The Regulation of Food Intake: the Brain-Endocrine Network?
Maria Sorrentino* and Giovanni Ragozzino
Department of Environmental, Biological and Pharmaceutical Sciences and Technologies, University of Campania, I-81100 Caserta, Italy
*Address for Correspondence: Maria Sorrentino, Department of Environmental, Biological and Pharmaceutical Sciences and Technologies, University of Campania, I-81100 Caserta, Italy, Tel: +329-617-4462; E-mail: [email protected]
Submitted: 11 August 2017; Approved: 24 August 2017; Published: 29 August 2017
Citation this article: Sorrentino M, Ragozzino G. The Regulation of Food Intake: the Brain-Endocrine Network. Int J Clin Endocrinol. 2017;1(2): 041-048.
Copyright: © 2017 Sorrentino M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Food intake; Energy expenditure; Feeding behaviour
Download Fulltext PDF
The response in terms of eating habits is therefore that, in times of abundance, the introduction of food should be reduced and energy consumption should increase, while the opposite would occur in times of famine. In order to maintain the energy homeostasis, during the evolutionary phases of eating habits, complex regulatory mechanisms (hunger/ satiety system) have established, involving central (hypothalamus) and peripheral (gastrointestinal tract, adipose tissue) structures. Energy balance stems from the relationship between energy expenditure and energy availability. It is managed by the endocrine-metabolic and hunger/ satiety system, mainly ruled by the hypothalamic centers at the encephalic level. The hypothalamus receives impulses from periphery as metabolic and endocrine nervous stimuli, informing the central nervous system on the nutritional status of the body, and through which food intake is regulated. The senses involved in food contact - taste, smell, sight, tactile features and palatability - represent the encephalic responses affecting eating behaviour. Palatability depends not only on food organoleptic characteristics but also on emotional state as well as cultural and environmental factors. This is because the secondary taste area communicates with cortical areas expressing emotions and memory, and with subcortical structures regulating hunger and satiety. Often, however, environmental influences interact with physiological control and stimulate consumption independently of satiety or inhibit it regardless of hunger. The hedonic properties of food can in fact stimulate its consumption even when energy needs have been met, thus contributing to increased weight and obesity. This review aims to describe the main mechanisms and mediators involved in maintaining the energy homeostasis and regulating food intake, the intracellular actions with which these mediators influence food intake and metabolism, as well as the complex system of interactions between the central nervous system and periphery, highlighting the powerful enhancing effects of food in the central reward system.
Hunger-Satiety System
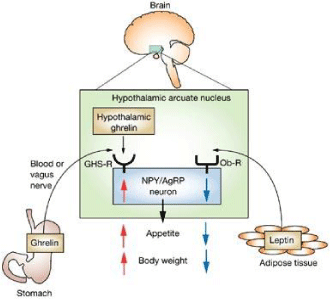
The metabolic energies introduced during the day are related to the amount and frequency of ingested meals [1]. Hunger occurs after a fasting period, or when nutrients have been absorbed. In order to avoid the excess consumption of food and control it over the long run, body activates peripheral satiety signals (metabolic, nervous and endocrine), which reach the cerebral centers (mainly the hypothalamus, cerebral trunk and “reward centers”) through the circulatory system. They also help the gastroenteric tract and central nervous system to communicate [2]. In addition to the amount, the composition and the caloric density of meals also play an important role in determining satiety [3]. The beginning and the end of meal are regulated by short-term neural (vagal efferences) and hormonal signals, whose reception and integration occur in the hypothalamic area, where the arcuate nucleus modulates its refined regulation [2,4]. For the first time, 1950s studies on the lesions and electrical stimulation of different hypothalamic nuclei attributed the “satiety centre” role to the Ventromedial Nucleus (VMN) and the “hunger centre” role to the Lateral Hypothalamic Area (LH) [5]. To support this thesis in laboratory animals, VMN destruction causes hyperphagia and obesity, while lateral hypothalamus lesions completely suppress the instinct of hunger and lead to a gradual reduction in the body weight, up to a cachetic state [6]. Currently, however, instead of imputing the energy homeostasis control to individual and specific hypothalamic nuclei, it has been found that neuronal circuits contribute to this regulation. These include not only multiple specialized hypothalamic areas, such as the Dorsomedial Nucleus (DMN), the Paraventricular Nucleus (PVN) and the Arcuate Nucleus (ARC), but also other brain regions [7,8]. Among these, we have the lower part of the encephalic trunk and, in particular, the vagal dorsal complex, which receives and integrates the incoming information from peripheral endocrine organs and from different areas of the CNS. Neuronal circuits of the Mesencephalon Bridge and thalamus then interpret this information in relation to the signals generated by the mechanical properties of food, detected at different levels of the G.I. tract. Amygdala and frontal cortex are instead responsible for higher functions involving the integration of cognitive information on the pleasantness or adversity to food [9]. The central control of food intake, however, is not enough to maintain a stable body weight over time. Indeed, body weight remains constant when the energy provided by diet is perfectly balanced by the one spent for metabolic activity and exercise. Specific peripheral inputs reach the hypothalamic control centre to regulate the energy homeostasis. There are a “short term” peripheral system, also referred to as “peripheral satiety system”, sending signals to the