Metabolic Encephalopathy vs Seizures: Distinguishing between Triphasic Waves and Epileptiform Discharges?
Jocelyn Y. Cheng*
Department of Neurology, NYU School of Medicine, 223 E. 34th Street, New York
*Address for Correspondence: Jocelyn Y. Cheng, NYU Comprehensive Epilepsy Center, Department of Neurology, NYU School of Medicine, 223 E. 34th Street, New York, NY 10016,Tel: +646-558-0797; Fax: +646-385-7166; E-mail: [email protected]
Submitted: 29 September 2017; Approved: 30 November 2017; Published: 05 December 2017
Citation this article: Cheng JY. Metabolic Encephalopathy vs Seizures: Distinguishing between Triphasic Waves and Epileptiform Discharges. Int J Neurol Dis. 2017;1(2): 044-047.
Copyright: © 2017 Cheng JY. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Triphasic Waves; Encephalopathy; Seizures; EEG; Hypoglycemia
Download Fulltext PDF
Continuous Video EEG (CVEEG) monitoring is often utilized in the evaluation of impaired consciousness. Non convulsive seizures may be distinguished from encephalopathies in this manner. However, Triphasic Waves (TWs), commonly associated with metabolic dysfunction, may assume a pattern concerning for epileptiform activity, calling into question the presence of electrographic seizures, and thus requiring further assessment for elucidation. The following case describes the transient resolution of quasiperiodic TWs after acute administration of glucose in a patient with hypoglycemic encephalopathy.
Abbreviations
