Euglycemic Diabetic Ketoacidosis Complicating use of an SGLT-2 Inhibitor in a Patient with Undiagnosed Type 1 Diabetes Mellitus: A Case Report?
Shahzeena Hafeez1* and Israel Orija2
1Department of Environmental, Biological and Pharmaceutical Sciences and Technologies, University of Campania, I-81100 Caserta, Italy
2Israel Orija, Internal Medicine, Wellstar Atlanta Medical Center, 303 Parkway Dr NE, Atlanta GA, 30312, USA
*Address for Correspondence: Shahzeena Hafeez, PG-Y 2 Resident Internal Medicine, Wellstar Atlanta Medical Center, 303 Parkway Dr NE, Atlanta GA, 30312, USA, Tel: +267-736-2470; E-mail: [email protected]/ [email protected]
Submitted: 13 July 2017; Approved: 28 August 2017; Published: 01 September 2017
Citation this article: Hafeez S, Orija I. Euglycemic Diabetic Ketoacidosis Complicating use of an SGLT-2 Inhibitor in a Patient with Undiagnosed Type 1 Diabetes Mellitus: a Case Report. Int J Clin Endocrinol. 2017;1(2): 049-052.
Copyright: © 2017 Hafeez S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Diabetic ketoacidosis; Euglycemia; Diabetes Mellitus; SGLT2 inhibitors
Download Fulltext PDF
Introduction: The use of sodium-glucose cotransporter 2 (SGLT-2) inhibitors have recently increased in patients with Diabetes Mellitus Type 2 (DM2). However their off label use in patients with Diabetes Mellitus Type 1 (DM1) is gradually increasing and thus exposing these patients to an increased risk of Euglycemic Diabetic Ketoacidosis (EuDKA). The aim of this case report is to emphasize the challenges encountered while treating a patient with EuDKA who was on an SGLT-2 inhibitor for presumptive DM2 and hope that such a complication can be avoided in the future.
Case report: We report a patient with DM2, being treated with Glyburide, Glucophage and recently added Canagliflozin, with clinical findings suggestive of diabetic ketoacidosis. Despite appropriate treatment and return of her blood glucose to normal, her anion gap remained persistently elevated, with low serum bicarbonate and positive urinary ketones and glucose, thus confirming the diagnosis of EuDKA that required prolonged intravenous insulin therapy.
Conclusion: This case reinforces the fact that SGLT-2 inhibitors should be withheld or used with extreme caution in treating DM1 patients.
Introduction
With the ever increasing burden of Diabetes Mellitus throughout the world, the use of new Oral antihyperglycemic agents have been increasing widely. SGLT-2 inhibitors are a class of drugs that block the protein channels in the proximal renal tubules that are responsible for reabsorption of about 90% of the glucose [1]. When used alone or in conjunction with another class of antihyperglycemics, they are known to reduce HbA1c levels by about 0.5-0.7% [1-4]. While the use of SGLT2 inhibitors in patients with DM2 has been shown to provide increased benefit by its gradual dose-dependent decrease in HbA1c and its secondary effects causing reduction in weight, blood pressure and improved lipid profile, its efficacy to achieve glycemic control in DM1 remains controversial and thus has not received approval from the FDA [5-7]. However the off label use of these agents for management of DM1, in an attempt to reduce insulin doses cannot be overlooked. And more importantly, its unintentional use in those incorrectly diagnosed as DM2 remains a concern for its precipitating serious side effects related to euglycemic acidosis [8]. The case below describes a patient with DM1, who was recently started on an SGLT-2 inhibitor, who presented initially as hyperglycemic DKA, but later evolved into a challenging case of EuDKA requiring treatment with intravenous insulin for almost a week.
