Ptosis as the Main Presenting Sign in a Patient with Leigh Syndrome?
Shira L. Robbins1* and Cristina Menicacci2
1Ratner Children’s Eye Center at the Shiley Eye Institute, University of California San Diego, La Jolla, California
2Department of Ophthalmology, University of Siena, Siena, Italy
*Address for Correspondence: Shira L. Robbins, Shiley Eye Institute, University of California San Diego, 9415 Campus Point Drive, La Jolla, CA 92093, Tel: +858-822-4333, Fax: +858-246-1193; E-mail: srobbins@ucsd.edu
Submitted: 01June 2018; Approved: 28 June 2018; Published: 29 June 2018
Citation this article: Robbins SL, Menicacci C. Ptosis as the Main Presenting Sign in a Patient with Leigh Syndrome. Int J Ophthal Vision Res. 2018;2(1): 005-007.
Copyright: © 2018 Robbins SL, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
Leigh Syndrome is a fatal mitochondrial disease with variable ophthalmologic manifestations. Ptosis can be the initial sign in patients with this rare inherited neurometabolic disorder. Misdiagnosis of conditions including juvenile myasthenia gravis and congenital ptosis delay proper identification and palliative care. We present a noteworthy case of a 16-month-old girl with acquired progressive bilateral ptosis who was diagnosed with Leigh Syndrome after further work up suggested by our examination.
Introduction
This case report highlights a rare but lethal disease which can have ophthalmic signs as the presenting signs. Leigh Syndrome (LS, subacute necrotizing encephalopathy) is a progressive neurodegenerative disorder with onset usually in infancy or early childhood [1-2]. It is characterized by basal ganglia, brainstem and thalamus changes (symmetrical hypo densities in the basal ganglia on CT or hyperintense lesions on T2-weighted MRI) but with considerable clinical and genetic heterogeneity [3-4]. The estimated incidence of LS is 1:40,000 live births. LS is associated with defects in mitochondrial energy production [5]. Defects of the mitochondrial respiratory chain are associated with a diverse spectrum of clinical phenotypes, and may be caused by mutations in either the nuclear or the mitochondrial genome (Mt-DNA)[5]. Frequently encountered clinical signs include motor and/or intellectual delay and signs of brainstem dysfunction like respiratory abnormalities [6]. Other neurological manifestations include ataxia and dystonia. Ophthalmologic manifestations are nystagmus, ophthalmoparesis, strabismus, pigmentary retinopathy, optic atrophy and ptosis [7]. Typically, symptoms begin in the first months and progress to death within 2 years, but later onset and/or slower progression can occur [6].
Report of a Case
A 16-month-old girl presented with a history of progressive drooping of the left upper eyelid over a 5-month period which worsened after a head concussion 3 months prior to presentation. Her primary care physician diagnosed developmental delay. There was no discernable eyelid ptosis on photographic review of the patient’s early infancy however there was an esotropia.
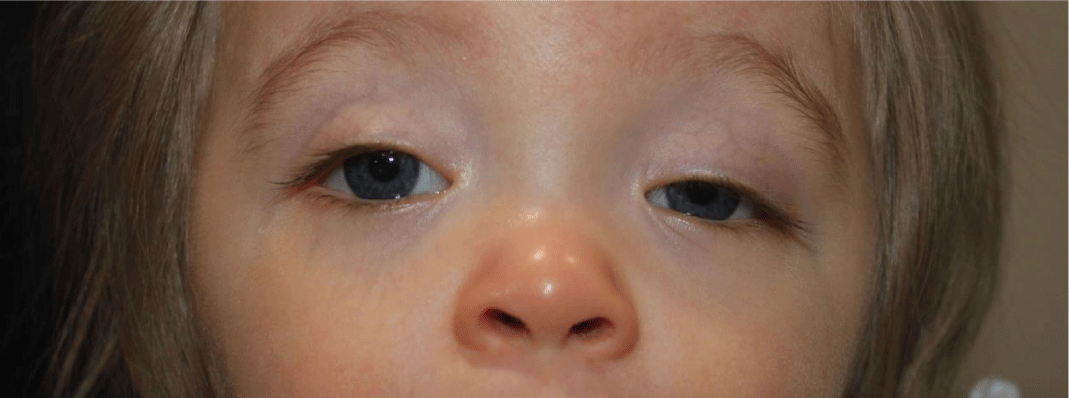
On ophthalmologic evaluation, visual acuity was decreased with fixation but only brief following behavior in both eyes. Her pupils were reactive to light with no relative afferent pupillary defect. On external exam, bilateral ptosis (left upper eyelid more than the right upper eyelid), asymmetric Bruckner’s reflexes and a 15 Prismatic Diopters (PD) exotropia were noted (Figure 1).
Bilateral optic nerve pallor was noted on fundus examination. Cycloplegic retinoscopy revealed mild ‘against the rule’ hyperopic astigmatism. Due to findings on our examination including variable strabismus during infancy, bilateral optic nerve pallor, developmental delay and acquired bilateral ptosis, neurologic and metabolic evaluation were recommended.
