Surgical Tips for Neurofibromatosis: Synopsis of Surgery with Technology?
Selma Sonmez Ergun1*, Halil Ibrahim Canter2, Kemalettin Yildiz3
1Bezmialem Vakıf University, Faculty of Medicine, Professor Department of Plastic Reconstructive and Aesthetic Surgery, Istanbul-TURKEY
2Acibadem University, Faculty of Medicine, Department Professor of Plastic Reconstructive and Aesthetic Surgery, Istanbul- TURKEY
3Acibadem University, Faculty of Medicine, Department Professor of Plastic Reconstructive and Aesthetic Surgery, Istanbul- TURKEY
*Address for Correspondence: Selma SÖNMEZ ERGÜN, Estonşehir 3. Mahalle Ilgın Sokak, CD Villa 7/2, TURKEY. Tel: + 90 212 691 52 56; Fax: + 90 212 621 75 80; E-mail: [email protected]
Submitted: 05 April 2017; Approved: 24 August 2017; Published: 29 August 2017
Citation this article: Ergun SS, Canter HI, Yildiz K. Surgical Tips for Neurofibromatosis: Synopsis of Surgery with Technology. Int J Neurol Dis. 2017;1(1): 018-027.
Copyright: © 2017 Ergun SS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
Background: Plexiform neurofibromas of the head and neck region inneurofibromatosis-1 (NF-1) presentwith varying degrees of disfigurement according to the extent andlocation of the tumor due to its growing continously.
Objective: Plexiform neurofibromas of the head and neck in NF-1 carry a significant morbidity with substantial loss of function as well assignificant cosmetic problems. We describe our experience with early aggressivesurgical intervention in such patients in order to avert these problems.
Methods: Retrospective review offive consecutive patients with head and neck plexiform neurofibromas who underwent single stage near total or subtotaltumor resections.
Results: One patient had local flap necrosis, which was covering the implant. Therefore free tissue transfer needed to be done. Otherwise, there were just two minor complications and no majör complications in rest of the operated patients. There have been no recurrences up to date, withfollow-up ranging from 15 months to 5 years.
Conclusions: Early surgical intervention of NF-1 patients with plexiform neurofibromasof the head and neck with a goal of near total resection avoids the loss of functionassociated with these tumors, such as tracheostomy dependence, swallowing difficulty,and speech problems, and prevents the inexorable progression of substantialcosmetic deformity. Successful management of these complex lesions requiresdetailed preoperative planning, advanced surgical techniques, and vigilant postoperativecare. Custom-made CAD/CAM implants makes the skelotal reconstruction easier and more symmetrical.
Introduction
Neurofibromatoses, a group of inherited disorders with established diagnosis criteria based on symptoms of presentation, are subdivided NF-1, neurofibromatosis-2 (NF-2) and schwannomatosis. NF-1, also known as von Recklinghausen disease, is the most common type. The hallmarks of NF-1 are the multiple café-au-lait macules and neurofibromas. The condition is called segmental NF-1 when clinical features are limited to one area of the body [1].
The incidence of NF-1 is approximately 1 in 2600 to 3000 individuals [2,3]. Chromosome 17 is involved. Although NF-2 was originally thought to be extremely rare, after two large population-based studies it was found that the incidence might be as high as 1 in 25,000 [4-6]. It is associated with a gene mutation on chromosome 22.Schwannomatosis is an uncommon disorder, with an annual incidence estimated at 0.58 cases per 1,000,000 persons [7].
Plexiform neurofibromas as an uncommon variant of NF-1 clinically represent themselves as bulging and deforming masses involving also connective tissue and skin folds in the craniomaxillofacial region [8]. Craniomaxillofacial lesions can be divided into massive plexiform, cranio-orbital, and cervical neurofibromas [9]. Plexiform neurofibromas are usually treated with surgically. Although with this treatment modality aimed at resecting deforming masses and cancerous tissue when malignant transformation occurs, these masses tend to recur in 20% of cases despite an appropriate approach [9]. Good results can be obtained after the administration of interferon-a in unrespectable, progressive and symptomatic lesions [7-11]. Due to the high risk of disease progression and its variable expressivity the prognosis of the disease is still unpredictable [12].
The discussion below pertains mainly to the treatment craniofacial manifestations of NF-1. The cranio-orbit-temporal involvement is seen in 1% to 4% of the patients with NF-1 [13,14]. Depending on the extent of the tumor involvement, varying degrees of facial and auricular disfigurement, asymmetry and sagging, upper eyelid ptosis, and vision problems can be seen. 15In severe cases with extensive involvement of the eyelids or intra orbital extension of the tumor, there can bebuphthalmos and blindness. Secondary developmental problems on the per orbital bony structures can lead to partial or complete absence of the greater wing of the sphenoid, which in turn leads to expansion of the middle cranial fosse into the posterior orbit and causes proptosis.16Orbital rims and the zygomatic arch can also be affected. Remodeling and/or decalcification of the posterior orbit, thinning of the lateral and inferior orbital rims, depression of the orbital floor and elevation of the orbital roof and supraorbital rim, which increases orbital volume and dysplasia and downward dislocation of the zygoma can be seen. Supraorbital tumors, which cause defects of the orbital roof and lead to a downward and outward displacement of the globe. Progressive plexiform neurofibromatosis of the temporal area, which leads to continued enlargement of the eyelids, excessive mechanical ptosis, pulsating proptosis, eye pain, and epiphora.
