Is 25- Hydroxy Vitamin D a Real Index of Vitamin D Status in Pre-Dialysis Chronic Kidney Disease Patients??
Ahmed Fayed1, Mahmoud M. El Nokeety1, Ahmed A. Heikal2, Hany Hammad1, Dina O. Abdulazim3, Mona M. Salem4, Mervat M. Naguib4, Usama A. Sharaf El. Din1* : on behalf of the Vascular Calcification Group (VCG)
1Nephrology Unit, Internal Medicine Department, School of Medicine, Cairo University, Egypt
2Internal Medicine Department, School of Medicine, Cairo University, Egypt
3Rheumatology and Rehabilitation Department, School of Medicine, Cairo University, Egypt
4Endocrinology Unit, Internal Medicine Department, School of Medicine, Cairo University, Egypt
*Address for Correspondence: Usama AA. Sharaf El Din, International Kidney Center, 58th Abbas El Akkad St., Nasr City, Cairo, Egypt. Post Code: 11759, Tel: +20-1111333800; Fax: +20-222753890; ORCID: orcid.org/0000-0001-9492-9336; E-mail: [email protected]
Submitted: 26 January 2018; Approved: 03 February 2018; Published: 04 February 2018
Citation this article: Fayed A, El Nokeety MM, Heikal AA, Hammad H, El. Din UAS, et al. Is 25- Hydroxy Vitamin D a Real Index of Vitamin D Status in Pre-Dialysis Chronic Kidney Disease Patients? Int J Nephrol Ther. 2018;4(1): 001-006.
Copyright: © 2018 El. Din UAS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords : CKD; Vitamin D deficiency; MBD; Uric acid; Parathormone; Urine albumin excretion; FGF23Z
Download Fulltext PDF
Background: The prevalence of vitamin D deficiency among chronic kidney disease (CKD) patients is high worldwide. Diagnosis of vitamin D status is based on the estimation of serum 25-hydroxy vitamin D (25 OH D) level.
Objective: We looked for the different determinants of serum 25 OH D among pre-dialysis stage 3-5 CKD patients.
Patients and Methods: 1624 stage 3-5 CKD adults (689 male and 935 female) were selected beside 200 normal control subjects. Patients and control subjects were tested for Body Mass Index (BMI), Serum Urea Nitrogen (BUN), Creatinine, Calcium (Ca), Phosphorus (P), Parathormone (PTH), 25 OH vit D, albumin, and Uric Acid (UA), urine Albumin/Creatinine Ratio (ACR), and estimated Glomerular Filtration Rate (eGFR). Beside all these parameters we studied serum fibroblast growth factor-23 (FGF23) in further 100 CKD cases.
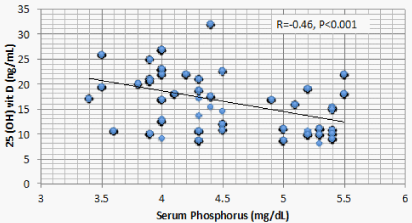
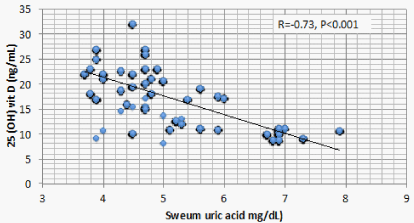
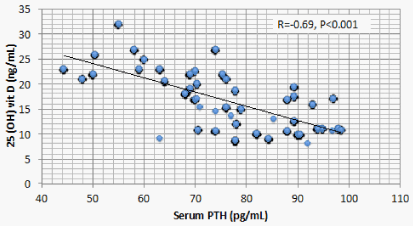
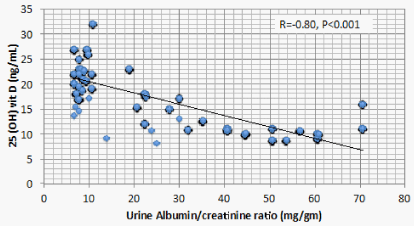
Results: Optimal level of vitamin D is encountered in 1.4% of CKD patients’ vs 52% of the control. 1107 (68.2%) of CKD patients vs 23 (11.5%) of the control group had serum 25(OH) vit D ≤ 20 ng/mL (mean ± S.D = 16.8 ± 5.8 vs 37.3±7.6 ng/mL for CKD vs control group respectively, P < 0.001). There is a significant positive correlation between serum 25(OH) vit D and serum Ca (r = 0.299, P < 0.001), and significant negative correlation between serum 25 OH vit D on one hand, and serum P, PTH, UA, and urine ACR on the other (r = -0.46, -0.69, -0.73, and -0.8 respectively, P < 0.001). On the other hand, BMI, age and FGF-23 did not have significant correlation with 25 OH vit D. Multivariate linear regression model confirmed these significant associations.