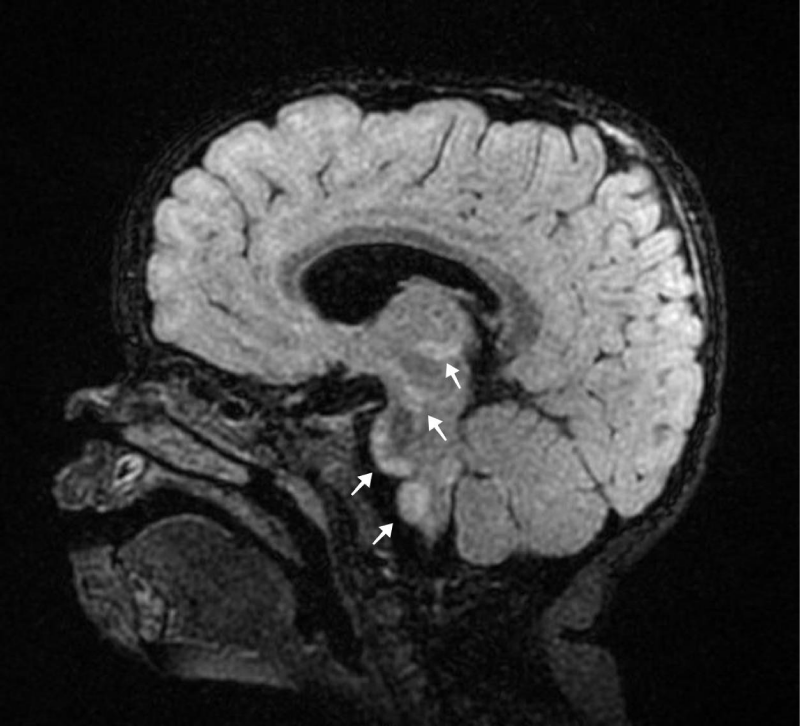
Neurologic exam revealed: upper extremity hypotonia with occasional head titubation and normal symmetrical reflexes. Brain MRI revealed “Symmetric reduced diffusion [and T2/FLAIR hyperintense lesions] were noted in bilateral medial thalami, cerebral peduncles, periaqueductal grey, ventral pons and medulla, substantia nigra and inferior cerebellar peduncles” (Figure 2). This pattern of involvement was concerning for an underlying metabolic etiology, in particular Leigh syndrome. Genetic testing was performed. Mitochondrial-DNA (Mt-DNA) sequencing revealed ND5 gene 13094T > C Val253Ala mutation.
A few days after the MRI scan, the patient developed a rapid and progressive deterioration systemically due to her mitochondrial disease. She quickly developed abnormal breathing, associated with congenital stenosis of her pulmonary valve and brainstem dysfunction caused by the LS, as well as paralysis of the eye muscles due to a progressive ophthalmoplegia. Dyspnea led to hospitalization where the patient unfortunately passed after a few days secondary to cardiorespiratory arrest, as was consistent with the natural history of her newly diagnosed disease. Exome sequencing was not able to be completed due to her sudden worsening status.
Discussion
LS is a little known but fatal disease that can present with ophthalmic signs. Eyelid ptosis can be the main presenting sign in patients with LS as in other mitocondrial disorders. These patients can initially be misdiagnosed as having juvenile myasthenia gravis because the presentation of ptosis in LS is variable. Han et al. [7] reported ophthalmologic manifestations in 44 patients with childhood onset LS. Among them, only seven patients (15.9%) had a ptosis phenotype while ptosis was the initial sign in four patients. Sudo et al. [5] reported on 6 patients with LS caused by ND5 gene mutation in the Mt-DNA. Among these 5/6 (83.3%) patients had eyelid ptosis which was the initial sign in most (4/5) of these patients. Rahman et al. [8] found the ptosis phenotype in 6/35 patients (17.1%), almost the same percentage of Han et al. [7].
Therefore, LS should be included in the differential diagnosis of patients who develop eyelid ptosis or gaze-palsy, and these patients should be followed to check for developmental delay, gait disturbance, or other neurologic symptoms [7]. In conclusion, acquired eyelid ptosis is a clinical manifestation that an ophthalmologist must examine in depth and carefully. Multidisciplinary management with a pediatric ophthalmologist, pediatric neurologist, geneticist, neuroradiologist and possibly a metabolic specialist is fundamental in patient assessment due to the lethality of the disease.
- Leigh D. Subacute necrotizing encephalomyelopathy in an infant. J Neurol Neurosurg Psychiatry.1951; 14: 216-221. https://goo.gl/eAkuzc
- Phillips PH, Newman NJ. Mitochondrial diseases in pediatric ophthalmology. JAAPOS. 1997; 2: 115-122. https://goo.gl/3sdVze
- Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, Darin N, Cohen BH, et al. Mitochondrial disease: a practical approach for primary care physicians. Pediatrics. 2007; 120: 1326-1333. https://goo.gl/3WGzZD
- Wallace DC, Brown MD, Melov S, Graham B, Lott M. Mitochondrial biology, degenerative diseases and aging. Biofactors. 1998; 7: 187-190. https://goo.gl/uWqavg
- Sudo A, Honzawa S, Nonaka I, GotoY. Leigh syndrome caused by mitochondrial DNA G13513A mutation: frequency and clinical features in Japan. J Hum Genet. 2004; 49: 92-96. https://goo.gl/FttLki
- Swalwell H, Kirby DM, Blakely EL, Mitchell A, Salemi R, Sugiana C, et al. Respiratory chain complex I deficiency caused by mitochondrial DNA mutations. Eur J Hum Genet. 2011; 19: 769-775. https://goo.gl/Rxtviw
- Han J, Lee YM, Kim SM, Han SY, Lee JB, Han SH. Ophthalmological manifestations in patients with Leigh syndrome. Br J Ophthalmol. 2015; 99: 528-535. https://goo.gl/t9zvgu
- Rahman S, Blok RB, Dahl HH, Danks DM, Kirby DM, Chow CW,et al. Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann Neurol. 1996; 39: 343-351. https://goo.gl/pK1vdg

Sign up for Article Alerts