and monitoring a new target for therapy. Curr Opin Pharmacol. 2013; 13: 128-133. https://goo.gl/r466ZY
- Yücel YH, Gupta N. Paying attention to the cerebrovascular system in glaucoma. Can J Ophthalmol. 2008; 43: 342-346. https://goo.gl/pTvRcH
- Kremmer S, Tolksdorf Kremmer A, Stodtmeister R. Simultaneous registration of VECP and pattern ERG during artificially raised intraocular pressure. Ophthalmologica. 1995; 209: 233-241. https://goo.gl/Sr2fem
- Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007; 114: 1965-1972. https://goo.gl/uVur9f
- Karakucuk S, Goktas S, Aksu M, Erdogan N, Demirci S, Oner A, et al. Ocular blood flow in patients with obstructive sleep apnea syndrome (OSAS). Graefe’s Arch Clin Exp Ophthalmol. 2008; 246: 129-134. https://goo.gl/HmCYNs
- Girkin CA, McGwin G, McNeal SF, Owsley C. Is there an association between pre-existing sleep apnoea and the development of glaucoma? Br J Ophthalmol. 2006; 90: 679-681. https://goo.gl/UNqYjZ
- Attarian H, Viola-Saltzman M, Jay WM. Ophthalmic and neuro-ophthalmic complications of obstructive sleep apnoea. Neuro-Ophthalmology. 2011; 35: 236-241. https://goo.gl/KiHRQa
- Wenner JB, Cheema R, Ayas NT. Clinical manifestations and consequences of obstructive sleep apnea. J Cardiopulm Rehabil Prev. 2009; 29: 76-83. https://goo.gl/m9fgwh
- Mohsenin V. Sleep-related breathing disorders and risk of stroke. Stroke. 2001; 32: 1271-1278. https://goo.gl/YtyMtd
- Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005; 28: 499-521. https://goo.gl/s1zkGZ
- Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: Towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002; 11: 1-16. https://goo.gl/RWtGFt
- Ayalon L, Peterson S. Functional central nervous system imaging in the investigation of obstructive sleep apnea. Curr Opin Pulm Med. 2007; 13: 479-483. https://goo.gl/8gQTht
- Knecht KM, Alosco ML, Spitznagel MB, Cohen R, Raz N, Sweet L, et al. Sleep apnea and cognitive function in heart failure. Cardiovasc Psychiatry Neurol. 2012; 2012: 402079. https://goo.gl/RoX19W
- Canessa N, Castronovo V, Cappa SF, Aloia MS, Marelli S, Falini A, et al. Obstructive Sleep Apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011; 183: 1419-1426. https://goo.gl/TGVwye
- Faridi O, Park SC, Liebmann JM, Ritch R. Glaucoma and obstructive sleep apnoea syndrome. Clin Experiment Ophthalmol. 2012; 40: 408-419. https://goo.gl/7MhaJR
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000; 342: 1378-1384. https://goo.gl/RNZvPd
- Lattimore J DL, Celermajer DS, Wilcox I. Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol. 2003; 41: 1429-1437. https://goo.gl/5dQnej
- Koskenvuo M, Kaprio J, Telakivi T, Partinen M, Heikkila K, Sarna S. Snoring as a risk factor for ischaemic heart disease and stroke in men. Br Med J. 1987; 294: 16-19. https://goo.gl/uZddGm
- Norton PG, Dunn E V. Snoring as a risk factor for disease: an epidemiological survey. Br Med J. 1985; 291: 630-632. https://goo.gl/8Q85si
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005; 365: 1046-1053. https://goo.gl/PAkDbw
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005; 353: 2034-2041. https://goo.gl/ABXeXQ
- Li M, Hou WS, Zhang XW, Tang ZY. Obstructive sleep apnea and risk of stroke: a meta-analysis of prospective studies. Int J Cardiol. 2014; 172: 466-469. https://goo.gl/HgdoQh
