Kratom Induced Hepatotoxicity: A Case Report
Jonathan Quinonez2* and Tegpal Atwal2
1General Practitioner, Lakeland, FL, United States of America
2Adventist Health, Department of Gastroenterology, Saint Helena, CA, United States of America
*Address for Correspondence: Jonathan Quinonez, Department of Osteopathic Medicine, Lakeland, United States of America, 33809, Tel: +1-801-425-1132, E-mail: [email protected]
Submitted: 23 December 2019; Approved: 20 January 2020; Published: 28 January 2020
Citation this article: Quinonez J, Atwal T. Kratom Induced Hepatotoxicity: A Case Report. Int J Hepatol Gastroenterol. 2020; 6(1): 001-004.
Copyright: © 2020 Quinonez J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
Kratom is an herbal product that is derived from Southeast Asian Mitragyna speciose tree leaves [1-10]. This compound is used for many purposes such as stimulation, euphoria, or analgesia [1-10]. It has been recently identified as a drug of abuse by the United States Drug Enforcement Administration [2,8]. Side-effects from this compound have not been well documented. We describe a case of a 36-year-old female who develop nephrotoxicity after taking an herbal supplement. She took kratom as an adjunctive therapy for back pain management. She developed right upper quadrant pain and nausea. Laboratory tests showed elevated liver enzymes without evidence of bile duct obstruction. Liver enzymes normalized several weeks after Kratom discontinuation. We advise clinicians to be vigilant about Kratom’s hepatotoxic potential on patient health.
Introduction
Kratom is an herbal product that is derived from Southeast Asian Mitragyna speciosa tree leaves [1-10]. Such leaves contain psychoactive opioid compounds that can be utilized for many purposes such as stimulation, euphoria, or analgesia [1-10]. As a popular drug, Kratom is used widely for conditions such as chronic pain, diarrhea, or fatigue management. Patients have used this drug for chronic pain management when interchanging with opiates. Kratom can also be used recreationally in teas consisting of Kratom leaves, cough syrup, Coca-Cola, and ice; this can induce euphoria and hallucinations when consumed [3,8,9].