The main treatment protocol of cranio-orbit-temporal NF depends on the extent of orbital involvement based on Jackson’s classification [14-17]. Characteristics of stage I disease is significant soft-tissue involvement with minimal bone involvement and normal vision. Treatment in this stage involves de bulking of the soft-tissue component of the tumor through an anterior, lateral, or anterolateral orbitotomy. If blepharoptosis is present, levator resection is performed. In cases where only a partial resection is carried out, a technique of netting the remaining tissue using Teflon mesh has been proposed. In stage II, bone tissue is involved along with the soft tissue but vision is intact. Treatment involves an intracranial approach for tumor de bulking and posterior orbital wall reconstruction. After the tumor is de bulked; the herniated temporal lobe is reduced and bone grafts, obtained from splitting the contra lateral frontal bone, are used to reconstruct the posterior and superior orbital walls. Orbital volume is increased with osteotomies and the globe is elevated by raising the catchall ligaments and building the floor. A two-stage approach has been suggested in which the intracranial portion of tumor de bulking and orbital reconstruction is completed in stage I. A second-stage procedure is performed in which subcutaneous de bulking, eyelid, face, and orbital reconstruction is completed. Stage III is characterized with soft-tissue and bone involvement with blindness or absence of the globe. Treatment involves de bulking the tumor with exenteration. An orbital approach is used to reduce the herniated temporal lobe into the middle cranial fosse. The bony defect is covered with a split-rib/bone graft. The orbital volume is reduced and its position is adjusted with osteotomies and bone grafts. Finally, an orbital prosthesis is fitted.
Although these are main outlines, treatment of each patient should be tailored individually. Some patients may refuse the radical excision of the tumor or complete excision is almost impossible without creating a major defect, therefore serial excisions to decrease the amount of tumor becomes the most common treatment modality. The remaining tumor has a tendency to re grow and sagging of the tumor due to gravity with facial disfigurement is one of the main