Conclusion: Low serum 25 OH vit D is very common among CKD patients. Serum P, UA, and urine ACR are the most important determinants of serum 25(OH) vit D. These results might cast a doubt on the significance of serum 25 OH vit D as diagnostic measure of vitamin D status among pre-dialysis CKD patients.
Introduction
Beside the pivotal role in calcium and phosphate metabolism, Vitamin D plays an important mechanism in musculoskeletal development and health [1]. Vitamin D deficiency has an impact on different body systems beside the musculoskeletal system. The hepatic 25-hydroxylase enzyme (CYP2R1) converts vitamin D to 25 OH vit D. So far, estimation of serum 25 OH vit D is considered the appropriate diagnostic test for vitamin D status. The normal level is above 30 ng/mL, while a level below 20 ng/mL is considered as vitamin D deficiency [2]. The consequences of vitamin D deficiency include increased risks of cardiovascular, neoplastic, infectious, metabolic, and autoimmune diseases. Vitamin D deficiency is also incriminated in the progression of CKD [3,4]. CKD patients have impaired renal 1-α hydroxylase (CYP27B1) activity with a consequent decrease in the rate of conversion of 25(OH)D to calcitriol [2]. This can lead to secondary hyperparathyroidism (2ry HPT). Superimposed deficiency of 25 OH vit D may aggravate 2ry hyperparathyroidism. Calcitriol level should not be used to diagnose vitamin D deficiency as it can lead to erroneous interpretations of vitamin D status. Calcitriol levels are usually normal or even elevated in vitamin D deficiency patients as a result of elevated PTH levels [2]. This observed discrepancy between serum 25 OH vit D and serum calcitriol among CKD patients [2] should cast doubt about the value of 25 OH D as a real indicator of vitamin D deficiency.
Different factors have been suggested as causes of increased prevalence and severity of low 25 OH vit D among CKD patients, namely, anorexia, dietary restriction, diabetes and increased body mass index [5].
Patients and Methods
1624 (688 male and 936 female) CKD patients were initially included in this study. Their ages ranged between 18 and 55 years. The underlying etiology of the renal disease is summarized in table 1. According to KDOQI CKD staging, 271 (16.7%) were in stage 3, 1290 (79.4%) were in stage 4 and 63 (3.9%) were in stage 5. For comparative purposes, 200 normal control subjects were included. Recruitment of these patients spent 18 months. Further 100 CKD patients (57 male and 43 female) were later studied to highlight if there’s a role of FGF-23 in the observed association of serum P with 25 OH vit D.
All CKD subjects were newly diagnosed cases. They were individually interviewed for history taking, clinical examination, and a written consent was obtained from every candidate after discussing the mission of the study. Recruited candidates were clinically examined and a blood sample and urine sample were collected for laboratory assessment of the different studied parameters. Intact PTH level was determined by enzyme-amplified sensitivity immunoassay (Roche Diagnostics, IN, USA). 25 OH vit D was assessed using HPLC [6]. eGFR was measured using MDRD equation [7]. Intact FGF23 was determined using a two-site (NH2-terminal/C-terminal) enzyme-linked immunosorbent assay (Immutopics, CA, USA). As recommended by manufacturer, samples were collected in the morning after 12 h fasting. The collected samples were centrifuged, and the plasma was separated from the cells. Samples were assayed immediately or stored at −70 °C or below.