Recently, it has been identified as an emerging drug of abuse. Liver toxicity and histopathology from Kratom has not been documented extensively despite the United States Drug Enforcement Administration listing this drug on its “Drugs and Chemicals of Concern” list [8]. Research about Kratom’s potential toxicities is scarce except for scattered case reports [1,2,4-6]. Given the scarce literature of Kratom, this report describes a novel case of Kratom induced hepatotoxicity disguised as choledocholithiasis in a young female with chronic back pain.
Case Report
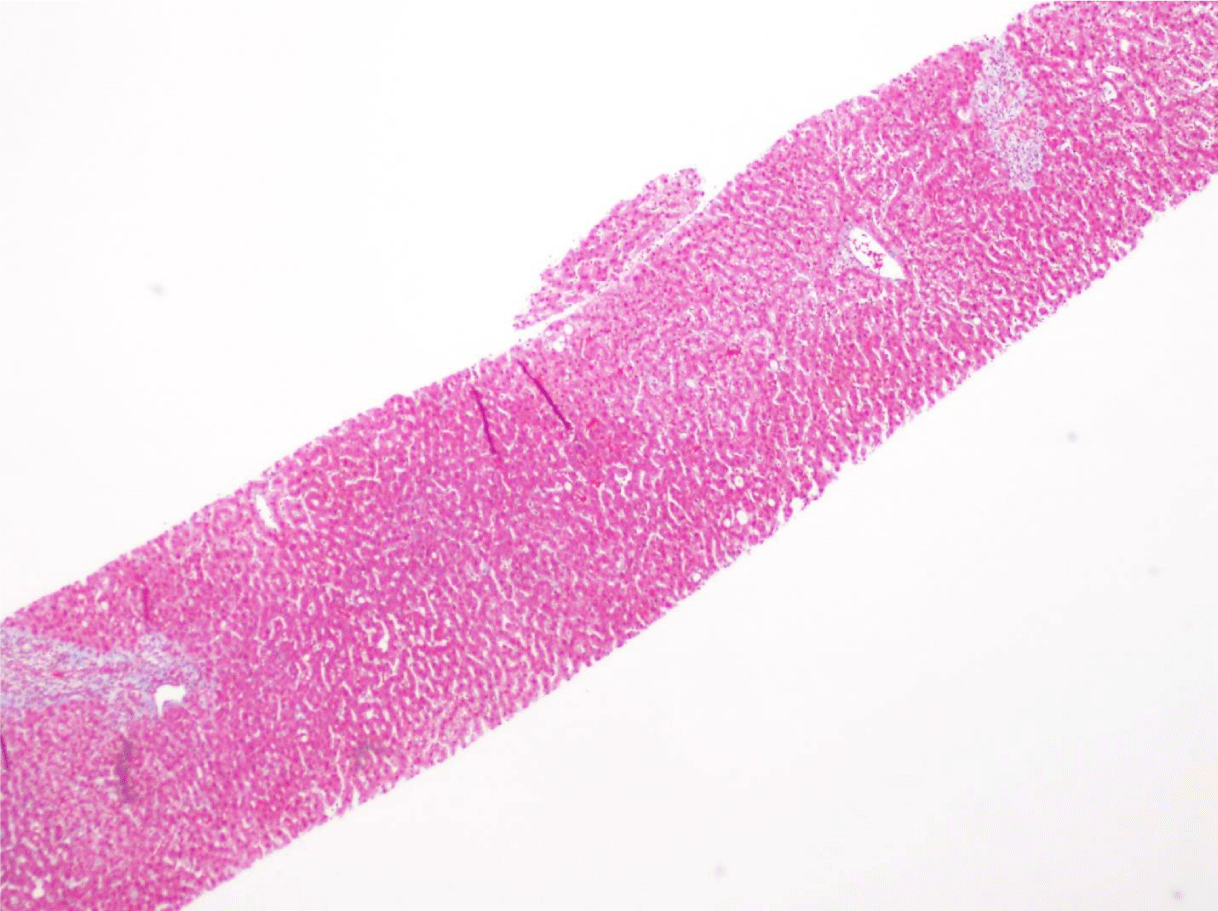
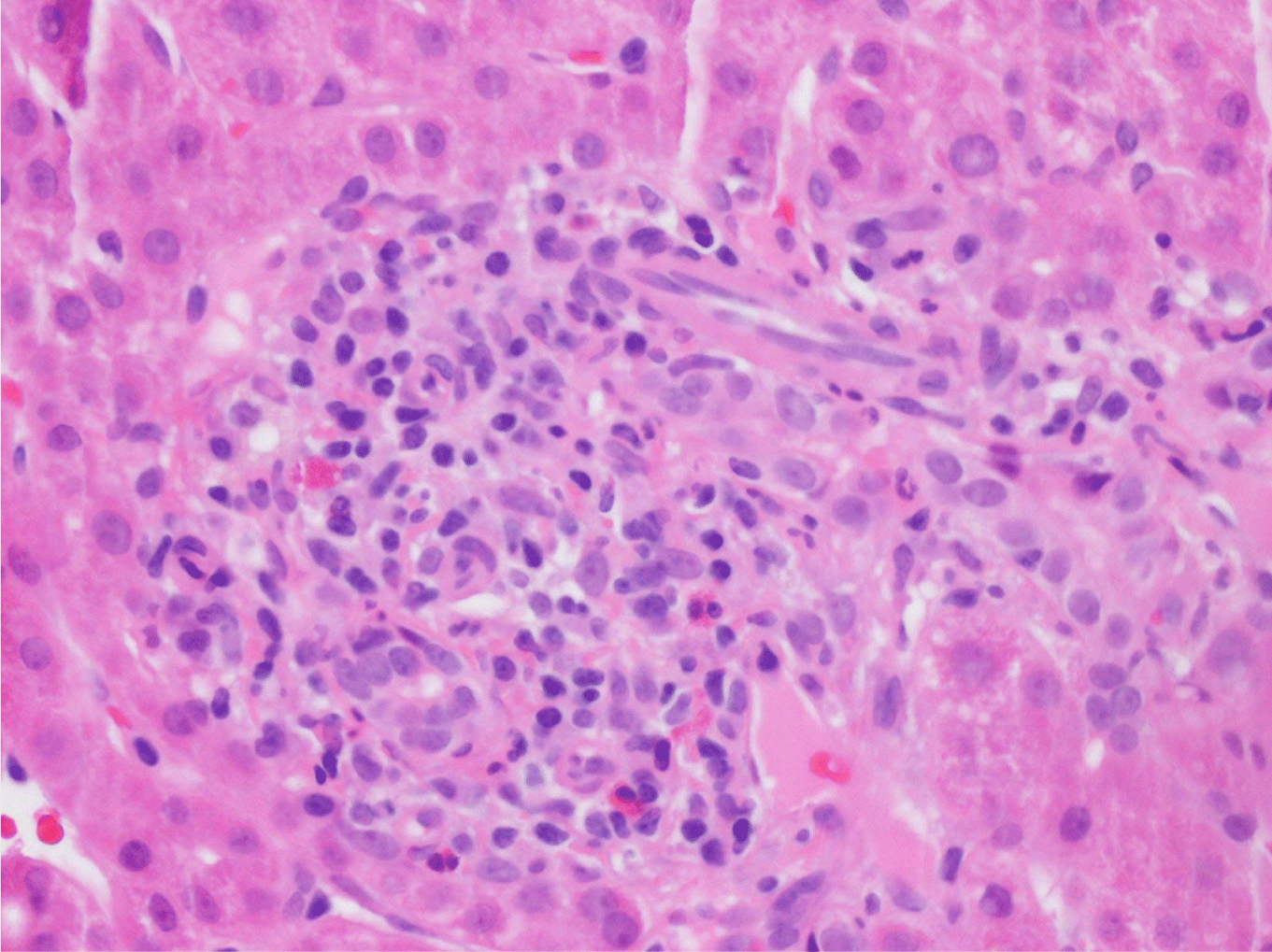
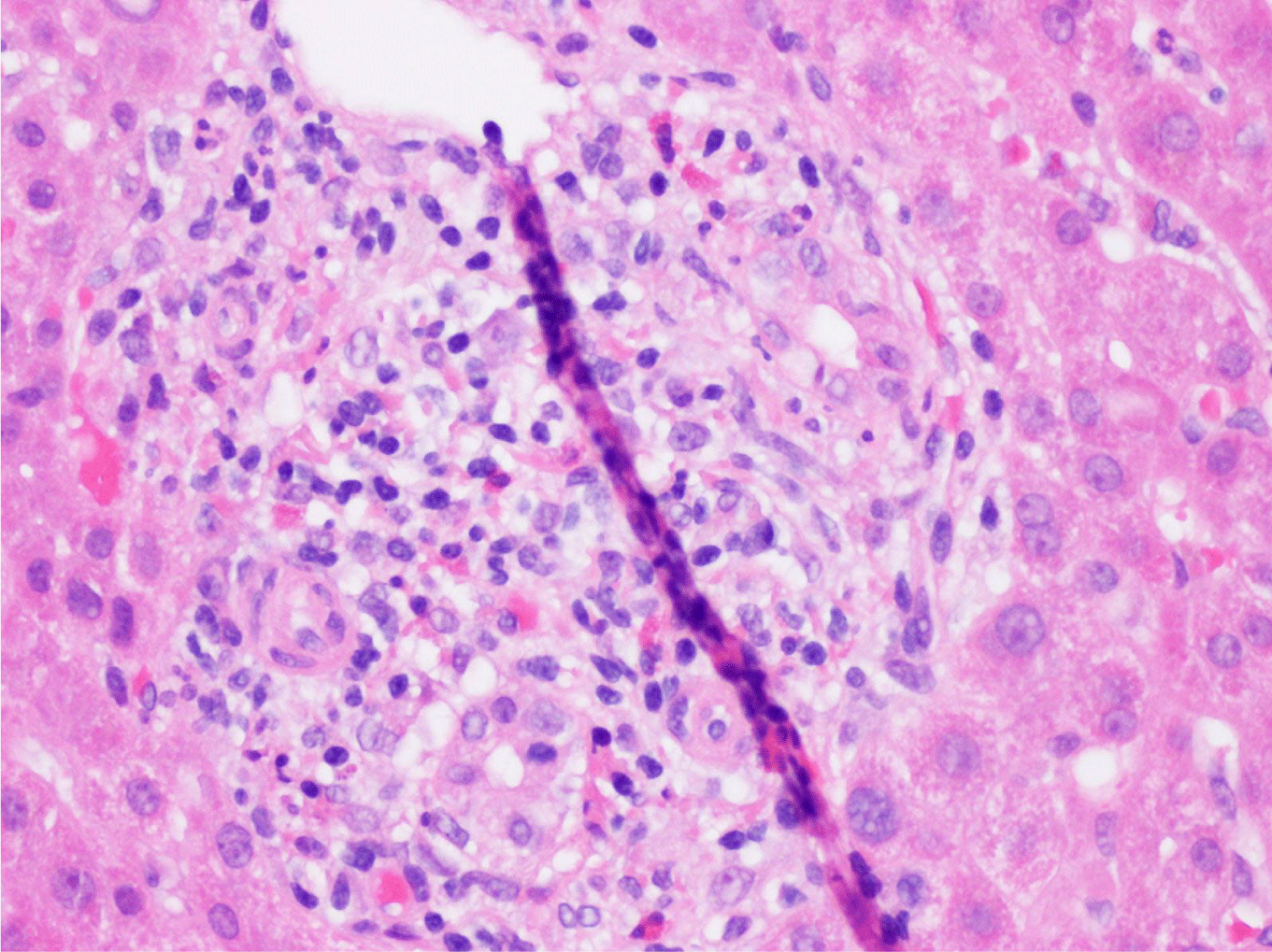
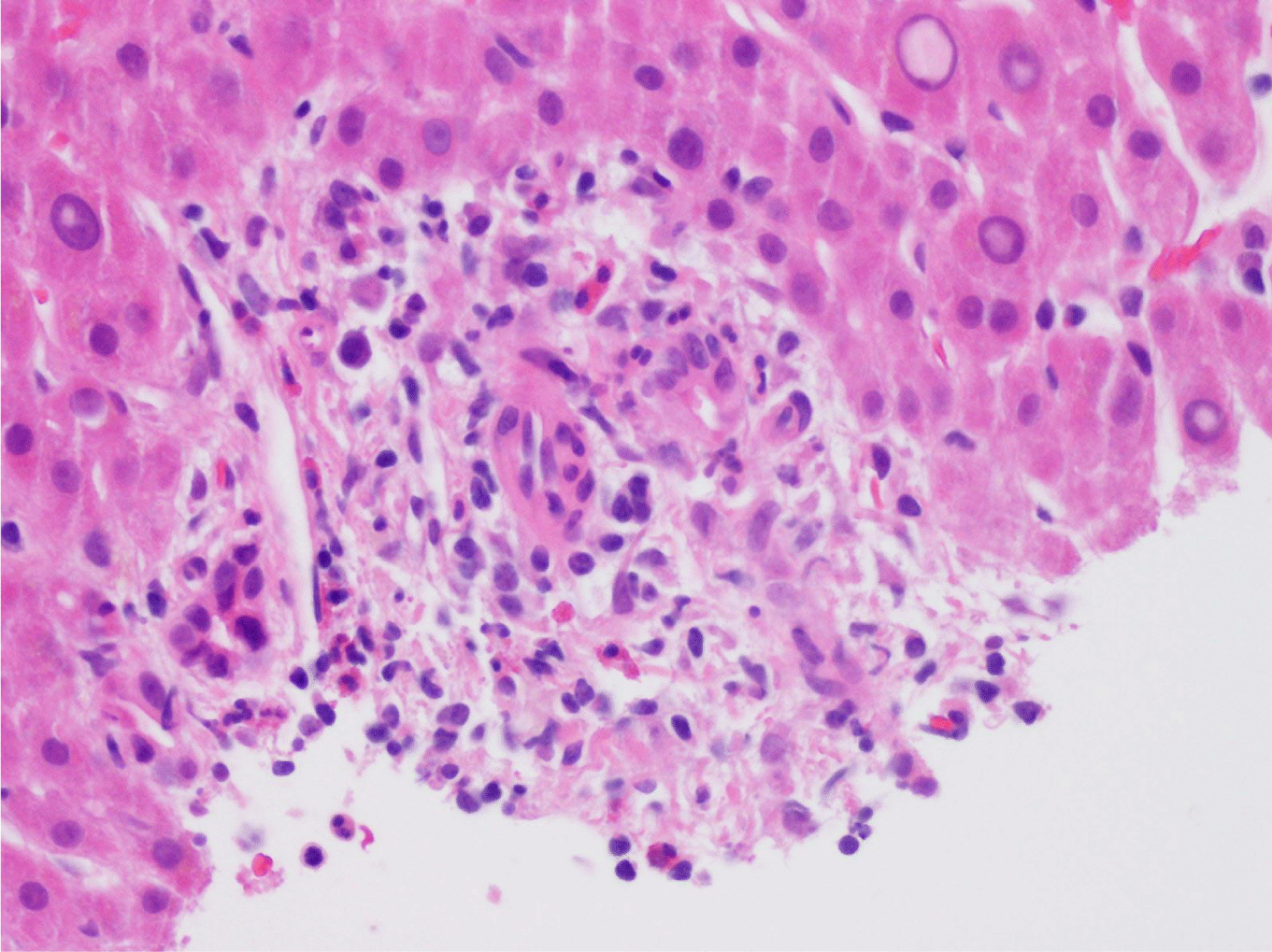
This case presents a 36-year-old female with chronic back initially managed with Gabapentin. Her baseline laboratory tests prior to starting Kratom us were within normal limits. She started Kratom for back pain. Two weeks later, she stopped taking Kratom due to experiencing vague epigastric symptoms such as severe nausea and vomiting. Her liver enzymes were elevated after stopping Kratom (Table 1). A right