- Hajak G, Klingelhofer J, Schulz Varszegi M, Sander D, Ruther E. Sleep Apnea Syndrome and Cerebral Hemodynamics. Chest. 1996; 110: 670-679. https://goo.gl/ZTqoLw
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993; 328: 1230-1235. https://goo.gl/QWnn6G
- Partinen M, Palomaki H. Snoring and Cerebral Infarction. Lancet. 1985; 2: 1325-1326. https://goo.gl/spS4RQ
- Pizza F, Biallas M, Wolf M, Werth E, Bassetti CL. Nocturnal cerebral hemodynamics in snorers and in patients with obstructive sleep apnea: a near-infrared spectroscopy study. Sleep. 2010; 33: 205-210. https://goo.gl/xHt5RT
- Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax. 1991; 46: 85-90. https://goo.gl/YeFUdS
- Hoffstein V, Rubinstein I, Mateika S, Slutsky AS. Determinants of blood pressure in snorers. Lancet. 1988; 2: 992-994. https://goo.gl/Uu3n6j
- Lindberg E, Janson C, Gislason T, Svardsudd K, Hetta J, Boman G. Snoring and hypertension: a 10 year follow-up. Eur Respir J. 1998; 11: 884-889. https://goo.gl/rV3m4u
- Gislason T, Benediktsdóttir B, Bjornsson JK, Kjartansson G, Kjeld M, Kristbjarnarson H. Snoring, hypertension, and the sleep apnea syndrome: an epidemiologic survey of middle-aged women. Chest. 1993; 103: 1147-1151. https://goo.gl/u5dUsf
- Koskenvuo M, Kaprio J, Partinen M, Langinvainio H, Sarna S, Heikkila K. Snoring as a risk factor for hypertension and angina pectoris. Lancet. 1985; 1: 893-896. https://goo.gl/Ys3NNi
- Parish J, Shepard JW. Cardiovascular effects of sleep disorders. Chest. 1990; 67: 1220-1226. https://goo.gl/3myCdv
- Fraser CL. Obstructive sleep apnea and optic neuropathy: is there a link? Curr Neurol Neurosci Rep. 2014; 14: 465. https://goo.gl/PNr1Sh
- Tsang CS, Chong SL, Ho CK, Li MF. Moderate to severe obstructive sleep apnoea patients is associated with a higher incidence of visual field defect. Eye. 2006; 20: 38-42. https://goo.gl/7CGiQw
- Sergi M, Salerno DE, Rizzi M, Blini M, Andreoli A, Messenio D, et al. Prevalence of normal tension glaucoma in obstructive sleep apnea syndrome patients. J Glaucoma. 2007; 16: 42-46. https://goo.gl/Xw5W2t
- Lin CC, Hu CC, Ho JD, Chiu HW, Lin HC. Obstructive sleep apnea and increased risk of glaucoma: a population-based matched-cohort study. Ophthalmology. 2013; 120: 1559-1564. https://goo.gl/Ziq3Ey
- Onen SH, Mouriaux F, Berramdane L, Dascotte JC, Kulik JF, Rouland JF. High prevalence of sleep-disordered breathing in patients with primary open-angle glaucoma. Acta Ophthalmol Scand. 2000; 78: 638-641. https://goo.gl/hD4fPQ
- Boyle Walker M, Semes LP, Clay OJ, Liu L, Fuhr P. Sleep apnea syndrome represents a risk for glaucoma in a veterans’ affairs population. ISRN Ophthalmol. 2011; 2011: 920767. https://goo.gl/WMTJzB
- Mojon DS, Hess CW, Goldblum D, Fleischhauer J, Koerner F, Bassetti C, et al. High prevalence of glaucoma in patients with sleep apnea syndrome. Ophthalmology. 1999; 106: 1009-1012. https://goo.gl/xdwrba
- Hashim SP, Al Mansouri FA, Farouk M, Al Hashemi AA, Singh R. Prevalence of glaucoma in patients with moderate to severe obstructive sleep apnea: ocular morbidity and outcomes in a 3 year follow-up study. Eye. 2014; 28: 1-6. https://goo.gl/pPC2Bp
- Bilgin G. Normal-tension glaucoma and obstructive sleep apnea syndrome: a prospective study. BMC Ophthalmol. 2014; 14: 27. https://goo.gl/PuWUNJ