complaints. This is especially true when only skin sutures are placed to hold the tissues against gravity. The patients, who prefer to precede with serial excision and palliative treatment in their life span and/or whose tissues are inset with only skin sutures, often have recurrent sagging within a short period after surgery and typically undergo multiple surgeries.
The problem of recurrent skin sagging after incomplete excisions may be overcome partially with suspension of the soft tissues with fascia grafts. Now it is possible to inset these fascia grafts to the bone tissue by using anchor sutures, which consist of a screw component to affix the permanent suture material component deep into the bone tissue. The fascia grafts spread the tension to larger surface and incorporate with the healing surrounding soft tissues so that maintains the suspension effect on soft tissues longer.
Another obstacle in stage II or III disease is to achieve perfect symmetry with conventional osteotomies and bone grafting techniques. It may not be possible to create the perfect tridimensional symmetry no matter how talented the surgeon is, the achieved symmetry may not last after bone healing process due to the risk of bone resorption. With the advances in computer-assisted design and computer-assisted manufacturing technologies (CAD/CAM) it became possible to manufacture the perfect fitting titanium implants to de facial defects, created by the osteotomies done by the help of the cutting guides, which are manufactured according to the preoperative virtual planning on computers. These titanium implants resist to the corrosive effects of the interstitial fluids and can be stable mechanically years after the surgery depending on how perfect they are designed preoperatively.
In a series of 5 patients with NF-1 of the orbital-temporoauricular area, we have used a combination of functional de bulking and lifting the sagging skin. In two patients, facial titanium implants were designed to achieve skeletal symmetry. The aim was to prevent or minimize sagging and maximize the facial symmetry in between anticipated serial excisions.
Cases
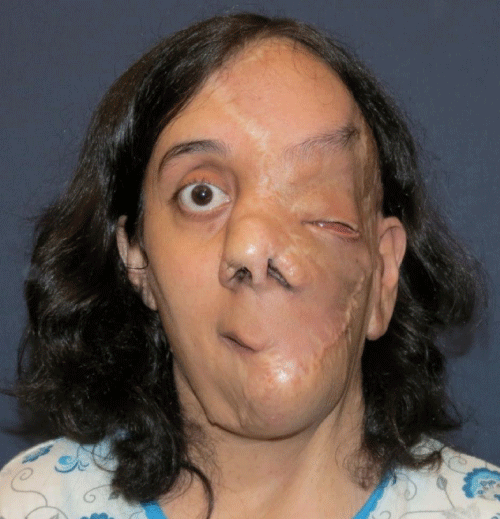
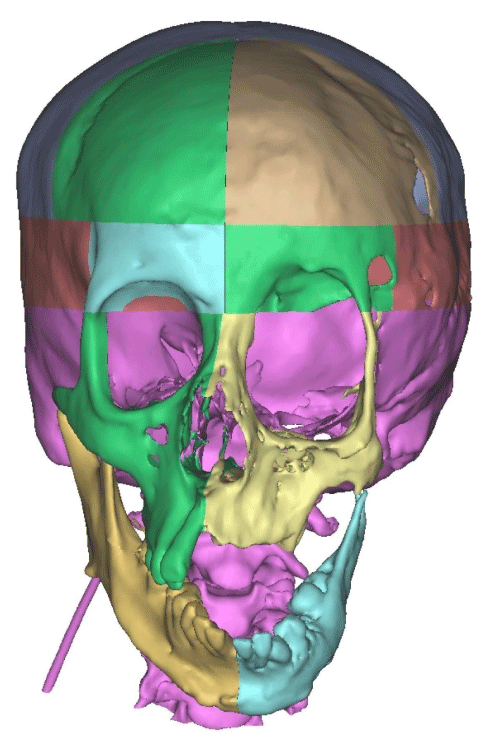
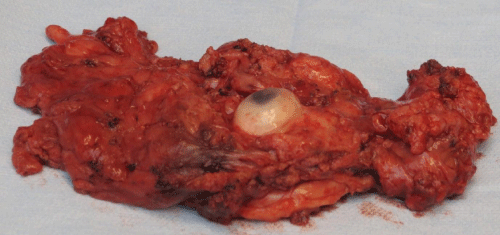
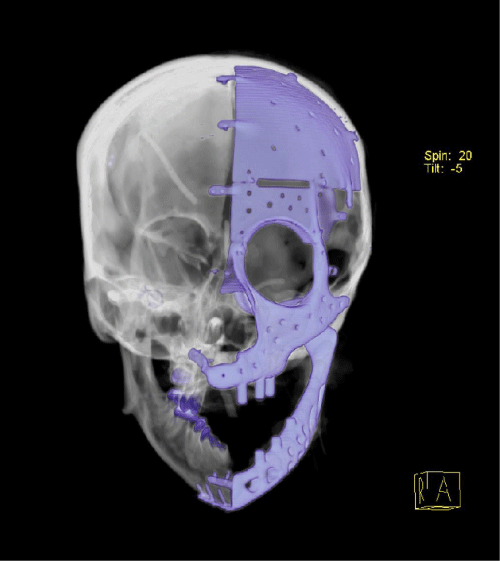
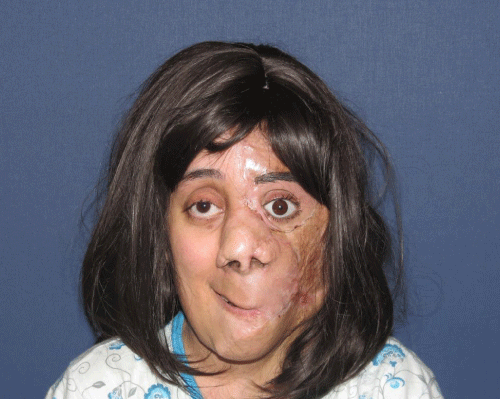