upper quadrant abdominal ultrasound showed mild non-specific thickening of the gallbladder wall with no gallstones present. An initial diagnosis of choledocholithiasis was suspected based on her symptoms and laboratory tests (Table 2). A liver biopsy was performed and it ruled out both infectious and autoimmune hepatitis; results demonstrated drug-induced liver injury (Figures 1-5). Soon after, her liver function tests were monitored every three days. She saw gradual improvement over the course of six weeks and eventually returned to normal limits.
Discussion/Conclusion
Kratom is an herbal product that is derived from Southeast Asian Mitragyna speciosa tree leaves [1-10]. The effects of this compound are mediated by mitragynine and its active metabolites which are intrinsic in antagonizing opioid receptors [3,7]. The adverse effect to a compound may include confusion, coma, respiratory arrest whereas milder symptoms may include right upper quadrant pain and elevated liver function tests [3]. The injury that occurred to this patient in this case report over a period of several months was primarily due to drug-induced liver injury from Kratom usage. Her liver function tests reflect such changes over a period of months. With right upper quadrant pain and elevated liver function tests, both can create a broad differential diagnosis that can include choledocholithiasis. She underwent workup for cholestatic injury along with a liver biopsy. Her work-up resulted in drug-induced liver injury. Her symptoms and liver function tests resolved after one month.The rise in Kratom usage in the United States and other Western countries has been attributed to supplement stores and over the internet as a sedative with euphoric effects [8,10]. Kratom, surprisingly, has been used in Southeast Asia for centuries but is now under consideration to be banned; Thailand is one country that has a ban on Kratom usage [8]. Attention is now being warranted to Kratom and similar compounds due to its deleterious side effect profile after its use. The side effect profile of this compound is dose dependent, which may include [3,8]:
• Low dose (1-5 g of raw leaves): Nausea, loss of appetite, blushing, anxiety, agitation.