- Balbay EG, Balbay O, Annakkaya AN, Suner KO, Yuksel H, Tunç M, et al. Obstructive sleep apnoea syndrome in patients with primary open-angle glaucoma. Hong Kong Med J. 2014; 20: 1-7. https://goo.gl/eTvzuT
- Khandgave TP, Puthran N, Ingole AB, Nicholson AD. The assessment of sleep apnoea as a risk factor in glaucoma. J Clin Diagnostic Res. 2013; 7: 1391-1393. https://goo.gl/Z8JygA
- Geyer O, Cohen N, Segev E, Rath EZ, Melamud L, Peled R, et al. The prevalence of glaucoma in patients with sleep apnea syndrome: same as in the general population. Am J Ophthalmol. 2003; 136: 1093-1096. https://goo.gl/Rk5gq3
- Aptel F, Chiquet C, Tamisier R, Sapene M, Martin F, Stach B, et al. Association between glaucoma and sleep apnea in a large French multicenter prospective cohort. Sleep Med. 2014; 15: 576-581. https://goo.gl/scykbt
- Kadyan A, Asghar J, Dowson L, Sandramouli S. Ocular findings in sleep apnoea patients using continuous positive airway pressure. Eye. 2010; 24: 843-850. https://goo.gl/kaYvtT
- Roberts TV, Hodge C, Graham SL, Burlutsky G, Mitchell P. Prevalence of nocturnal oxygen desaturation and self-reported sleep-disordered breathing in glaucoma. J Glaucoma. 2009; 18: 114-118. https://goo.gl/MgBssH
- Wu X, Liu H. Obstructive sleep apnea / hypopnea syndrome increases glaucoma risk : evidence from a meta-analysis. Int J Clin Exp Med. 2015; 8: 297-303. https://goo.gl/LpHCR5
- Shi Y, Liu P, Guan J, Lu Y, Su K. Association between glaucoma and obstructive sleep apnea syndrome: a meta-analysis and systematic review. PLoS One. 2015; 10: 0115625. https://goo.gl/189TLV
- Abdal H, Pizzimenti JI, Pruvis CC. The eye in sleep apnea syndrome. Sleep Med. 2006; 7: 107-115. https://goo.gl/5Kwif5
- Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med. 2003; 4: 451-454. https://goo.gl/8J32Qt
- Villringer A, Planck J, Hock C, Schleinkofer L, Dirnagl U. Near infrared spectroscopy (NIRS): a new tool to study hemodynamic changes during activation of brain function in human adults. Neurosci Lett. 1993; 154: 101-104. https://goo.gl/yN2kGm
- Gatto R, Hoffman WE, Mueller M, Paisansathan C, Charbel F. Age effects on brain oxygenation during hypercapnia. J Biomed Opt. 2007; 12: 62113. https://goo.gl/LV5ZMX
- Safonova LP, Michalos A, Wolf U, Wolf M, Hueber DM, Choi JH, et al. Age-correlated changes in cerebral hemodynamics assessed by near-infrared spectroscopy. Arch Gerontol Geriatr. 2004; 39: 207-225. https://goo.gl/vwUTuu
- Zhang Z, Schneider M, Fritschi U, Lehner I, Khatami R. Near-infrared spectroscopy (NIRS) as a useful tool to evaluate the treatment efficacy of positive airways pressure therapy in patients with obstructive sleep apnea syndrome (OSAS): A pilot study. J Innov Opt Health Sci. 2014; 7: 1450014. https://goo.gl/xoyUdf
- Ward LM, Aitchison RT, Tawse M, Simmers AJ, Shahani U. Reduced haemodynamic response in the ageing visual cortex measured by absolute fNIRS. PLoS One. 2015; 10: 125012. https://goo.gl/2rvWzw
- Wijeakumar S, Shahani U, Simpson WA, McCulloch DL. Localization of hemodynamic responses to simple visual stimulation: an fNIRS study. Investig Opthalmology Vis Sci. 2012; 53: 2266-2273. https://goo.gl/RQaCGu
- Chen LC, Sandmann P, Thorne JD, Herrmann CS, Debener S. Association of concurrent fNIRS and EEG signatures in response to auditory and visual stimuli. Brain Topogr. 2015; 28: 710-725. https://goo.gl/n2NWWR