Case Presentation
Our subject is a 52 year old African American female with past medical history of primary hypothyroidism and poorly controlled DM2 for a duration of two years. She presented with two days history of nausea, vomiting and extreme lethargy along with altered mentation. Prior to her admittance, she was being managed with Metformin 1 g twice a day and Glyburide 10 mg twice a day and she was noted to have been recently started on Canagliflozin 100 mg once daily. She only had one prior episode of DKA a few years ago. She denied non-compliance with her medications. There was no history of recent illness, starvation or alcohol use. On initial examination, her vitals showed blood pressure of 102/61 mmHg, pulse of 61/min, respiratory rate of 32/min and temperature of 97°F. She was lethargic, had Kussmaul respiratory pattern, and was oriented only to person. The rest of the physical examination was unremarkable.
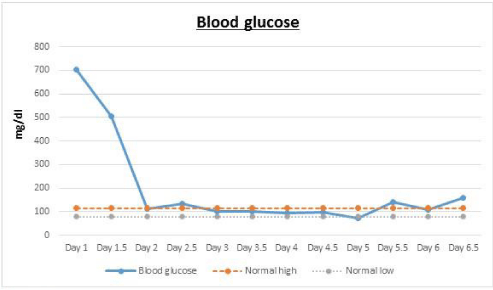
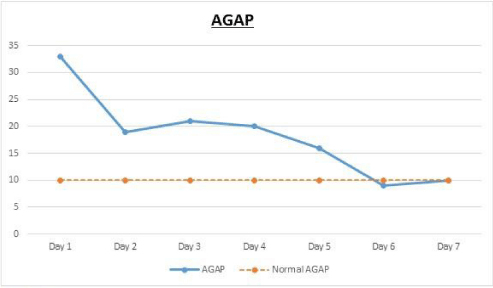
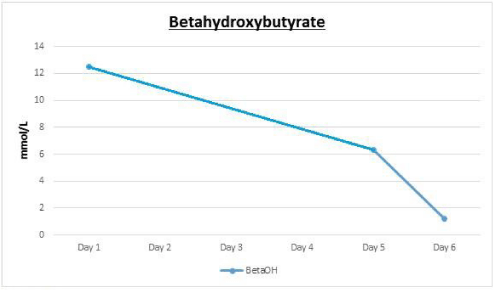
Body Mass Index (BMI weight in kg/ height in meters squared) was calculated as 24 kg/ m². Her initial laboratory tests are summarized as follows: white blood cells, 31/ cumm; sodium,130 mEq/ L; potassium, 5.6 mEq/ L; bicarbonate, 4.3 mEq/ L; BUN, 34 mg/dL; creatinine, 2 mg/ dL; glucose, 705 mg/ dL; anion gap, 33; serum betahydroxybutyrate, 12.2 mmol/ L (N < 0.27 mmol/ L); lactic acid, 0.6 mmol/ L; HbA1c, 13.9 % (128 mmol/ mol). Urinalysis was positive for 2+ ketones and >500 glucose; Urine drug screen was negative.
She was admitted to the intensive care unit with the diagnosis of DKA and was started on Intravenous fluids and insulin drip. The insulin requirement was determined using the Glucommander protocol.
Her hyperglycemia resolved in 8-10 hours and she was noted to require almost 23U of insulin in the first 24 hours (Figure 1). However her anion gap remained elevated in the range of 22-29 with bicarbonate levels dropping to as low as 9.1 mEq/L in the following days for which she also briefly required intravenous bicarbonate drip but without much improvement (Figure 2).