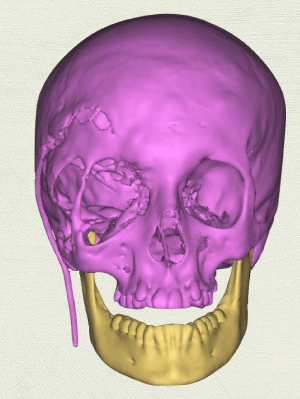
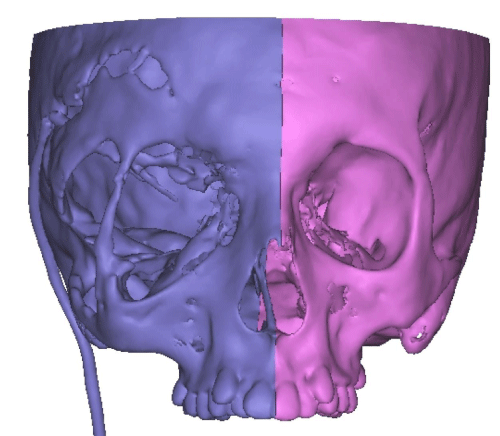
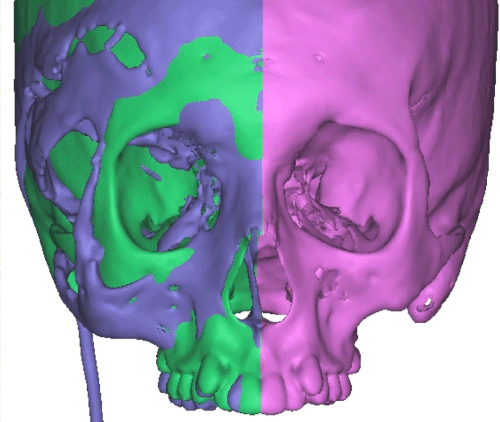
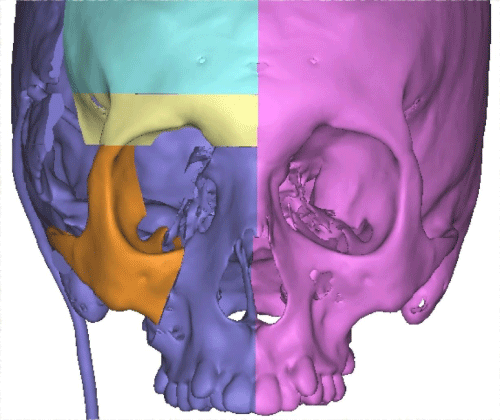
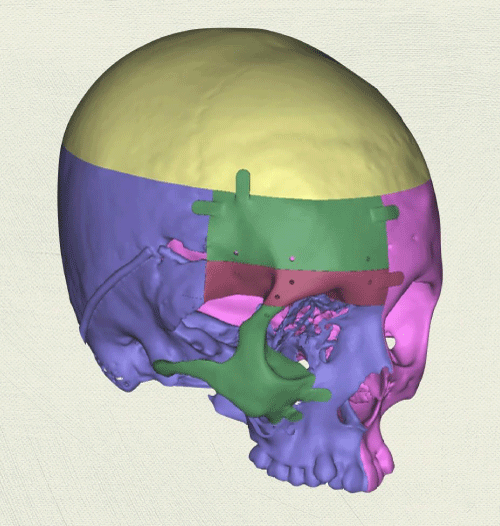
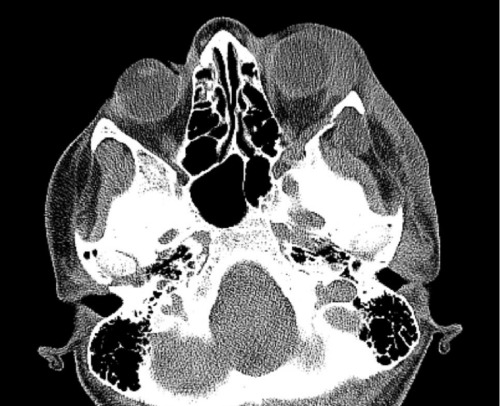
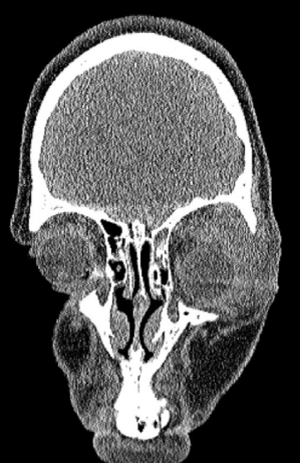
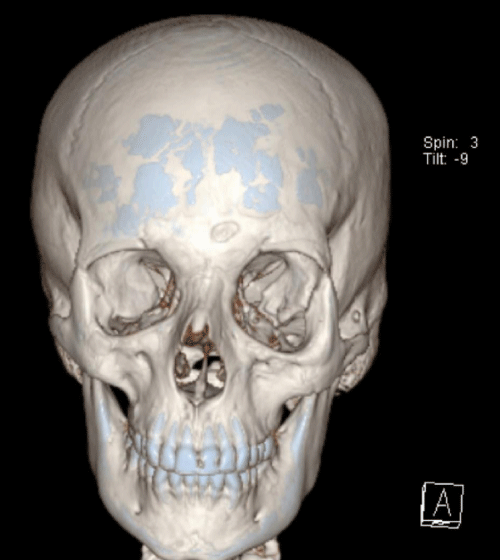
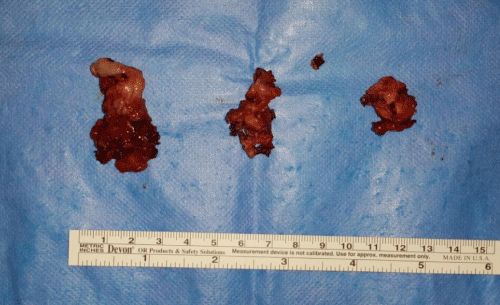
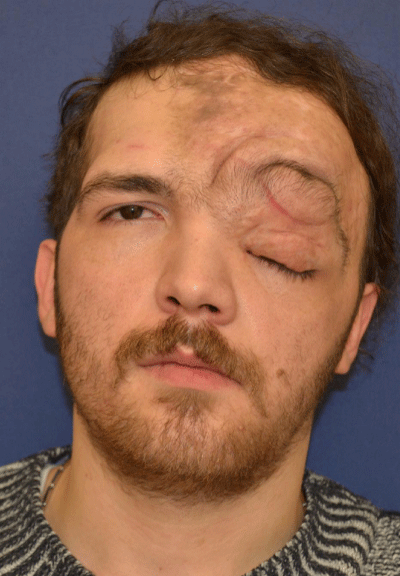
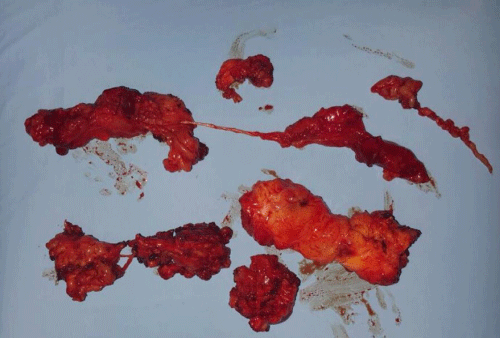
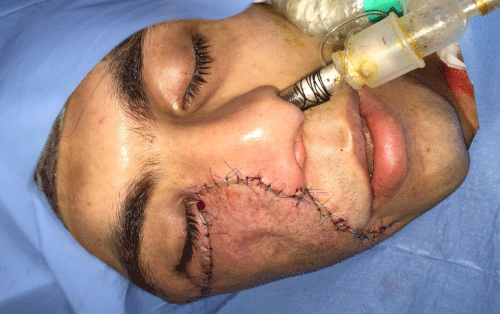
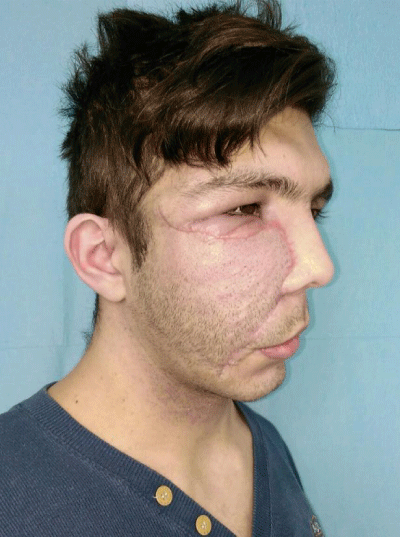
Case 1: 22-year old female patient with NF-1 evaluated for the facial deformity (Figure 1A). She had been operated several times before for removal of the tumor and defects were closed with local flaps. Preoperative imaging with CT scan revealed extends of the facial skeletal deformity predominantly on the left side and severity increasing from cranial to caudal. Her left eye was blind and involved in the tumoral mass. Additionally her left TMJ along with the left mandibular condoyle was eroded (Figure 1B). DICOM data of the CT scan was transferred to MIMICS software for Computer Assisted Design (CAD) and Manufacturing (CAM) of the tentative titanium implant. The midline was determined according to the vertebral and cranial base structures, which were relatively spared from the pathology. Then the anterior part of the skull was subdivided into the 5 units according to the Tarsier’s craniofacial framework (Figure 1C). Mirror imaging of the right side skeletal structures were used for designing the titanium implant for the left side (Figure 1D).The old scars of the previous surgeries were used to get access to the tumoral mass; therefore the facial skin flap was elevated as flap on caudal pedicle although the flap would still depend on a scar based pedicle. Tumoral mass extending from cranium to the cheek including the left orbit was removed en bloc (Figure 1E). Structurally deformed skeletal segments were replaced with the custom-made titanium implant in order to reconstitute the hard tissue symmetry (Figure 1F). In the postoperative period, however, the facial skin flap had circulatory problem and replaced with latissimus dorsi free muscle flap. Treatment was completed with left orbital prosthesis and a wig. Follow-up period was five years (Figure 1G).