Microsoft computer statistics package was used for data analysis. Data were summarized as a mean and standard deviation. Comparison between groups was evaluated using Student’s t-test. Correlation coefficient between different parameters of mineral metabolism and kidney function tests was also performed. Multivariate regression analysis was performed looking for the predictors of 25 OH vit D.
Results
Results are summarized in tables 1-8 and figure 1-4. Table 1 summarizes the etiology of renal disease among this group. 1602 of the 1624 patients (98.6%) had suboptimal levels of 25 (OH) vit D (<30 ng/ml). The CKD group was subdivided based on serum 25 OH vit D into 4 subgroups. There was no difference between these 4 groups regarding male/female distribution or presence vs absence of diabetes (Table 3). A similar insignificant difference was observed between normal weight and overweight patients and between diabetic versus non-diabetic patients. In addition, there is no significant difference in between these subgroups in patients’ age, body mass index, blood urea nitrogen, serum creatinine, serum albumin, and estimated glomerular filtration rate (Table 4). On the other hand, there was a significant difference in serum Ca, P, PTH, UA and urine ACR (Table 4).
Correlation studies showed significant positive association between serum 25 OH vit D on one hand and serum albumin and Ca , and significant negative association with age, BUN, P, PTH, UA and urine ACR on the other hand (Table 5). On multivariate regression analysis, positive predictors of 25 OH vit D were serum alb and Ca and negative predictors included serum P, PTH, UA and urine ACR (Table 6).
In a trial to find out an explanation for the negative association between serum P and 25 OH vit D, further 100 CKD patients were recruited in order to study the impact of FGF23 on serum 25 OH vit D. Statistical studies in this new group confirmed the negative association between serum P and 25 OH D but failed to disclose a significant association between FGF23 and serum 25 OH vit D (Table 8).
Discussion
Vitamin D plays a central role in calcium homeostasis and maintaining bone health. A serum level of 25 (OH) vit D of > 30 ng/mL is required to maximize the beneficial effects of vitamin D for health [8]. 98.6% of the CKD patients in the current study have suboptimal levels of 25 (OH) vit D. This figure was never met within the literature. When the prevalence of vitamin D deficiency was studied in sunny Louisiana, 77% had suboptimal levels of 25 (OH) vit D [9]. When a similar study was done in Italy, a more sunny country compared to Louisiana, only 39.6% of the studied CKD patients were considered vitamin D-insufficient [10]. The etiology of vitamin insufficiency among normal healthy subjects includes inadequate exposure to sun and poor fortification of food with vitamin D [11]. Additional factors were accused as causes of increased prevalence and severity of vitamin D insufficiency among CKD patients. These factors include anorexia, dietary restriction [5], old age [11-13], increased body weight [13,14], and diabetic status [9,13,15,16]. However, the association between these different factors and serum level of 25 (OH) vit D is not consistent. Many studies failed to find an association between vitamin D status and age, body mass index, urine albumin excretion or eGFR. In the present study, we did not encounter significant association between vitamin D status, on one hand, and either age, body mass index, or e GFR on the other.
It seems that the climate has no role on serum 25 OH vit D among Egyptian CKD patients. For culture and religious reasons, most of the Egyptian adult females lack adequate exposure to the sun. However, statistical analysis failed to encounter any appreciable difference in serum 25 OH vit D between male and female CKD patients.
The current study discloses a highly significant association between serum 25 OH D and serum Ca, P, PTH, Alb, UA, and urine ACR. Two of these associations, namely, the positive association with Ca and the negative association with PTH are attributed to the physiologic role of vitamin D. The negative association between 25 OH vit D and serum P observed in the current study was also reported in a previous study [14]. This negative association looks odd and raises possible explanations. FGF23 is triggered by P retention in CKD patients. FGF23 was found to negatively correlate with serum 25 OH D in mice. However, this same study, beside the current one, failed to find similar relation in human CKD patients [17]. These findings raise a possible direct effect of P on 25 OH D. Serum P correlates with 24 hydroxylase RNA [18]. This relation means that high serum P can increase catabolism of 25 OH D by the excess activation of Cyp24a1 24 hydroxylase.
