Surgical Management of Patients with Cerebellopontine Angle Meningiomas in a 15 Year Period?
Manochehr Shirvani1,2, Mohammadreza Shahmohammadi3, Nima Mohseni Kabir1 and Yasaman Arjmand4
1Department of Neurosurgery, Shohada Tajrish Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Tehran Clinic Hospital, Tehran, Iran
3Functional Neurosurgery Research Center, Shohada Tajrish Neurosurgical Center of Excellence, Shahid Beheshti University of Medical Sciences, Tehran, Iran
4Shahid Beheshti University of Medical Sciences, Tehran, Iran
*Address for Correspondence: Manochehr Shirvani, Tehran Clinic Hospital, Tehran, Iran, E-mail: dmshirvani@gmail.com
Submitted: 12 September 2017; Approved: 26 September 2017; Published: 28 September 2017
Citation this article: Shirvani M, Shahmohammadi M, Kabir NM, Arjmand Y. Surgical Management of Patients with Cerebellopontine Angle Meningiomas in a 15 Year Period. Sci J Neurol Neurosurg. 2017;3(3): 048-051.
Copyright: © 2017 Shirvani M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Cerebellopontine Angle; Meningioma; Suboccipital Retrosigmoid Approach; Gross Total Removal
Download Fulltext PDF
Background: Cerebellopontine Angle (CPA) meningiomas comprise 10% of all intracranial meningiomas and due to their location, are producing different surgical challenges. This study is evaluating surgical management and clinical outcome of CPA meningiomas operated during 15 years.
Materials and Methods: In a 15year period, 29 patients with definite CPA meningioma enrolled in the study. Most of the patients underwent suboccipital retrosigmoid approach (n=24), followed by combined retrolabyrinthine-retrosigmoid and translabyrinthine (n=5). Demographic data as well as neurological status and imaging features were recorded and analyzed in pre and postoperative period.
Results: Headache (n=17), hearing loss (n=15) and facial hyposthesia (n=10) were the most common presenting symptoms. Gross total resection (GTR) and subtotal resection was achieved in 79.3% and 17.2% respectively. Trigeminal (V) nerve function was significantly improved after operation (62% vs. 34.4%, p value < 0.05). Vestibulocochlear (VIII) nerve function was also significantly improved in postoperative assessment (51.72% vs. 20%, P value<0.05). 1 patient discharged with multiple cranial nerve deficits and another patient expired after operation. Postoperative complications included cerebrospinal fluid leakage, pneumonia, deep vein thrombosis and hydrocephalus. Tumor volume > 2.5 cm3 was significantly associated with higher incidence of postoperative neurological deficits.
Conclusion: CPA meningiomas are challenging to excise. Selection of appropriate surgical approach as well as GTR may decrease postoperative neurological complications and increase survival in this group of patients.
Introduction
The lesion most comparable with a meningioma was first reported by Felix Plater in 1614 [6]. In 1915, Harvey Cushing considered that meningeal tumors arose from arachnoidal cap cells. Seven years later, Cushing proposed the term meningioma for these tumors and the term achieved global acceptance. Working together with his student, Percival Bailey, he adopted a histopathological classification system composed of meningothelial, fibroblastic, angioblastic, and osteoblastic. Later, Bailey and Bucy expanded the initial histopathological classification of meningiomas, which was similar to the new WHO histopathological classification 2007 [7]. According to the literature, meningiomas account for 26% of primary intracranial neoplasms, 10% being located in the Cerebellopontine Angle (CPA) [1]. Sekhar and Wright have classified meningiomas into 6 types, depending on their location and anatomical extension. Cerebellar convexity and lateral tentorial meningiomas extend to tentorium, transverse and sigmoid sinus; CPA meningiomas extend to petrous ridge and Internal Auditory Canal (IAC); jugular foramen meningiomas may extend to cerebello-medullary angle, internal jugular vein and extracranial sites; petroclival meningiomas extend to upper 2/3rd clivus, cavernous sinus, meckle’s cave and petrous ridge; foramen magnum meningiomas may extend to lower third of clivus and C1 and C2 area and finally, the unclassified meningiomas have desire for entire clivus [2]. The location of a CPA meningioma affects clinical outcome. The most important involvement of anatomical locations includes: IAC, jugular foramen, cranial nerves involvement and bony invasion [3]. Patients commonly complain from long standing slow progressive symptoms, such as headache and cerebellar symptoms and most commonly, cranial nerve involvement with hearing loss, facial pain and numbness [4]. Microsurgical resection is the treatment of choice for the majority of these lesions; however, variable locations, large size at diagnosis and frequent neurovascular invasion are challenging factors to the surgeons [5]. In this study, we have described the surgical and clinical post-operative outcome of 29 patients surgically treated for CPA meningioma over a 15-year period.