Her urinalysis remained positive for glucose and ketones with persistent elevation of beta hydroxybutyrate up until day 5 (Figure 3). As a result of which she was continued on IV insulin for almost 6 days. Her daily insulin requirement as per the Glucommander readings remained in the range of 0.2-0.4 units/ hour for day 2and 3 and around 0.4-0.8 units/ hour for day 4 to 6. She also received continuous IV dextrose 5% with 0.45% normal saline at the rate of 150cc/ hour for almost 3 days to allow for continuation of the insulin drip, since her blood glucose remained below 200mg/ dl. On day 3, her symptoms had completely resolved and so she was started on oral diabetic diet which she tolerated without any nausea or vomiting. Subsequently her IV fluids were decreased to half the rate and then eventually discontinued the following day. On day 6, her anion gap was noted to have closed, she was appropriately transitioned to subcutaneous insulin and was discharged a day later. Also, it was strongly suspected that she may have DM1, in view of her lean body and prolong history of hypothyroidism that was suggestive of an underlying autoimmune disorder. Glutamic Acid Decarboxylase (GAD) antibody was tested, which was elevated at 1459 units/ ml (N: 0.0-5.0), consistent with the diagnosis of DM1. C-Peptide levels were not tested.
Discussion
With the increasing use of SGLT-2 inhibitors in the management of DM2 and off-label use in DM1, its association with euglycemic DKA is being recognized lately [9]. It has been proposed that these agents tend to delay the diagnosis of DKA posing a challenge to physicians, as these patients mostly present with borderline high to normal serum glucose levels [10]. SGLT-2 inhibitors are known to cause euglycemia through various pathways. Studies suggest that these agents lead to suppression of insulin levels in the body. They increase urinary glucose excretion, thus resulting in reduction in total circulating insulin levels as a result of which under any stressful condition, the overall metabolism is more prone to shift towards lipolysis and production of free fatty acids and ketones [11]. There is evidence that SGLT-2 inhibitors promote glucagon secretion through alpha cells that further stimulates ketogenesis [12]. There has been some recent data that reviewed the correlation between SGLT-2 inhibitors and EuDKA. In a case series by Peters et al, nine patients, two with DM2 and seven with DM1, developed 13 episodes of either EuDKA or ketosis on treatment with SGLT-2 inhibitors in conjunction with either insulin pump or multiple daily insulin [13]. Various aetiopathogenetic mechanisms have been proposed for EuDKA. These include increased renal glucose clearance associated with euglycemia and subsequent reduction in insulin doses, acute illness, reduction of calorie intake and use of alcohol.
Similarly some additional individual cases have been reported over the span of 2 years that reinforce the same association of EuDKA with this class of drug [14,15]. However, unlike in the index case, all of these patients presented with diabetes ketoacidosis without significant hyperglycemia as an initial presentation and management with IV insulin and fluids achieved biochemical correction within few hours to days. None of them required that prolonged duration of IV insulin therapy to correct the acidosis. An executive summary published by AACE in June 2016 reviewed the data from UK and USA on this subject. It outlines that it remains unclear whether incidence of DKA in DM2 patient is reported more than it did before the use of SGLT-2 inhibitors. But at the same time, it did comment on the fact that DKA occurs more commonly in individuals with insulin deficiency and thus its risk with the use of SGLT-2 inhibitors specifically in DM1 patient should be further evaluated [16]. In the clinical trials carried out so far in DM1 patients on SGLT-2 inhibitors, 9.4% develop ketosis and 6% develop DKA. Overall incidence of EuDKA in these trials was not reported [17,18].
It still remains unclear why our patient required such prolonged duration of IV insulin therapy to achieve metabolic correction. Our patient had no acute illness, had no alcohol ingestion, no evidence of illicit drug use and was not on insulin at the time of presentation. Resolution of DKA is typically associated with normalization of the anion gap and transition of IV to SQ insulin, so it is unclear if IV insulin therapy needs to be continued for persistently elevated anion gap despite resolution of symptoms and hyperglycemia in patients with EuDKA. Thus, it is in this context that we need to attend to this potential problem of DKA with hyperglycemia as well as with normal blood glucose levels with the use of SGLT2 inhibitors. Despite the fact that FDA has not approved its use in DM 1, its promising benefits related to optimum glycemic control with low risk of hypoglycemia, reduced insulin doses and weight loss should encourage further clinical trials in this area to meet challenging goals in management of diabetes as well as diabetic ketoacidosis [19,20]. We agree with Peters, et al. [13] that patients with DM1 or DM2 who develop nausea, vomiting, or malaise, or develop a metabolic acidosis with SGLT-2 inhibitor use, should be promptly evaluated for EuDKA. SGLT-2 inhibitors should only be used with great caution, extensive counselling, and close monitoring in the setting of DM1 and DM2 patients on insulin.
- Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open. 2012; 2: e001007. https://goo.gl/NEHKME
- Musso G, Gambino R, Cassader M, Pagano G. A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co-transport (SGLT) inhibitors: systematic review and meta-analysis of randomized trials. Ann Med. 2012; 44: 375. https://goo.gl/HWoX21
- Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013; 159: 262. https://goo.gl/djtkRi
- Sun YN, Zhou Y, Chen X, Che WS, Leung SW. The efficacy of dapagliflozin combined with hypoglycaemic drugs in treating type 2 diabetes mellitus: meta-analysis of randomised controlled trials. BMJ Open. 2014; 4: e004619. https://goo.gl/NE7kCS
- Idris I, Donnelly R. Sodium-glucose co-transporter-2 inhibitors: an emerging new class of oral antidiabetic drug. Diabetes Obes Metab. 2009; 112: 79–88. https://goo.gl/ozA5Vd
- Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013; 154: 372-382. https://goo.gl/Hyq7kq
- FDA Drug Safety Communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. 2015/12/04: https://goo.gl/g6d2rr
- Erondu N, Desai M, Ways K, et al. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015; 38: 1680-1686. https://goo.gl/uJgi2g
- Bell DS. Case reports that illustrate the efficacy of SGLT2 inhibitors in the type 1 diabetic patient. Case Reports Endocrinology. 2015; 2015: 676191. https://goo.gl/4JQwTq
- Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: A potential complication of treatment with sodium–glucose cotransporter 2 inhibition. Diabetes Care. 2015; 38: 1687-1693. https://goo.gl/EQ1eaG
- Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016; 7: 135-138. https://goo.gl/KyKDB5
- Bonner C, Kerr-Conte, Gmyr, Queniat, Moerman E, Thévenet J, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nature Medicine. 2015; 21: 512-7. https://goo.gl/ujPCg2
- Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015; 38: 1687–1693. https://goo.gl/xod7kM
- Singh AK. Sodium-glucose co-transporter-2 inhibitors and euglycemic ketoacidosis: Wisdom of hindsight. Indian J Endocrinol Metab. 2015; 19: 722-730. doi:10.4103/2230-8210.167554. https://goo.gl/8XxJiw
- Thawabi M, Studyvin S. Euglycemic diabetic ketoacidosis, a misleading presentation of diabetic ketoacidosis. N Am J Med Sci. 2015; 7: 291-294. https://goo.gl/Q1jyBz
- Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, et al. American association of clinical endocrinologists and american college of endocrinology position statement on the association of sglt-2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016; 22: 753–762. https://goo.gl/BUWKxV
- Henry RR, Thakkar P, Tong C, Polidori D, Alba M, et al. Efficacy and safety of canagliflozin, a sodium glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care. 2015; 38: 2258- 2265. https://goo.gl/QKz9oP
- Henry RR, Rosenstock J, Edelman S, Mudaliar S, Chalamandaris AG, Kasichayanula S, et al. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015; 38: 412-419. https://goo.gl/wjEHkG
- Tamez HE, Tamez AL, Garza LA, Hernandez MI, Polanco AC. Dapagliflozin as an adjunct therapy to insulin in the treatment of patients with type 1 diabetes mellitus. Journal of Diabetes and Metabolic Disorders. 2015; 14:78. https://goo.gl/fQqUZZ
- Perkins BA, Cherney DZ, Partridge H, Soleymanlou N, Tschirhart H, Zinman B, et al. Sodium glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of concept trial. Diabetes Care. 2014; 37: 1480-1483. https://goo.gl/Z9T925

Sign up for Article Alerts