- Colier WN, Quaresima V, Wenzel R, van der Sluijs MC, Oeseburg B, Ferrari M, et al. Simultaneous near-infrared spectroscopy monitoring of left and right occipital areas reveals contra-lateral hemodynamic changes upon hemi-field paradigm. Vision Res. 2001; 41: 97-102. https://goo.gl/P2o9WV
- Fabiani M, Gordon BA, Maclin EL, Pearson MA, Brumback Peltz CR, Low KA, et al. Neurovascular coupling in normal aging: a combined optical, ERP and fMRI study. Neuroimage. 2014; 85: 592-607. https://goo.gl/bKY7DY
- Gratton G, Goodman-Wood MR, Fabiani M. Comparison of neuronal and hemodynamic measures of the brain response to visual stimulation: an optical imaging study. Hum Brain Mapp. 2001; 13: 13-25. https://goo.gl/8mQAUc
- Hallacoglu B, Sassaroli A, Wysocki M, Guerrero Berroa E, Schnaider Beeri M, Haroutunian V, et al. Absolute measurement of cerebral optical coefficients, hemoglobin concentration and oxygen saturation in old and young adults with near-infrared spectroscopy. J Biomed Opt. 2012; 17: 81406-1. https://goo.gl/DwaeC7
- Kato T, Kamei A, Takashima S, Ozaki T. Human visual cortical function during photic stimulation monitoring by means of near-infrared spectroscopy. J Cereb Blood Flow Metab; 13: 516-520. https://goo.gl/WuJR6o
- Jasdzewski G, Strangman G, Wagner J, Kwong KK, Poldrack RA, Boas DA. Differences in the hemodynamic response to event-related motor and visual paradigms as measured by near-infrared spectroscopy. Neuroimage. 2003; 20: 479-488. https://goo.gl/kJEdbR
- Kate Lorig, Anita Stewart, Philip Ritter, Virginia Gonzalez, Diana Laurent, John Lynch. Outcome measures for health education and other health care interventions. SAGE, 1996. https://goo.gl/HLSPDw
- Jasper H. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalogr Clin Neurophysiol. 1957; 10: 370-375. https://goo.gl/rR257V
- Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Tormene AP, et al. ISCEV standard for clinical visual evoked potentials (2009 update). Documenta Ophthalmologica. 2010; 120: 111-119. https://goo.gl/j1iMhr
- Sergio Fantini, Maria Angela Franceschini, John S. Maier, Scott A. Walker, Beniamino B. Barbieri, Enrico Gratton. Frequency-domain multichannel optical detector for noninvasive tissue spectroscopy and oximetry. Opt Eng. 1995; 34: 32-42. https://goo.gl/fu3XLS
- Gatto R, Hoffman W, Mueller M, Flores A, Valyi Nagy T, Charbel FT. Frequency domain near-infrared spectroscopy technique in the assessment of brain oxygenation: a validation study in live subjects and cadavers. J Neurosci Methods. 2006; 157: 274-277. https://goo.gl/zDJeh8
- McIntosh MA, Shahani U, Boulton RG, McCulloch DL. Absolute quantification of oxygenated hemoglobin within the visual cortex with functional near infrared spectroscopy (fNIRS). Investig Opthalmology Vis Sci. 2010; 51: 4856-4860. https://goo.gl/kbrF9j
- Wijeakumar S, Shahani U, McCulloch DL, Simpson WA. Neural and vascular responses to fused binocular stimuli: a VEP and fNIRS study. Investig Opthalmology Vis Sci. 2012; 53: 5881-5889. https://goo.gl/Y9YK3r
- Herrmann MJ, Ehlis AC, Wagener A, Jacob CP, Fallgatter AJ. Near-infrared optical topography to assess activation of the parietal cortex during a visuo-spatial task. Neuropsychologia. 2005; 43: 1713-1720. https://goo.gl/uMs4V3
- Takahashi K, Ogata S, Atsumi Y, et al. Activation of the visual cortex imaged by 24-channel near-infrared spectroscopy. J Biomed Opt. 2000; 5: 93-96. https://goo.gl/U4JKyM