Materials and Methods
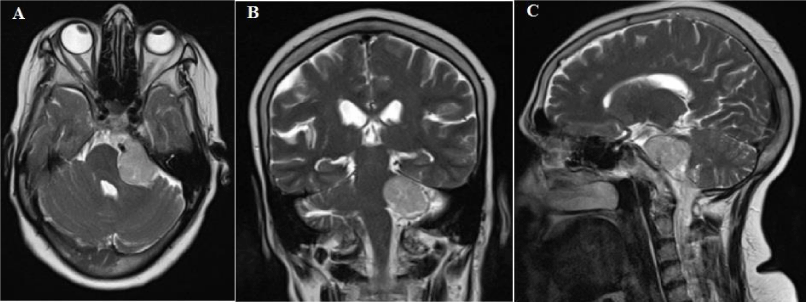
Twenty-nine meningiomas out of 187 patients with CPA tumors (%15.5) that were referred to Shohada Tajrish hospital and operated during 15 years (from 2000 to 2015) were included in this study. Our inclusion criteria were pre-operative Magnetic Resonance Imaging (MRI) investigation of the CPA area and definite post-operative histopathological diagnosis of meningioma (Figure 1: A-C). Patients without pre- and post-operative MRI, neurofibromatosis, multiple intracranial and intra-spinal neoplasms and previous radiotherapy were excluded. Patient’s data, including surgical records, discharge letters, histological reports, follow-up records, and imaging studies, were analyzed. Collected data were studied for patient’s age and sex, tumor site of origin, duration of first presenting symptom, neurological deficits, neuro-imaging appearance, surgical approach, and outcome as well as clinical and neuro-imaging findings at follow-ups. Gross-total resection (GTR) was defined as the complete absence of the lesion on post-operative MRI. Additionally, imaging studies and operative reports were used to determine tumor extension into the IAC and jugular foramen. Retrosigmoid suboccipital approach was the standard surgical procedure. Data were analyzed using SPSS (version 19, SPSS Statistics/IBM Corp, Chicago IL, USA) using Mann-Whitney, Student’s t-test and Chi-square tests. This study was conducted under the principles of the Helsinki Declaration and approved by the Ethics Committee of Shohada Tajrish hospital.
Results
From the 29 patients, 22 were female (75.8%) and female to male ratio of 3.1:1. Mean age of patients was 49.27 (±1.27) years. Most of the patients presented with cranial nerve dysfunction. Based on World Health Organization (WHO) classification, 18 patients (62%) have meningo the liomatous type and 6 patients (20.6%) have mixed type. Fibrous and psammomatous were observed in 3 (10.3%) and 2 (6.9%) patients respectively.
Presenting symptoms included headache that was observed in 17 patients (58.6%) followed by hearing loss in 15 patients (51.7%), vertigo in 11 cases (37.9%) and facial pain and facial hyposthesia in 10 cases (34.4%). Other less common presenting symptoms were tinnitus in 5 (17.2%) and dysphagia, diplopia and limb paresis, each in 2 (6.8%) patients (Table 1).