CVEEG: Continuous Video EEG; NCSE: Non Convulsive Status Epilepticus; TWS: Triphasic Waves
Introduction
Continuous Video EEG (CVEEG) monitoring is often utilized in the evaluation of impaired consciousness. Non convulsive seizures may be distinguished from encephalopathies in this manner. However, Triphasic Waves (TWs), commonly associated with metabolic dysfunction, may assume a pattern concerning for epileptiform activity, calling into question the presence of electrographic seizures, and thus requiring further assessment for elucidation. The following case describes the transient resolution of quasiperiodic TWs after acute administration of glucose in a patient with hypoglycemic encephalopathy.
Case
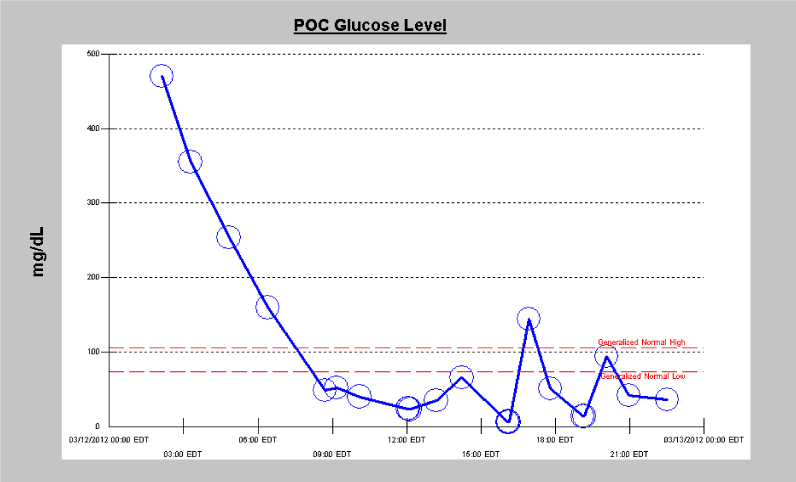
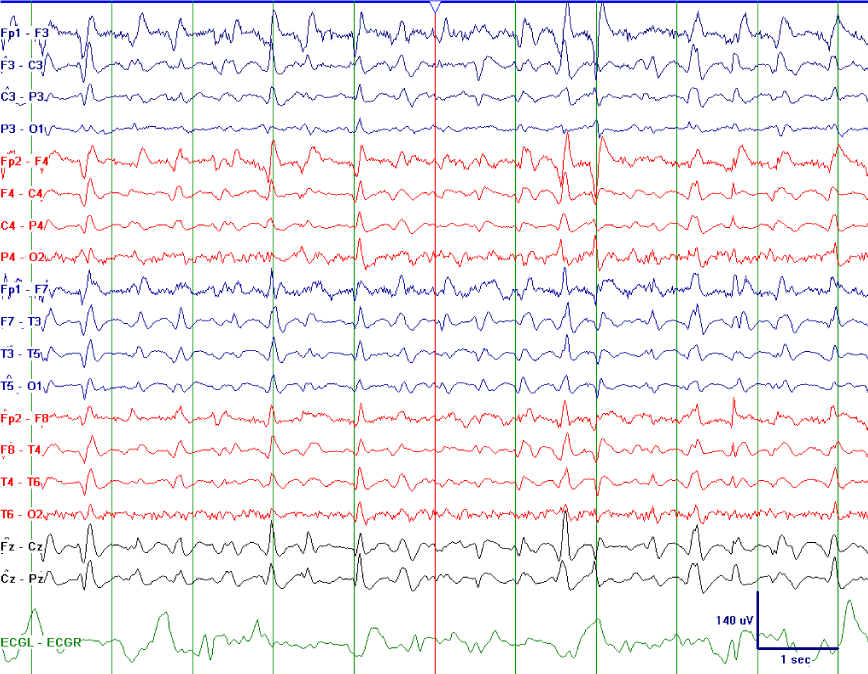
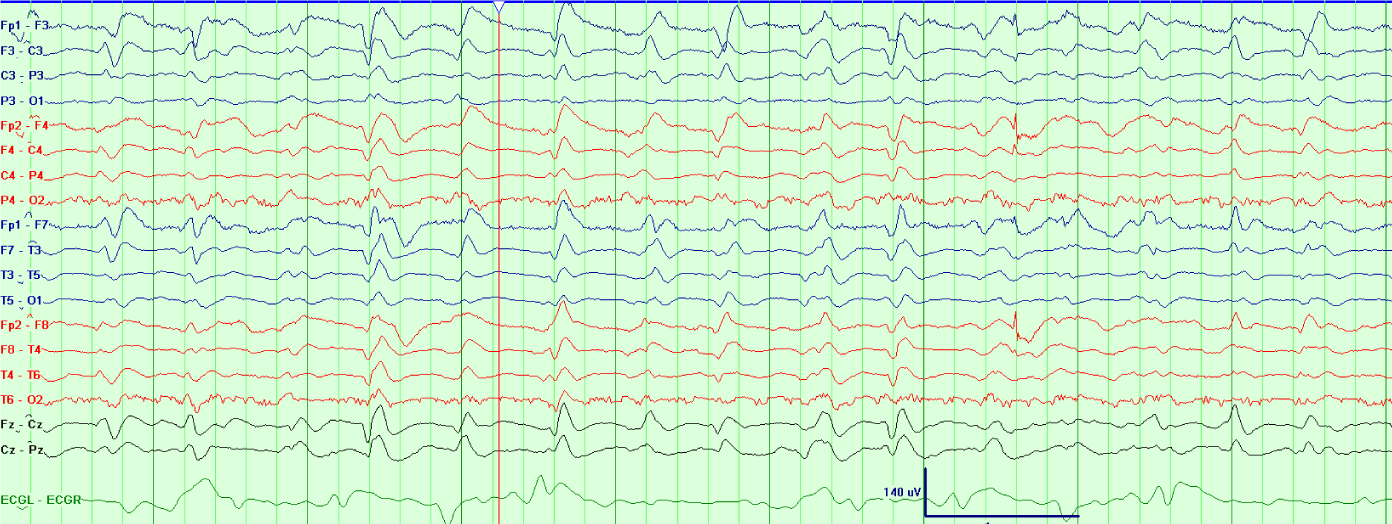
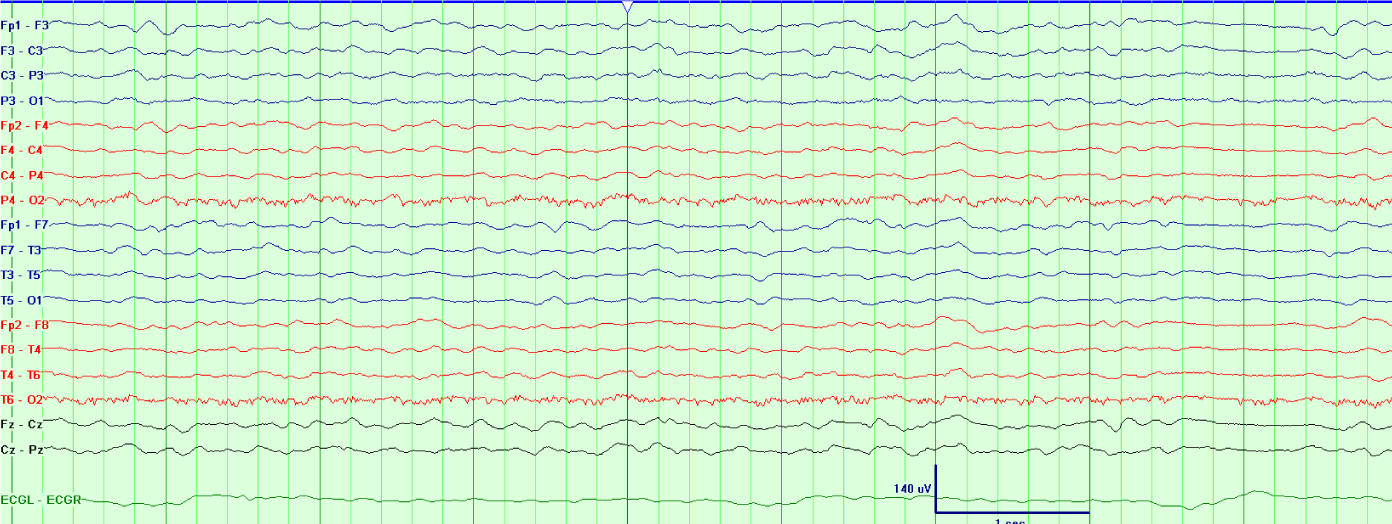
A 66 year old woman with type 1 diabetes mellitus presented with decreased responsiveness after being found at home by a friend. She was subsequently intubated upon arrival of emergency medical services. She was stuporous. Cranial nerves were intact. All four extremities withdrew to noxious stimulation. Plantar responses were flexor. In the hospital, MRI brain exhibited leptomeningeal enhancement consistent with meningitis. Serum blood glucose was 392 mg/dL, with a corresponding CSF glucose of 16mg/dL. Studies from CSF were positive for Streptococcus pneumoniae, and the patient was treated with