hypothalamus about the presence of food in the G.I. tract and nutrients in the systemic circulation, and a “long-term” peripheral system, providing the hypothalamus with information on the amount and consistency of the adipose tissue and, above all, on the overall body energy balance [10]. Hypothalamic neurons involved in the appetite control synthesize a large number of neuropeptides and / or neurotransmitters capable of stimulating or inhibiting food intake, depending on the case. They can do this by interacting with the signals coming from periphery through the afferent nerve endings and the systemic circle. In the arcuate nucleus, two populations of neurons, named 1st order nuclei and responsible for the appetite regulation, are traced: the neurons co-expressing the neuropeptide Y (NPY) and the “Agouti-related” peptide (AgRP), stimulating appetite (these neuropeptides are inhibited by leptin and insulin and stimulated by ghrelin), as well as neurons expressing Proopiomelanocortin (POMC) and the Cocaine and Amphetamine Transcript (CART) with inhibiting hunger function [2]. The latter are inversely regulated by the leptin, insulin and ghrelin hormones [11]. The peripheral stimuli of any origin arriving to the arcuate nucleus are transmitted to another group of neurons (2nd order neurons). From here efferent pathways, mediated by the autonomic nervous system (ANS) and hormones, transmit the efferences to periphery [12]. 2nd order neurons are also contained in hypothalamic nuclei and intervene in controlling appetite, forming an extremely refined network. 1st order neurons produce two classes of neuropeptides targeting secondary neurons: anorexigenic and orexigenic neuropeptides. The former are produced by POMC / CART neurons and, after satiety signals, inhibit food intake. The most studied anorectic neurons in the ARC are nearly exclusively localized in the ARC, the Paraventricular Nucleus (PVN), the lateral hypothalamus (LH): all cerebral system areas associated with energy balance control. Leptin and insulin can influence these neurons’ signalling pathways. The most widespread anorexigenic neuropeptide in the ARC is α-MSH (melanocyte stimulating hormone), derived from the POMC precursor after its post-translational cleavage. It acts as an agonist of melanocortin 3 (MC3R) and melanocortin 4 (MC4R) receptors in the hypothalamus. These receptors are highly expressed in the 2nd order neurons in the PVN and their activation leads to a reduction in food intake and in an increased use of energy. The other important anorexigenic neuropeptide in the ARC is CART [13]. CART is released by the ARC neurons, which release POMC and has a similar effect in reducing food intake and in increasing energy expenditure [14]. Orexigenic neuropeptides are instead produced by the AgRP / NPY neurons after signals of hunger. They stimulate food intake. They are represented by AgRP, α-MSH inverse agonist, and NPY. Satiety center nuclei express two types of receptors, the MC4R receptor and the receptor for NPY, Y1R. The first binds the α-MSH neuropeptide with inhibitory function and AgRP, inverse agonist that binds the receptor by occupying the α-MSH binding site, thus preventing the inhibition of these neurons. These two neuropeptides are part of the melanocortin pathway. The binding of NPY to the Y1R receptor disinhibits these neurons. Instead, the center which controls appetite is made up of two groups of neurons: those producing orexin and those producing MCH (melanin concentrating hormone), hormones with orexigenic action. Both neurons are uniformly expressed within the LHA and synapsed with different brain areas. Here the receptors for respective hormones are located. The action on these targets results in salivation, gastric motility and secretion of insulin and glucagon, which push for food. The neurons producing orexin and MCH receive different inputs. The former are activated by the nucleus of the solitary tract, with which they are synapsed, and by the NPY neurons of the arcuate nucleus. The latter also activate MCH neurons. There is a crosstalk between the two groups of neurons. In particular, the neurons producing orexin would be able to influence, by direct and indirect mechanisms, the expression of MCH [15]. The information recorded in the two hypothalamic centers of hunger and satiety translates into the activation of the hormonal scale ruling our appetite: if the satiety signals are strong, the scale will fall on the side of the alpha-MSH, if the hunger signals prevail it will fall on the side of NPY. The α-MSH signal reaches the pancreas and the liver through the autonomic nervous system. On the pancreas it regulates the insulin production, on the liver it blocks the release of glucose. The overall effect is to regulate glycaemia and insulinemia. Signals from periphery regulate the activity of these neuronal subpopulations [2]. Ingested food evokes satiety through gastric distension and the release of peptides by enteroendocrine cells [2]. Unlike leptin and insulin (hormones informing about the long-term energy state), intestinal hormones play an important role in determining the beginning and end of meals [16]. As the stomach fills, the brain is reached through the autonomic nervous system by a mechanical signal due to the relaxation of the bowels. Chemical signals cross the hemato-encephalic barrier and reach the neurons located in the ARC, mechanical ones reach the NTS [17] through the vague nerve. From these brain areas, links to the satiety (paraventricular hypothalamic nucleus) and hunger centers (lateral hypothalamus area) spread out. Mechanical signals are transmitted to the solitary tract nucleus (an area of the brain stem) by the autonomic nervous system fibres, which record the dilation or contraction of the stomach walls depending on its state, whether full or empty. From the solitary tract nucleus, these signals will then reach the hypothalamus.