Twenty four patients underwent suboccipital retrosigmoid approach (82.8%), followed by combined retrolabyrinthine-retrosigmoid and translabyrinthine in 5 patients (17.2%).
Gross total removal (GTR) was achieved in 23 cases (79.3%), followed by subtotal resection (STR) in 5 cases (17.2%). Due to surgical complications, one patient underwent biopsy only (Table 2).
Based on pre-operative and intra-operative evaluation, mean tumor volume was 2.93 (±0.38) cm3, with 3.75 cm3 and 1.11 cm3 being the maximum and minimum volumes. IAC and jugular foramen invasion was observed in 7 (24.1%) and 2 (6.9%) patients respectively.
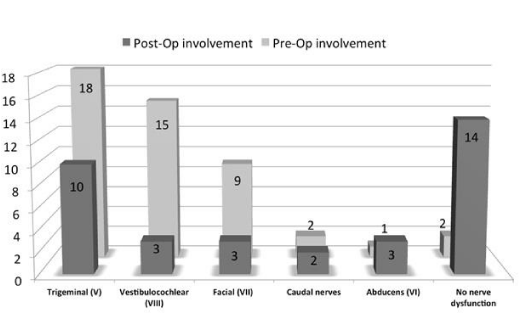
In pre-operative evaluation, trigeminal (V) was the most common involved nerve in 18 patients (62%), followed by vestibule-cochlear (VIII) nerve dysfunction in 15 patients (51.7%), facial (VII) nerve in 9 (31%), caudal cranial nerves in 2 (6.9%) and abducens (VI) nerve in 1 (3.5%). Two cases (6.9%) showed no nerve dysfunction in pre-operative cranial assessment. Post-operative assessment of cranial nerves revealed 14 cases (48.2%) with no nerve dysfunction, trigeminal (V) nerve as the most frequent involved nerve in 10 cases (34.4%), facial (VII) and vestibulocochlear (VIII) nerve each in 6 patients (20%) and abducens (VI) nerve in 3 patients (10.3%). Caudal nerve involvement and partial facial nerve paresis were present in 2 (6.9%) and 1 patient (3.5%) respectively (Figure 2). Trigeminal (V) nerve function was significantly improved after operation (62% vs. 34.4%, pre- and post-operative; P value<0.05). Vestibulocochlear (VIII) nerve function was also significantly improved in post-operative assessment (51.7% vs. 20.6%; P value<0.05).
There was one mortality (3.5%), 1 patient showed multiple cranial nerve deficits and other post-operative complications were observed in 7 patients (24.13%), including cerebrospinal fluid (CSF) leakage in 3 (10.3%), pneumonia in 2 (6.9%), deep vein thrombosis in 1 (3.5%) and hydrocephalus in 1 (3.5%) patients (Table 3).