For long time, serum Alb was considered as the principal marker used to identify malnutrition in CKD patients if such cases have normal liver function and do not suffer heavy proteinuria. However, further studies and observations have reconsidered serum Alb. as a marker of illness rather than nutrition [19]. The significant positive association of serum Alb. with serum 25 OH vit D denotes that this level might be affected by the health status of CKD patients.
Low 25 OH D was previously reported in CKD patients having sub-nephrotic range of proteinuria [13] and even in those having microalbuminuria [20]. In the current study, CKD patients with increased urine ACR were in the range of microalbuminuria. Serum 25 OH D in these patients has a significant negative association with urine ACR. The Molecular Weight (MW) of Vitamin D Binding Globulin (VDBG) is 52 kDa, a little bi-lower than albumin [21]. 25 OH D bound to VDBG is filtered at glomeruli. Under physiologic conditions, reabsorption of 25 OH D- VDBG complex at proximal tubular cells is mediated by megalin present at the brush border [22]. Whether increased filtration of 25 OH D- VDBG complex or its decreased reabsorption by proximal tubular cells due to down regulation of megalin in CKD patients still needs further studies. On the other hand, rats develop podocyte injury and renal dysfunction if they are 1,25 (OH)2 D -deficient. 1,25 (OH)2 D -deficient animals develop decreased expression of nephrin, podocin, and desmin in the podocyte [23]. In 1,25 (OH)2 D -deficient mice, glomerular transient receptor potential cation channel 6 (TRPC6) expression is increased, accompanied by podocyte foot process effacement and proteinuria [24]. Being the substrate of 1,25 (OH)2 D, deficiency of 25 OH D can result in injury of the renal filtration barrier, leading to proteinuria and consequent renal dysfunction.
The significant negative association between 25 OH vit D and serum UA is more difficult to explain. This association was once observed in old Chinese women with normal kidney functions [25]. The liver is the major if not the sole source of 25 OH vit D production [26]. UA is an important factor underlying Non-Alcoholic Fatty Liver Disease (NAFLD) in patients with or without CKD [27,28]. The significant association of NAFLD and low 25 OH vit D is reported in many recent studies [29-23]. Taken together, these studies raise strongly the possibility of inhibition of hepatic 25 hydroxylation of vitamin D by the high serum UA in patients with or without CKD. A second possibility might be through the recently discovered down regulation of the urate exporter, ATP-Binding Cassette Transporter G2 (ABCG2), by PTH [33]. ABCG2 is responsible for intestinal excretion of UA [34]. PTH induced down regulation of ABCG2 might thus cause retention of UA. In other words, low 25 OH vit D stimulates PTH secretion that in turn causes UA retention. In the current study, the relative weight of 25 OH vit D in association with PTH versus UA criticizes this possibility.
Finally, it seems that serum 25 OH is not a mere indictor of vitamin D status among pre-dialysis CKD patients. At least 3 factors that are commonly encountered in these patients would interfere between real vitamin D input and the estimated level of 25 OH vit D. Future experimental and clinical trials are still needed to explain the discrepancy in the prevalence and the severity of vitamin D deficiency that can not be explained by the environmental and nutritional factors, the inconsistency in the association between vitamin D deficiency and the different factors accused in different studies, and to confirm the or obviate the conclusion of the current study.
Ethical Committee Approval
The local ethical committee of the Internal Medicine department, School of Medicine, Cairo University, approved this work.
Acknowledgments
Professor Usama and Dr. Ahmed Fyed suggested the hypothesis and objectives of this study, Dr. Dina collected the necessary literature, Dr. Mahmoud El Nokeety and Dr. Ahmed Heikal collected the study subjects, Dr. Ahmed Fyed collected the samples and made the statistics prof Usama wrote the manuscript, Dr. Mervat shared in collection of samples and doing the statistics, Dr. Dina made the final revision, Professor Mona supervised the different steps of manuscript creation and finalization.