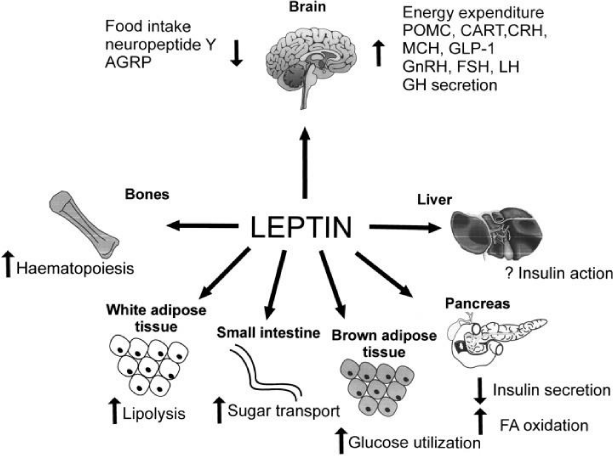
Schematic illustration of leptin actions at central and peripheral levels
Adipokine
Leptin: Figure 1 shows the main leptin actions. It plays a key role in regulating the energy balance as it calibrates food intake and energy expenditure. It also affects the brain regions involved in food reward, modulating the preference for food, related to the activation of the nucleus accumbens by dopamine. As showed in Figure 2, leptin expresses its action by interacting with the long form of the Ob-Rb receptor at the ARC level, where it activates the POMC neurons and inhibits the AgRP / NPY ones with an overall reduction of food intake [18]. By acting on the melanocortin receptors (especially MC4R), these peptides regulate in turn other neuromediators (MCH, orexin, CRH and TRH) at the level of different hypothalamic nuclei, which condition in various way caloric intake and energy deposits. Leptin and insulin are released together, after an increase in the blood glucose levels, one from the adipose tissue, the other from the endocrine pancreas [19]. At peripheral level, leptin, if in physiological amounts, increases glucose, fat metabolism and energy consumption. Insulin mainly regulates the carbohydrate homeostasis. Peripheral insulin resistance can determine the well-known phenomena resulting from glucotoxicity [20]. Leptin regulates the lipid homeostasis and the states of relative leptin-resistance may determine an accumulation of intracellular lipids. These generate lipotoxicity at pancreas beta cells, myocardium and other tissues level, damaging organs and apparatus as described in the obese [21]. The circulating levels of leptin are directly related to the mass of the adipose tissue and are reduced during fasting periods, while increasing after food intake [22]. Leptin or its receptor deficiency, in both man and animal models, translates into an obese phenotype characterized by hyperphagia, diabetes, and infertility. The presence of a “gastric” leptin secreted by the stomach mucosa has recently been confirmed. Its function is twofold: once released into the stomach after the arrival of food, it reaches the duodenum, where it promotes digestion. Then it leaves the digestive tract and reaches the hypothalamic satiety center through the bloodstream, like the one derived from the adipose tissue. The effect of gastric leptin is “quicker”. It triggers the sense of satiety in the short term, while the action of adipose leptin is calibrated over the long run in regulating energy expenditure [23]. The circulating levels of leptin are therefore a relatively accurate marker of the nutritional and metabolic state of the body. People who lose weight after a low-calorie diet usually reduce the circulating levels of leptin. This leptin reduction seems to mediate the reversible decrease in the thyroid activity, sympathetic tone, and basal energy expenditure [24].