- Efron B. Better bootstrap confidence intervals. J Am Stat Assoc. 1987; 82: 171 - 185. https://goo.gl/abMvKX
- Artes PH, Nicolela MT, LeBlanc RP, Chauhan BC. Visual field progression in glaucoma: total versus pattern deviation analyses. Investig Opthalmology Vis Sci. 2005; 46: 4600. https://goo.gl/8FZN5L
- Yucel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003; 22: 465-481. https://goo.gl/t1xy6A
- Dai H, Mu KT, Qi JP, Wang CY, Zhu WZ, Xia LM, et al. Assessment of lateral geniculate nucleus atrophy with 3T MR imaging and correlation with clinical stage of glaucoma. Am J Neuroradiol. 2011; 32: 1347-1353. https://goo.gl/gEppzq
- Lestak J, Tintera J, Svata Z, Ettler L, Rozsival P. Glaucoma and CNS. Comparison of fMRI results in high tension and normal tension glaucoma. Biomed Pap. 2014; 158: 144-153. https://goo.gl/Nn4poG
- Qing G, Zhang S, Wang B, Wang N. Functional MRI signal changes in primary visual cortex corresponding to the central normal visual field of patients with primary open-angle glaucoma. Investig Opthalmology Vis Sci. 2010; 51: 4627-4634. https://goo.gl/UKDgyc
- Duncan RO, Sample PA, Weinreb RN, Bowd C, Zangwill LM. Retinotopic organization of primary visual cortex in glaucoma: Comparing fMRI measurements of cortical function with visual field loss. Prog Retin Eye Res. 2007; 26: 38-56. https://goo.gl/79U3uL
- Duncan RO, Sample PA, Bowd C, Weinreb RN, Zangwill LM. Arterial spin labeling fMRI measurements of decreased blood flow in primary visual cortex correlates with decreased visual function in human glaucoma. Vision Res. 2012; 60: 51-60. https://goo.gl/2xwWTC
- Chen Z, Lin F, Wang J, Li Z, Dai H, Mu K, et al. Diffusion tensor magnetic resonance imaging reveals visual pathway damage that correlates with clinical severity in glaucoma. Clin Experiment Ophthalmol. 2013; 41: 43-49. https://goo.gl/nVi7KG
- Garaci FG, Bolacchi F, Cerulli A, Melis M, Spano A, Cedrone C, et al. Optic nerve and optic radiation neurodegeneration in patients with glaucoma: In vivo analysis with 3-T diffusion-tensor MR Imaging. Radiology. 2009; 252: 496-501. https://goo.gl/NTE29w
- Olopade CO, Mensah E, Gupta R, Huo D, Picchietti DL, Gratton E, et al. Noninvasive determination of brain tissue oxygenation during sleep in obstructive sleep apnea: a near-infrared spectroscopic approach. Sleep. 2007; 30: 1747-1755. https://goo.gl/7VwBDy
- Matsuo A, Inoue Y, Namba K, Chiba H. Changes in cerebral hemoglobin indices in obstructive sleep apnea syndrome with nasal continuous positive airway pressure treatment. Sleep Breath. 2011; 15: 487-492. https://goo.gl/wVDQvc
- Hayakawa T, Terashima M, Kayukawa Y, Ohta T, Okada T. Changes in cerebral oxygenation hemodynamics during obstructive sleep apneas. Chest. 1996; 109: 916-921. https://goo.gl/L4SNYB
- Valipour A, McGown AD, Makker H, O'Sullivan C, Spiro SG. Some factors affecting cerebral tissue saturation during obstructive sleep apnoea. Eur Respir J. 2002; 20: 444-450. https://goo.gl/Scg986
- Spaeth G, Walt J, Keener J. Evaluation of quality of life for patients with glaucoma. Am J Ophthalmol. 2006; 141: 3-14. https://goo.gl/JLkVr7
- Goldberg I, Clement CI, Chiang TH, Walt JG, Lee LJ, Graham S, et al. Assessing quality of life in patients with glaucoma using the glaucoma quality of life-15 (GQL-15) questionnaire. J Glaucoma. 2009; 18: 6-12. https://goo.gl/t7wUnm

Sign up for Article Alerts