penicillin G for a total of 14 days after initially receiving empiric treatment with vancomycin, ampicillin and cefepime for 2 days. Three weeks after hospital admission and one week status post completion of antibiotics, her mental status fully returned to baseline. However, during recovery, she developed sudden onset stupor. A CT head was negative for acute pathology, as was subsequent MRI brain, which no longer demonstrated leptomeningeal enhancement. Laboratory evaluation was significant for low blood glucose (50 mg/dL) (Figure 1). She underwent cVEEG monitoring. The EEG showed generalized, polymorphic delta/theta slowing intermixed with TWs that demonstrated a quasiperiodic pattern (Figure 2). During cVEEG, TW activity fluctuated, with the emergence of increasing frequencies, coinciding with worsening hypoglycemia, and a blood glucose nadir of 14mg/dL (Figure 3). Electrographic activity was not induced or exacerbated by tactile or auditory stimulation. Administration of 50 ml of D50 (25 g D - glucose) at bedside resulted in transient resolution of TWs within minutes, which corresponded to a blood glucose of 64 mg/dL (Figure 4). However, background slowing remained on cVEEG, with gradual re-emergence of infrequently occurring TWs despite normoglycemia (Figure 5). Mental status returned to baseline approximately 72 hours after blood glucose stabilization. This was associated on cVEEG with gradual resolution of TWs and an increase in background frequencies.