Discussion
CPA meningiomas tend to show indolent growth pattern and most of our cases were diagnosed in advanced stages. Headache, cranial nerve dysfunction and cerebellar symptoms were the most common presenting symptoms. Of the cranial nerves, trigeminal (V) and vestibulocochlear (VIII) are the most affected nerves [8]. 18 trigeminal (62%) and 15 vestibulocochlear nerves involvement in our cases is confirmatory to the literature findings. Due to the prognostic effect of the tumor pathology on post-operative neurological outcome, differentiating CPA meningioma from vestibular schwannoma is extremely important. Broad based with dural tail along with the presence of hyperostosis are the most important radiological differentiating factors between CPA meningioma and vestibular schwannoma [9]. One of the most important factors, both of technical value for surgeon and prognostic value for the patient, is the attachment site. CPA meningiomas are mostly originating from the dura mater of tentorium and anterior petrous bone, with trigeminal nerve displaced caudally or posterior petrous area with attachment posterior to the internal auditory meatus [10]. This characteristic site of origin differentiates them from petroclival and Meckel’s cave counterparts that have different presentations and need different approaches. MRI provides useful information about tumor site and attachment, secondary intra- and extra-dural tumor invasion, along with important data for best approach by the surgeon [11]. Retromastoid craniotomy is the common surgical approach for the CPA meningioma. Its advantages are simplicity and familiarity of approach to the surgeons, provides wide exposure of the CPA structures [12], generous drainage of the CSF at the beginning of the intervention by opening of the cisterna magna which avoids cerebellar retraction and increased chance of hearing preservation [13]. This was the procedure of choice in our patients that was used in 83% of them. In a study conducted by Roser et al, 72 patients with CPA meningioma involving IAC underwent operation. GTR and STR were achieved in 86% and 14% respectively. Hearing was preserved in %90 of those with pre-operative intact hearing status, along with facial nerve function in 89% of patients. Best hearing preservation achieved in those tumors originating from superior or posterior IAC [3]. In another study by Kane et al, 24 patients with CPA meningioma, with 62% involvement of IAC, mostly presented with decreased hearing. After operation, 90% of the patients achieved stable status or improved hearing. However, vagus (X) and glossopharyngeal (IX) nerves were damaged after operation, in 33% and 17% of patients, respectively [14].
In our study, we achieved GTR and STR in 79.3% and 17.2% of the cases. From 18 preoperative trigeminal nerve involvements 8 and from 15 preoperative vestibulocochlear nerve involvements 6 were improved both significantly meaningful (P value <0.05), but from 9 preoperative facial nerve involvements 6 had no change and 2 cases recovered partially only and both cases of caudal nerves dysfunction before operation none were recovered making them resistant to change by operation if involved preoperatively. Abducens nerve palsy present in only one patient before operation was involved in 3 cases after surgery that signaled us in being more careful about its dissection from tumor in future [15]. Overall from 29 patients with only 2 cases (6.9%) of intact cranial nerves preoperatively 14 (48.3%) had intact and improved cranial nerves functions (p value = 0.0008). Although GTR is the ultimate goal in management of CPA meningioma and carries a significant prognostic value; however, it is not always feasible and majority of the authors recommend STR for old patients or when there are factors defying complete removal [16,17]. Even though local tumor recurrence is common in STR, addition of stereotactic radiosurgery may decrease recurrence and improve survival time [18,19].
Various factors, such as tumor size/extension and the extent of resection, were found to affect the incidence of complications. Patients with tumors larger than 2.5 cm3 had a significantly higher incidence of permanent cranial nerve deficits than those with smaller tumors (34.4% vs. 3.5% respectively; P value<0.05).
In general, surgical approach should depend on maximal exposure for the particular tumor location and probability of maximal extent of resection.
Conclusion
Although CPA meningiomas are difficult to excise because of their adjacent neurovascular structures, most WHO grade1 meningiomas could be resected without post-operative complications. GTR by suitable retrosigmoid suboccipital approach do not increase post-operative neurological deficits and may increase symptom free survival along with decreased tumor recurrence.