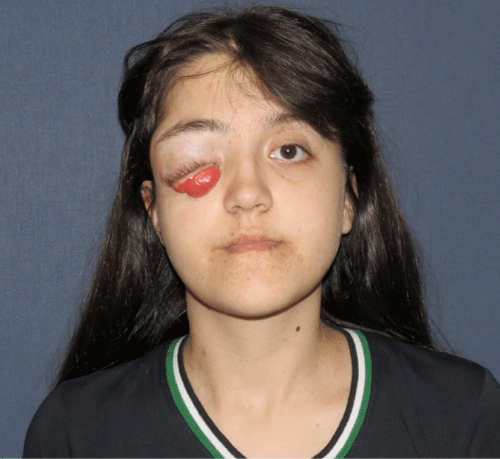
Case 2: 18-year old female patient with NF-1 evaluated for the facial asymmetry and blind eye of right side (Figure 2A). She had not been operated before. DICOM data obtained from preoperative CT scan transferred to MIMICS software and segmentation of the mandible from the rest of the skull was done (Figure 2B). The midline was determined according to the vertebral and cranial base structures (Figure 2C). Mirror image of the left facial skeletal was superimposed on the right side (Figure 2D). Then it became apparent that the facial skeletal deformity was mainly around the right eye. Right alveolar process of her maxilla was not involved. Therefore in order to spare the dental structures and the occlusal relation of the upper and lower teeth; only the forehead area, supraorbital bar and the lateral part of the zygoma of the right side of her facial skeletal were planned to be operated (Figure 2E) and the custom made facial implant was designed accordingly (Figure 2F). Then patient did not want to proceed with the operation and the procedure was brought to a stand.
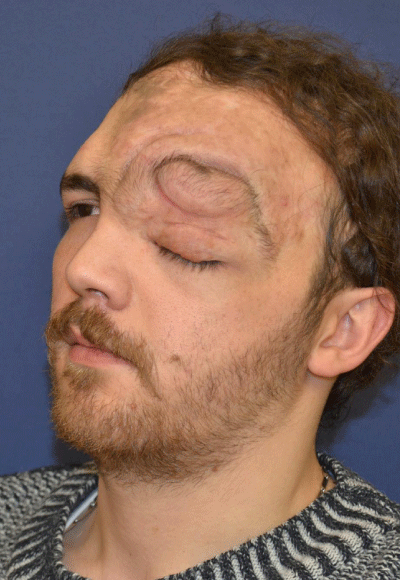
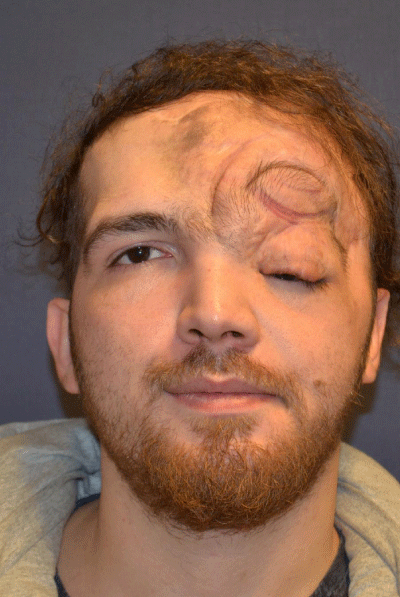
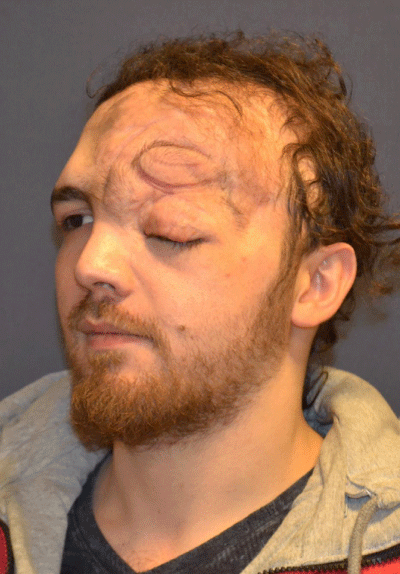
Case 3: 16-year old female patient with NF-1 evaluated for the left upper eyelid fullness, ptosis, and facial asymmetry (Figure 3A). She did not any complaint of vision or visual field defect. Although preoperative CT scan revealed the presence of per orbital soft tissue mass of the right intraorbital space, it did not cause the ostensible skeletal deformity (Figure 3 B-D). Intraorbital mass was removed through the upper eyelid incision and ptosis was managed with frontalis suspension surgery. Follow-up period was three years (Figure 3E).