Human and Animal Rights
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
- Holick MF. Medical progress: vitamin D deficiency. N Engl J Med. 2007; 357: 266-281. https://goo.gl/iehJZ2
- Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc. 2010; 85: 752-757. https://goo.gl/QsZFsn
- Plum LA, Deluca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010; 9: 941-955. https://goo.gl/LCRf55
- DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004; 80: 1689-1696. https://goo.gl/rwTLSo
- Echida Y, Mochizuki T, Uchida K, Tsuchiya K, Nitta K. Risk factors for vitamin D deficiency in patients with chronic kidney disease. Intern Med. 2012; 51: 845-850. https://goo.gl/zJVBSu
- Neyestani TR, Gharavi A, Kalayi A. Determination of serum 25-hydroxy cholecalciferol using high-performance liquid chromatography: a reliable tool for assessment of vitamin D status. Int J Vitam Nutr Res. 2007; 77: 341-346. https://goo.gl/TKQehd
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130: 461-470. https://goo.gl/oh4Eur
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008; 87: 1080-1086. https://goo.gl/7Zmzwn
- Yaturu S, Davis J. Prevalence of Decreased Vitamin D Levels is High among Veterans with Diabetes and/or CKD. ISRN Endocrinol. 2011; 2011: 109458. https://goo.gl/vzeCNk
- Cuppari L, Carvalho AB, Draibe SA. Vitamin D status of chronic kidney disease patients living in a sunny country. J Ren Nutr. 2008; 18: 408-414. https://goo.gl/2gtY7L
- Urena Torres P, Metzger M, Haymann JP, Karras A, Boffa JJ, Flamant M, et al. Association of kidney function, vitamin D deficiency, and circulating markers of mineral and bone disorders in CKD. Am J Kidney Dis. 2011; 58: 544-553. https://goo.gl/tvNXuP
- Cupisti A, Vigo V, Baronti ME, D'Alessandro C, Ghiadoni L, Egidi MF. Vitamin D status and cholecalciferol supplementation in chronic kidney disease patients: an Italian cohort report. Int J Nephrol Renovasc Dis. 2015; 8: 151-157. https://goo.gl/S73d5w
- Rodriguez Villarreal I, Ortega O, Gallar P, Sanchez M, Callejas R, Gracia C, et al. Clinical and biochemical characteristics of predialysis patients in terms of 25 hydroxy vitamin D levels. Nefrologia. 2011; 31: 185-191. https://goo.gl/W9rMFX
- Barreto Silva MI, Cavalieri VV, Lemos CC, Klein MR, Bregman R. Body adiposity predictors of vitamin D status in nondialyzed patients with chronic kidney disease: A cross-sectional analysis in a tropical climate city. Nutrition. 2017; 33: 240-247. https://goo.gl/1LPRij
- Rozita M, Noorul Afidza M, Ruslinda M, Cader R, Halim AG, Kong CT, et al. Serum Vitamin D levels in patients with chronic kidney disease. EXCLI J. 2013; 12: 511-20. https://goo.gl/U2FMU4
- Yuste C, García De Vinuesa S, Goicoechea M, Barraca D, Panizo N, Quiroga B, et al. Vitamin D deficiency in a Spanish cohort of patients with chronic kidney disease. Med Clin (Barc). 2013; 141: 338-342. https://goo.gl/74PGA6
- Dai B, David V, Alshayeb HM, Showkat A, Gyamlani G, Horst RL, et al. Assessment of 24, 25(OH)2D levels does not support FGF23-mediated catabolism of vitamin D metabolites. Kidney Int. 2012; 82: 1061-1070. https://goo.gl/YMYns8
- Wu S, Finch J, Zhong M, Slatopolsky E, Grieff M, Brown AJ. Expression of the renal 25-hydroxyvitamin D-24-hydroxylase gene: regulation by dietary phosphate. Am J Physiol. 1996; 271: 203-208. https://goo.gl/sHKo2z