• Moderate (5-15 g of raw leaves): tachycardia, constipation, dry mouth, sweating.
• Heavy (greater than 15 g of raw leaves): Similar to opioid overdose – Respiratory depression, liver toxicity, and death.
Although Kratom is listed on the “Drugs and Chemicals of Concern” list, this drug remains rampant as it is sold on the internet as an alternative to pain control [8]. Many websites exist that include articles that claim its anti-analgesic properties and its use in opioid withdrawal. Here, attention should be given to fully analyze the effects of Kratom.
Kratom use has been on the rise for the past few years for management of conditions such as stimulation, euphoria, or analgesia. The side-effect profile of this compound has not been well studied; this compound can mimic conditions such as choledocholithiasis. Clinicians should monitor for Kratom usage in patients and advise against its use.
Acknowledgement
The authors of this article would like to thank the sponsoring institution Trios Health for making substantive contribution to the research of this manuscript.
Disclosure Statement
The authors have no conflicts of interest to declare. Verbal and written consent was obtained from a patient who participated in this case report.
Author Contributions
Dr. Tegpal Atwal provided the information needed for the creation of this case report along with supervision. Drs. Jonathan Quinonez helped to create the article.
- Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged Kratom use: A case report. Hepatology. 2014; 61: 1086-1087. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25418457
- Galbis-Reig D. A Case Report of Kratom Addiction and Withdrawal. WMJ. 2016; 115: 49-52. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27057581
- Hassan Z, Muzaimi M, Navaratnam V, Yusoff NH, Suhaimi FW, Vadivelu R, et al. From Kratom to mitragynine and its derivatives: Physiological and behavioral effects related to use, abuse, and addiction. Neurosci Biobehav Rev. 2013; 37: 138-151. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23206666
- Kapp FG, Maurer HH, Auwärter V, Winkelmann M, Hermanns-Clausen M. Intrahepatic cholestasis following abuse of powdered Kratom (Mitragyna speciosa). J Med Toxicol. 2011; 7: 227-231. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21528385
- McIntyre IM, Trochta A, Stolberg S, Campman SC. Mitragyne ‘Kratom’ Related Fatality: A Case Report with Postmortem Concentrations. Journal of Analytical Toxicology. 2015; 39: 152-155. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25516573
- McWhirter L, Morris S. A Case Report of Inpatient Detoxification after Kratom (Mitragyna speciosa) Dependence. Eur Addict Res; 2010; 16:229-231. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20798544
- Pantano F, Tittarelli R, Mannocchi G, Zaami S, Ricci S, Giorgetti R, et al. Hepatotoxicity Induced by “the 3Ks”: Kava, Kratom, and Khat. Int. J. Mol. Sci. 2016; 17: 580. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27092496
- Prozialeck WC. Update on the Pharmacology and Legal Status of Kratom. J Am Osteopath Assoc. 2016; 116: 802-809. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27893147
- Nelsen JL, Lapoint J, Hodgman MJ, Aldous KM. Seizure and Coma Following Kratom (Mitragynina speciose Korth) Exposure. J Med Toxicol. 2010; 6: 424-426. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20411370
- Swogger MT, Hart E, Erowid F, Erowid E, Trabold N, Yee K, et al. Experiences of Kratom Users: A Qualitative Analysis. J Psychoactive Drugs. 2015; 47: 360-367. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26595229



Sign up for Article Alerts