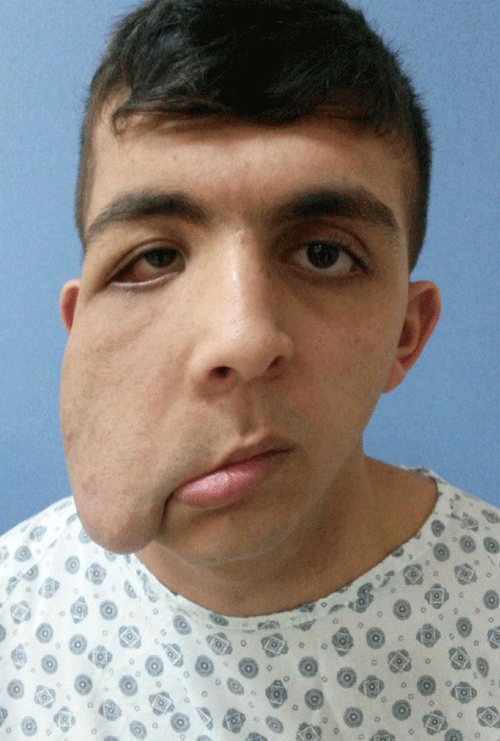
Case 4: 26-year old male patient with NF-1 who had operated several times before for removal of the mass, evaluated for recurrence of the left orbital mass, ptosis of the upper eyelid, orbital dystopia and facial asymmetry (Figure 4 A,B). Although the preoperative CT scan revealed soft tissue infiltration of left superior orbit and enlargement of orbital cavity, patient refused the proposed treatment with de bulking of orbital mass along with correction of left upper eyelid ptosis with frontalis suspension surgery and insisted on to have more conservative surgical approach even if the cosmetic results was said to be less satisfactory. Then intraorbital mass was removed through the upper eyelid incision and ptosis was managed with removal of the excess of skin as in forehead lifting procedure. Follow-up period was two years (Figure 4 C,D).
Case 5: 26-year old male patient with NF-1 operated for facial disfigurement due to excessive weight of the soft tissue mass on the right side of his face (Figure 5 A,B). De bulking of the tumor was managed with right Weber-Ferguson incision (Figure 4C, D) and skin flap was anchored to the periosteum of the intraorbital rim with tensor facial late graft applied in strips, while excess of the skin was removed as done in facelift surgery). Follow-up period was fifteen months (Figure 4E).
Discussion
Café au lait spots, axillaries freckles, multiple neurofibromas, and Lisch nodules are the cardinal features of NF-1. Although intellectual handicap, scoliosis, and optic gliomas are common, peripheral nerve malignancy, bony deformities, and epilepsy are individually rare [18,19]. When the patient has two or more criteria that are developed by the National Institutes of Health (NIH), the diagnosis of NF-1 is usually achieved. Few patients have all of these cardinal symptoms. NF-1 is diagnosed in one-third of the patients based on family history and the presence of only one of the six cardinal features [20].
Neurofibromas have various subtypes such as cutaneous, subcutaneous, nodular and diffuse plexiform subtypes according to their histopathological appearance and locations. While cutaneous and subcutaneous neurofibromas arise from distal ends of the nerves and are localized to the cutaneous and subcutaneous layers of the skin respectively, nodular neurofibromas arise from the proximal nerve roots and grow within organs. Whereas Diffuse Plexiform Neurofibromas (DPNs) involve long segments of multiple nerves and can be found in any histological layer of the tissue where nerves are present [21].
DPNs develop in approximately 20-44% of NF-I patients [22]. They are slow-growing tumors and occur mostly in the head and neck region of the children and young adults. Clinically, these tumors are easily diagnosed due to their typical features, and they initially represent their selves as a plaque-like elevation of the skin but by the time due to growing mass they cause major disfigurement and morbidity. Although they have infiltrative growth pattern, they do not destroy but rather envelop normal structures. They have a 20% risk for malignant degeneration into peripheral nerve sheath tumor [23,24].
They are slow-growing tumors and occur mostly in the head and neck region of the children and young adults. Clinically, these tumors are easily diagnosed due to their typical features, and they initially represent themselves as a plaque-like elevation of the skin but by the time due to growing mass they cause major disfigurement and morbidity. Although they have infiltrative growth pattern, they do not destroy but rather envelop normal structures. They have a 20% risk for malignant degeneration into peripheral nerve sheath tumor [13-26].
Surgery is indicated when the tumor is severely disfiguring, when function is seriously compromised, when the patient has intractable pain, when a suspection of malignant transformation or when the tumor has progressive enlargement with compressive effects. DPNs have a recurrence rate of 20% because of complete removal is rarely achieved due to the extensive nature, multicentricity and significant vascularity of the tumor. While total or subtotal resections may be possible in superficial DPNs due to they arise from subcutaneous or cutaneous nerves and may remain within the upper skin layers, invasive DPNs infiltrate multiple tissue planes and cannot be completely resected without functional disturbance. Serial excision is the generally accepted mode of treatment for invasive DPNs due to large size of the tumors and the high risk of neurological and functional destruction upon tumor resection. However, malignant transformation rarely occurs in the DPNs, they metastasize widely, and present with poor outcomes. For that reason radical surgery is reserved for instances of malignant transformations with palliative chemotherapy [30-33].
Patients should be evaluated on individually taking into account of the patients’ needs. Therefore DPNs are prone to significant neovascularisation; the vascularity of DPNs should be distinguished by angiography in the preoperative period. If necessary, a combination of preoperative intra-arterial embolization should be performed. Adequate blood should also be arranged before taking up these cases for resection [27-29].
Hypotensive anesthesia has proven to be effective in decreasing intraoperative bleeding. Because the vessels in tumor or skin lesions are abnormal, incisions must be made on normal skin, which provides better bleeding control. Tumescent fluid infiltration can also be effective in minimizing the bleeding [34-36].