- Tomogane Y, Mori K, Izumoto S, Kaba K, Ishikura R, Ando K, et al. Usefulness of PRESTO magnetic resonance imaging for the differentiation of schwannoma and meningioma in the cerebellopontine angle. Neurol Med Chir(Tokyo). 2013; 53: 482–489. https://goo.gl/ZkWq6V
- Roberti F, Sekhar LN, Kalavakonda C, Wright DC. Posterior fossa meningiomas: surgical experience in 161 cases. Surg Neurol. 2001; 20: 20–31. https://goo.gl/2tRNjT
- Roser F, Nakamura M, Dormiani M, Matthies C, Vorkapic P, Samii M. Meningiomas of the cerebellopontine angle with extension into the internal auditory canal. J Neurosurg. 2005; 102:17–23. https://goo.gl/cxzp4v
- Okonkwo DO, Laws ER. Meningiomas: Historical perspective. In: Lee JH, editor. Meningiomas: Diagnosis, Treatment, and Outcome. London: Springer-Verlag; 2009. 3-11. https://goo.gl/zgZQ5o
- Sekhar LN, Jannetta PJ. Cerebellopontine angle meningiomas: Microsurgical excision and follow-up results. J Neurosurg. 1984; 60: 500–5. https://goo.gl/aWXQLM
- Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: A review. J Neurooncol. 1996; 29: 197–205. https://goo.gl/AnSZKm
- Kaye H. Posterior fossa meningiomas. In: Sindou M, editor. Practical Handbook of Neurosurgery: From Leading Neurosurgeons. Germany: Springer-Verlag; 2009; 2: 181. https://goo.gl/UBXURJ
- Bricolo AP, Turazzi S, Talacchi A, CristoFori L. Microsurgical removal of petroclival meningioma: A report of 33 patients. Neurosurgery. 1992; 31: 813–28. https://goo.gl/s6QRPY
- Kutz JW, Barnett SL, Hatanpaa KJ, Mendelsohn DB: Concurrent vestibular schwannoma and meningioma mimicking a single cerebellopontine angle tumor. Skull Base 2009; 19: 443-446. https://goo.gl/koAUAc
- Kunii N, Ota T, Kin T, Kamada K, Morita A, Kawahara N, et al: Angiographic classification of tumor attachment of meningiomas at the cerebellopontine angle. World Neurosurg. 2011; 75: 114-121. https://goo.gl/QEUAD1
- Canvalho GA, Marthles C, Tatagiba M, Eghhal, Samii M. Impact of computed tomography and maganetic resonance imaging findings on surgical outcome in petroclival meningioma. Neurosurgery. 2000; 47: 1287–94. https://goo.gl/aTiQHe
- Rosen CL, ammerman JM, Sekhar LN, Bank WO. Outcome analysis of preoperative embolization in cranial base surgery. Acta Neurochir. 2002; 144: 1157–64. https://goo.gl/qJ5GQt
- Chen CM, Huang AP, Kuo LT, Tu YK. Contemporary surgical outcome for skull base meningiomas. Neurosurg Rev. 2011; 34: 281–96. https://goo.gl/GnXorC
- Kane AJ, Sughrue ME, Rutkowski MJ, McDermott MW, Berger MS, Parsa AT. Clinical and surgical considerations for cerebellopontine angle meningiomas. J Clin Neurosci. 2011; 18: 755-9. https://goo.gl/91dKzJ
- Setty P, D'Andrea KP, Stucken EZ, Babu S, LaRouere MJ, Pieper DR. Fully Endoscopic Resection of Cerebellopontine Angle Meningiomas. Journal of Neurological Surgery Part A: Central European Neurosurgery. 2016; 77: 011-8. https://goo.gl/Bbi7ec
- Saleh EA, Taibah AK, Achilli V, Aristegui M, Mazzoni A, Sanna M. Posterior Fossa Meningioma Surgical Strategy. Skull Base Surg. 1994; 4: 202–12. https://goo.gl/W5ejfP
- Lobato RD, Gonzaaez P, Alday R, Ramos A, Lagares A, Alen JF, et al. Meningiomas of the basal posterior fossa. Surgical experience in 80 cases. Neurocirugia. 2004; 15: 525–42. https://goo.gl/cwyZf3
- Nikouei A, Seddighi A, Seddighi AS. The Results of Image Guided Surgery Using Neuron avigation in Resection of Cerebral Gliomas in Eloquent Cortical Areas. Archives of Physical Medicine and Rehabilitation. 2016; 97: 69-70. https://goo.gl/azHmmA
- Subach BR, Lunsford LD, Kondziolka D, Maitz AH, Flickinger JC. Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurgery. 1998; 42: 437–43. https://goo.gl/QgPd2T

Sign up for Article Alerts