Discussion
Triphasic Waves (TWs) have been described as early as the 1950s [1], and are commonly associated with metabolic encephalopathy [2]. The waveform is characterized by three phases: 1. an initial low amplitude negative deflection; 2. a dominant, high amplitude, steeply declining positive phase; and 3. a broad, slow rising component. Though greater than a half century of literature exists on TWs, controversies remain as to their definition, distinction from visually similar EEG activity, and significance. This leads to a diagnostic dilemma frequently encountered in the clinical setting: does the presence of TWs merely imply encephalopathy, or do they represent an ictal phenomenon such as Non Convulsive Status Epilepticus (NCSE)?. Several attempts have been made to distinguish TWs from epileptiform activity. While an anterior-posterior lag has been classically described, this is not a reliable feature [3]. In a series of 87 EEGs from 71 patients, noxious or auditory stimulation increased the TWs in 51% of those with metabolic encephalopathy, while there was no effect in patients with NCSE [4]. Kaplan and Schlattman studied the EEG of a patient with genetic epilepsy with generalized frontocentral spike waves who simultaneously developed TWs due to hepatic failure [5]. In using the patient’s prior and current EEGs as a comparative control, objective criteria were posited to distinguish TWs from generalized epileptiform discharges. Based on their analysis, generalized spike waves were more narrow-angled, briefer and front polar dominant, while TWs were characterized by longer duration, a frontocentral predominance, and blunting of morphology with stimuli. More recently, in 2012, the American Clinical Neurophysiology Society published recommendations for standardization of EEG patterns encountered in the critical care setting [6]. Though primarily targeted for research use, this introduced greater objectivity into EEG interpretation. In this context, the term “Triphasic waves” was replaced with “continuous 2/s generalized periodic discharges (with Triphasic morphology).” Criteria for identifying EEG patterns based on location, rhythmicity, periodicity, sharpness and baseline crossings, among other features, were established. In addition, the presence of unequivocal electrographic seizures and equivocal EEG patterns was acknowledged. While helpful in terms of defining clear criteria for electrographic seizures, the identification that an EEG pattern is equivocal leaves a clinician with the same diagnostic dilemma as previously mentioned. In the case described, this was the exact scenario that presented itself: altered mental status in a patient with quasiperiodic TWs (or continuous 2/s generalized quasiperiodic discharges with Triphasic morphology) on EEG. With the limitations of EEG interpretation as discussed above, the management of this equivocal EEG pattern required clinical intervention to elucidate its significance. Response to a benzodiazepine, which broadly suppresses EEG activity, would not electrographically distinguish whether this activity was an ictal or interictal phenomenon. Administration of a loading dose of an antiepileptic drug may have clarified the significance of EEG findings. However, electroencephalographic resolution would be delayed due to the time required for a drug’s pharmacologic actions to take effect. On the other hand, if hypoglycemia was the cause of the patient’s clinical condition and EEG activity, correction of the metabolic disturbance might be expected to ameliorate the quasiperiodic TWs, which it did, and in an immediate manner, thus avoiding unnecessary additional procedures or medications. Certain antibiotics, including cephalosporins and penicillins, have also been associated with TWs, encephalopathy and seizures [7]. As the patient had completed antibiotics one week prior to presentation with altered mental status, and as cephalosporin’s had last been administered for 2 days 3 weeks beforehand, antibiotic related encephalopathy was un likely. Hypoglycemia does not necessarily result in TWs. TWs are a nonspecific phenomenon associated with multiple medical