Surgical field reveals grossly thickened subcutaneous nerves and very friable neo-vessels which can bleed profusely during dissection in the tumoral mass, and tissue planes are not clearly identified. With using bipolar cauther dissection during tumor de bulking procedure, profuse bleeding can be minimized. The bleeding can also be controlled by systematic ligation of the venous confluents. During de bulking procedure facial nerve branches should be protected unless they were already completely destroyed by the tumor. Recurrent sagging occurs within a short period after surgery due to skin sutures are generally placed to hold the tissues against gravity after subtotal tumor excision. To avoid recurrent sagging, soft tissue suspension can be provided with fascia grafts or Teflon mesh (as a replacement material for fascia grafts), in setting them to the bone tissue by using anchoring sutures or bone-anchored prolene sutures. Thus it can be possible to prevent pulling down of some parts on face such as eyelids, nasolabial sulci. Fibrin sealant can be applied on the operated site before closure to control the diffuse bleeding that persisted after this de bulking procedure. After drains are placed skin flap should be re draped in small to medium defects. Large defects can be managed with a free flap [37,38].
In cases with destruction of skeletal system such as orbit, zygoma and mandible, proper preoperative planning should be considered. Recent advances in technology and the increase of knowledge about the virtual surgery provided pre surgical planning, design and manufacturing of custom implants. To achieve better symmetry and cosmetic results, these custom-made implants have been used. In this study, the use of CAD/CAM technology has been proposed for bone and contour restoration. The assessment of amount and extension of effected bone tissue and the design of implant for the bone defect may be performed virtually. The data from CT or MRI images of the patient provides us delicate planning and design of implant in size and contour. However, implants should be covered by healthy and well vascularized tissue. Inefficient and multiple surgical resection attempts may deteriorate the vascularity and perfusion of skin coverage. Therefore, in some cases, regional or free flaps may be considered for the coverage of implants. So, these operations should not be assessed as a resection only but also reconstruction of hard and soft tissue both. And microsurgical procedures may be needed in some cases. The other limitation of this procedure is the cost of implant design and manufacturing.
Visual acuity or visual loss should be evaluated before resection of NF. The possible risks about this issue should be discussed with patient. Because, the last decision will determine the extension of surgery and the limits of the outcome.
To obtain good cosmetic result, if possible incisions should be placed taking into consideration of the subunits of the face [37].
Skin rheological profiles such as distinctive softness and smoothness of the skin of NF-1 patients are different from normal population due to mutation seen in NF-1 that affects the structure of the connective tissues. These patients demonstrated hyper extensibility above neurofibromas and lack of elasticity in areas that are not affected by the tumor. The wounds of patients with NF-1 heal properly. Whereas solitary neurofibromas has a potential to cause hypertrophic scarring of a variable degree, DPN produced good scars without keloid or hypertrophic characteristics [39].
Patients should be followed up with periodic clinical examinations and MRI evaluations during this period about 2 years [37].
- Bruce R Korf. Neurofibromatosis type 1 (NF1): Pathogenesis, clinical features, and diagnosis. https://goo.gl/u6ukuR
- Lammert M, Friedman JM, Kluwe L, Mautner VF. Prevalence of neurofibromatosis 1 in German children at elementary school enrollment. Arch Dermatol. 2005; 141: 71-4. https://goo.gl/WgNCoQ
- Evans DG, Howard E, Giblin C, Clancy T, Spencer H, Huson SM,et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010; 152A: 327-32. https://goo.gl/cDMsbV
- Evans DG, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005; 26: 93-7. https://goo.gl/K1yKVH
- Evans DG, Howard E, Giblin C, Clancy T, Spencer H, Huson SM, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010; 152: 327-32. https://goo.gl/TZuA1a
- Evans DG, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R.Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005; 26: 93-7. https://goo.gl/makL68
- Antinheimo J, Sankila R, Carpén O, Pukkala E, Sainio M, Jääskeläinen J. Population-based analysis of sporadic and type 2 neurofibromatosis-associated meningiomas and schwannomas. Neurology. 2000; 54: 71-6. https://goo.gl/xw5PgS
- Tchernev G, Chokoeva AA, Patterson JW, Bakardzhiev I, Wollina U, Tana C. Plexiform Neurofibroma: A Case Report. Medicine (Baltimore). 2016; 95: e2663. https://goo.gl/bj2EoB
- Citak EC, Oguz A, Karadeniz C, Okur A, Memis L, Boyunaga O. Management of plexiform neurofibroma with interferon alpha. Pediatr Hematol Oncol. 2008; 25: 673-8. https://goo.gl/Xat6dK
- Needle MN, Cnaan A, Dattilo J, Chatten J, Phillips PC, Shochat S,et al. Prognostic signs in the surgical management of plexiform neurofibroma: the Children’s Hospital of Philadelphia experience, 1974–1994. J Pediatr. 1997; 131: 678-82. https://goo.gl/e4WmVi
- Kebudi R, Cakir FB, Gorgun O. Interferon-αfor unresectable progressive and symptomatic plexiform neurofibromas. J Pediatr Hematol Oncol. 2013; 35: e115-7. https://goo.gl/ksVUpH
- Park SJ, Sawitzki B, Kluwe L, Mautner VF, Holtkamp N, Kurtz A. Serum biomarkers for neurofibromatosis type 1 and early detection of malignant peripheral nerve-sheath tumors. BMC Med. 2013; 11:109. https://goo.gl/5xPTkE
- Adkins JC, Ravitch MM. The operative management of von Recklinghausen’s neurofibromatosis in children, with special reference to lesions of the head and neck. Surgery. 1977; 82: 342-8. https://goo.gl/nP6Y1f
- Jackson IT, Carbonnel A, Potparic Z,Shaw K. Orbitofrontal neurofibromatosis: classification and treatment. Plast Reconstr Surg. 1993; 92: 1-11.https://goo.gl/3Tr7ni
- Acartürk TO, Yiğenoğlu B, Pekedis O. Excision and "transcutaneous" lift in patients with neurofibromatosis of the fronto-temporo-orbital and auricular regions. J Craniofac Surg. 2009; 20: 771-4. https://goo.gl/J2VVyu
- Krastinova Lolov D, Hamza F. The surgical management of cranio-orbital neurofibromatosis. Ann Plast Surg. 1996; 36: 263-9. https://goo.gl/SRY6H3
- Kapur S, Bentz ML, Pediatric Tumors, In: Plastic Surgery, PC. Neligan (Ed). Third Edition, vol three, section 42, 2013, China: Elsevier: p. 877-892.