- Friedman AN, Fadem SZ. Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol. 2010; 21: 223-230. https://goo.gl/xu1qtZ
- Damasiewicz MJ, Magliano DJ, Daly RM, Gagnon C, Lu ZX, Ebeling PR, et al. 25-Hydroxyvitamin D levels and chronic kidney disease in the AusDiab (Australian Diabetes, Obesity and Lifestyle) study. BMC Nephrol. 2012; 13: 55. https://goo.gl/WQpuh6
- Imawari M, Kida K, Goodman DS. The transport of vitamin D and its 25-hydroxy metabolite in human plasma. Isolation and partial characteri-zation of vitamin D and 25-hydroxyvitamin D binding protein. J Clin Invest. 1976; 58: 514-523. https://goo.gl/LL7tZ2
- Fukagawa M, Komaba H, Hamano T. Vitamin D supplementation in renal disease: is calcitriol all that is needed? Scand J Clin Lab Invest Suppl. 2012; 243: 120-123. https://goo.gl/RiXBje
- Sonneveld R, Hoenderop JG, Stavenuiter AW, Ferrantelli E, Baltissen MP, Dijkman HB, et al. 1,25-Vitamin D3 Deficiency Induces Albuminuria. Am J Pathol. 2016; 186: 794-804. https://goo.gl/76mDML
- Sonneveld R, Ferre S, Hoenderop JG, Dijkman HB, Berden JH, Bindels RJ, et al. Vitamin D down-regulates TRPC6 expression in podocyte injury and proteinuric glomerular disease. Am J Pathol. 2013; 182: 1196-1204. https://goo.gl/EkftVU
- Peng H, Li H, Li C, Chao X, Zhang Q, Zhang Y. Association between Vitamin D Insufficiency and Elevated Serum Uric Acid among Middle-Aged and Elderly Chinese Han Women. PLoS One. 2013; 8: 61159. https://goo.gl/WVFfqb
- Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014; 21: 319-329. https://goo.gl/6sNUXu
- Liang J, Pei Y, Gong Y, Liu XK, Dou LJ, Zou CY, et al. Serum uric acid and non-alcoholic fatty liver disease in non-hypertensive Chinese adults: the Cardiometabolic Risk in Chinese (CRC) study. Eur Rev Med Pharmacol Sci. 2015; 19: 305-311. https://goo.gl/hX2aqc
- Jia G, Di F, Wang Q, Shao J, Gao L, Wang L et al. Non-Alcoholic Fatty Liver Disease Is a Risk Factor for the Development of Diabetic Nephropathy in Patients with Type 2 Diabetes Mellitus. PLoS One. 2015; 10: 0142808. https://goo.gl/uGbToX
- Eliades M, Spyrou E. Vitamin D: a new player in non-alcoholic fatty liver disease? World J Gastroenterol. 2015; 21: 1718-1727. https://goo.gl/91ykPX
- Hao YP, Ma XJ, Luo YQ, Ni J, Dou JX, Hu YQ, et al. Serum vitamin D is associated with non-alcoholic fatty liver disease in Chinese males with normal weight and liver enzymes. Acta Pharmacol Sin. 2014; 35: 1150-1156. https://goo.gl/CytzfJ
- Chen EQ, Shi Y, Tang H. New insight of vitamin D in chronic liver diseases. Hepatobiliary Pancreat Dis Int. 2014; 13: 580-585. https://goo.gl/h4uQic
- Barchetta I, Carotti S, Labbadia G, Gentilucci UV, Muda AO, Angelico F, et al. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology. 2012; 56: 2180-2187. https://goo.gl/1ADV83
- Sugimoto R, Watanabe H, Ikegami K, Enoki Y, Imafuku T, Sakaguchi Y, et al. Down-regulation of ABCG2, a urate exporter, by parathyroid hormone enhances urate accumulation in secondary hyperparathyroidism. Kidney Int. 2017; 91: 658-670. https://goo.gl/eSmEZh
- Takada T, Ichida K, Matsuo H, Nakayama A, Murakami K, Yamanashi Y, et al. ABCG2 dysfunction increases serum uric acid by decreased intestinal urate excretion. Nucleosides Nucleotides Nucleic Acids. 2014; 33: 275-281. https://goo.gl/PQtvci

Sign up for Article Alerts