conditions, including but not limited to uremia, sepsis, hypercalcemia, hepatic failure, dementia, post anoxia, and toxic exposure (i.e., lithium) [3,8]. In hypoglycemia, many EEG patterns have been described in addition to TWs, and individual EEG patterns can vary widely without consistent correlation to blood glucose levels. Alpha frequencies may slow to the theta range at 50-80 mg/dL, and below 40 mg/dL, there may be a mixture of theta and delta frequencies with intermixed rhythmic delta activity [9,10]. Others have found that mental status is more closely related to the rate of blood glucose decline than absolute level [11]. In a study of 5 nondiabetic subjects monitored with quantitative EEG, EEG power and blood glucose was tracked before and after acute administration of insulin to induce hypoglycemia [12]. Twenty to 35 minutes after insulin infusion, EEG power increased in beta, theta and delta frequencies, which was associated with declining blood glucose levels and nadir. Triphasic waves were present on EEG during this time interval. Between 45 and 50 minutes, total EEG power fell below baseline at all frequencies, reaching its nadir, and was associated with rising glucose levels. Nonetheless, as demonstrated by the current case, in certain individuals, hypoglycemia-associated Triphasic wave resolution may be demonstrated in the immediate setting with acute correction of blood glucose. This significantly affects the management of a patient presenting with altered mental status and TWs on EEG. Of note, however, the return of mentation to baseline may be delayed in comparison to EEG resolution of TWs, underscoring the fact that chronic effects from the inciting metabolic disturbance remain, and that EEG may lack the sensitivity and specificity to directly assess this.
Conclusions
Quasiperiodic Triphasic wave activity due to hypoglycemia may be distinguished from electrographic seizures after acute correction of blood glucose, with corresponding transient resolution of Triphasic Waves. However, clinical response to correction of metabolic dysfunction may be delayed for up to 72 hours.
- Sheridan PH, Sato S. Triphasic waves of metabolic encephalopathy versus spike-wave stupor. J Neurol Neurosurg Psychiatry. 1956; 49: 108-109. https://goo.gl/j7vprd
- Sutter R, Kaplan PW. Clinical and electroencephalographic correlates of acute encephalopathy. J Clin Neurophysiol. 2013; 30: 443-453. https://goo.gl/pxt76e
- Kaplan PW. The EEG in metabolic encephalopathy and coma. J Clin Neurophysiol. 2004; 21: 307-318. https://goo.gl/qgsfKG
- Boulanger JM, Deacon C, Lecuyer D, Gosselin S, Reiher J. Triphasic waves versus nonconvulsive status epilepticus: EEG distinction. Can J Neurol Sci. 2006; 33: 175-180. https://goo.gl/b5J78u
- Kaplan PW, Schlattman DK. Comparison of triphasic waves and epileptic discharges in one patient with genetic epilepsy. J Clin Neurophysiol. 2012; 29: 458-461. https://goo.gl/ZEJG9h
- Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American Clinical Neurophysiology Society’s standardized critical care EEG terminology: 2012 version. Clin Neurophysiol. 2013; 30: 1-27. https://goo.gl/a8Jrii
- Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol. 2011; 72: 381-393. https://goo.gl/n3dU1N
- Kaplan PW, Rossetti AO. EEG patterns and imaging correlations in encephalopathy: encephalopathy part II. J Clin Neurophysiol. 2011; 28: 233-251. https://goo.gl/SzDX4w
- Davis PA. Effect on the electroencephalogram of changing the blood sugar level. Arch Neurol Psychiatr. 1943; 49: 186-194. https://goo.gl/qSiyWw
- Saunders MG, Westmoreland BF. The EEG in Evaluation of Disorders Affecting the Brain Diffusely. In: Klass DW and Daly DD, eds. Current practice of clinical electroencephalography. New York: Raven Press. 1979; 343-378.
- Von Braunmu¨hl A. Insulinschock und Heilkrampf in der Psychiatrie. Stuttgart: Wissenschaftliche Verlagsgesellschaft. 1947.
- Chalew SA, Sakamoto RN, McCarter R, Hanukoglu A, Kowarski AA, Matjasko J. Quantitative monitoring of brain function, vital signs, ad hormonal response during acute insulin-induced hypoglycemia. J Clin Monit. 1989; 5: 229-235. https://goo.gl/ur1gWR

Sign up for Article Alerts