- Harper, J. Genetics and Genodermatoses. In: Textbook of Dermatology,RH. Champion, JL. Burton, and FJG. Ebling (Eds.), 5th Ed. Oxford: Blackwell Scientific Publications, 1992. p. 305.
- North, K. N. Neurofibromatosis 1 in childhood. Semin Pediatr Neurol. 1998; 5: 231-42. https://goo.gl/93fSeH
- DeBella K, Szudek J, Friedman JM. Use of the National Institutes of Health Criteria for Diagnosis of Neurofi bromatosis 1 in Children. Pediatrics. 2000; 105: 608-14. https://goo.gl/26Avht
- Yohay K. Neurofi bromatosis Types 1 and 2. Neurologist 2006;12:86-93. https://goo.gl/fNKJoM
- Huson SM, Harper PS, Compston DA. Von Recklinghausen neurofibromatosis: a clinical and population study in south-east Wales. Brain. 1988; 111: 1355-81. https://goo.gl/MWggx8
- Enzinger FM, Weiss SW. Soft Tissue Tumors. 1983; 2.
- Korf BR. Malignancy in neurofi bromatosis type 1. Oncologist. 2000; 5: 477-85. https://goo.gl/aSqyzd
- Zanella FE, Modder U, Benz-Bohm G, Thun F. Neurofibromatosis in childhood. Computed tomographic findings in skull and neck areas. Rofo. 1984; 141: 498-504. https://goo.gl/obrECb
- Ergün SS, Emel E, Karabekir S, Büyükbabani N. Extracranial diffuse neurofibroma with intracranial extension.Plast Reconstr Surg. 2000;105:801-3. https://goo.gl/oUWVNP
- Chowdary RP, Little BW. Large vascular plexiform neurofibroma of scalp: Excision and coverage with free transfer. Ann Plast Surg. 1990; 24: 75-9. https://goo.gl/SwFWZu
- de Varebeke SJ, De Schepper A, Hauben E, Declau,F, Van Marck E, Van de Heynin PH. Subcutaneous diffuse neurofibroma of the neck: A case report. J Laryngol Otol. 1996; 110: 182-4. https://goo.gl/jVBdBg
- Asha’ari ZA, Kahairi A, Shahid H Surgery for Massive Paediatric Head and Neck Neurofibroma: Two Case Reports. The International Medical Journal of Malaysia. 2012; 11: 54-61. https://goo.gl/LzhFT6
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004; 69: 335-49. https://goo.gl/jPQHLH
- Hoffman WY, Baker DC. Pediatric Tumors of the Head and Neck. Curr Opin Otolaryngol Head Neck Surg. 2009; 17: 471-6. https://goo.gl/EMiiop
- Friedrich RE, Schmelzle R, Hartmann M, Mautner VF.Subtotal and total resection of superficial plexiform neurofibromas of face and neck: four case reports. J Craniomaxillofac Surg. 2005; 33: 55-60. https://goo.gl/ASpXp3
- Chen YR, Chen KT, Noordhoff MS. Facial elephantiasis neurofibromatosa: Excision and skin graft. Ann Plast Surg. 1989; 23: 547-51. https://goo.gl/3BSk4S
- Tung, TC, ChenYR., Chen KT, Chen CT, Bendor-Samuel R. Massive intratumor hemorrhage in facial plexiform neurofibroma. Head Neck. 1997; 19: 158-62. https://goo.gl/kuiVQ8
- Friedrich RE, Korf B, Funsterer C, Mautner VF. Growth type of plexiform neurofibromas in NF1 determined on magnetic resonance images. Anticancer Res. 2003; 23: 949-52. https://goo.gl/T9UXCc
- Hivelin M, Wolkenstein P, Lepage C, Valeyrie-Allanore L, Meningaud JP, Lantieri L. Facial aesthetic unit remodeling procedure for neurofibromatosis type 1 hemifacial hypertrophy: report on 33 consecutive adult patients. Plast Reconstr Surg. 2010; 125: 1197-207. https://goo.gl/AiPQ8P
- Dogra BB, Rana KS. Facial plexiform neurofibromatosis: A surgical challenge. Indian Dermatol Online J. 2013; 4: 195-8. https://goo.gl/AmjwVs
- Janes LE, Sabino J, Matthews JA, Papadimitriou JC, Strome SE, Singh DP.Surgical management of craniofacial neurofibromatosis type 1 associated tumors. J Craniofac Surg. 2013; 24: 1273-7. https://goo.gl/tpsRLe
- Miyawaki T, Billings B, Har-Shai Y, Agbenorku P, Kokuba E, Moreira-Gonzalez A, et al. Multicenter study of wound healing in neurofibromatosis and neurofibroma. J Craniofac Surg. 2007; 18: 1008-11. https://goo.gl/XkMqnV




Sign up for Article Alerts