Adiponectin: In addition to leptin, the adipose tissue releases many other mediators called adipokines, involved in the energy homeostasis. They regulate energy intake and basal metabolic rate. Therefore, the adipose tissue is involved in the metabolic control of energy substrates such as glucose and lipids, and interacts with several hormonal systems. Such molecules act remotely, by an endocrine action, or locally through a paracrine and autocrine action on stroma, other components of the adipose tissue (blood vessels, inflammatory cells, etc.) and other tissues, such as the muscle [25]. ADP is a 244-amino acid protein, especially produced in mature adipocytes, and the concentration of coding RNA is higher in the peripheral than in the visceral adipose tissue [26]. If lean, healthy mice are injected with ADP in combination with a high-fat and high-sugar meal, the postprandial increases in plasma glucose levels, FFA and triacylglycerol are reduced. On the contrary, if insulin-resistant mice are treated with physiological concentrations of ADP, glucose tolerance is improved and insulin resistance is reduced [27]. Two different types of receptors ADP interacts with have been identified: AdipoR1 and 2. AdipoR1 is mainly expressed in the muscle and AdipoR2 is primarily expressed in the liver. The ADP binding with AdipoR1 and AdipoR2 results in an increased glucose uptake, in the oxidation of fatty acids in the skeletal muscle (AdipoR1) and in a decreased production of glucose in the liver (AdipoR2) [28,29]. Table 1 shows the effects of an increase or a decrease in the production of Adiponectin.
Main Actions of Adiponectin
The biological ADP effects depend on plasma concentrations, on the properties of various ADP isoforms and on the specific expression of the ADP receptor subtypes in the tissue. ADP has anti-atherogenic, anti-diabetic and anti-inflammatory properties [30].
Neuroendocrine factors secreted by the gastrointestinal tract
Ghrelin: Ghrelin is the only known orexigenic circulating hormone. It is a 28 aa peptide (endogenous agonist of the GHS-R receptor), involved in the control of food intake and energy balance. It is localized in distinct cells of the gastric mucosa, mainly distributed in the mid portion of the oxyntic gland characterized by P/D1 granules in man and X/A-like granules in rodents. For several years, the intestinal enteroendocrine cells have been considered as main source of peptides regulating food intake. However, recent evidence shows that the P / D1 of humans and X / A of rats at the stomach level also have an endocrine function and play a very important role in controlling appetite. Acylated ghrelin is produced in this kind of cells. The enzyme responsible for the ghrelin acylation has recently been identified in mice and humans and seems to belong to the superfamily of O-acyltransferases bound to the membranes (MBOATs). It has been called ghrelin-O-acyltransferase (GOAT) [31]. Due its marked pre-prandial increase, which seems to be part of the cephalic phase under the sympathetic nervous system stimulus [4], it is responsible for the start of meal. The circulating levels of ghrelin are instead suppressed by ingested macronutrients: to a greater extent than carbohydrates, followed by proteins and lipids [3]. Furthermore, ghrelin increases gastrointestinal motility and reduces insulin secretion [4]. If administered parenterally or centrally to rodents, it rapidly increases food intake and body weight.
In the research over the last few years, additional peptides produced in the X / A gastric cells of mouse have been identified to be able to regulate food intake: nesfatin, obestatin and des-acyl-ghrelin. However, while the functions of nesfatin-1 [31] and des-acyl-ghrelin [33] have recently been clarified, the obestatin functions remain controversial [34].
Des-acyl-ghrelin: The function of des-acyl-ghrelin on food intake is less clear than that of ghrelin. It is currently being discussed as a possible anorexigenic hormone, although some results are controversial. Furthermore, the receptor on which des-acyl ghrelin acts is still unidentified.
Nesfatin: Nesfatin was initially identified by Oh-I, et al. [35] in the rat hypothalamus. It derives the Nucleobindin-2 protein (NUCB2) from a precursor. Studies have shown that the intraperitoneal injection of nesfatin into mice reduces food intake and that its expression at the level of the oxyntic mucosa is 10 times higher than its expression at the hypothalamic level [36].
Obestatin: Obestatin is a peptide of 23-amino acids generated by the proteolytic cut of the primary transcript of the gene encoding for ghrelin. It is present not only in the G.I. tract, but also in the spleen, breast gland, breast milk and plasma. The action of this protein / hormone is both autocrine and paracrine. Recent studies have shown that the plasma levels of obestatin are significantly lower in obese, when compared to controls, indicating a role for obestatin in the long-term regulation of body weight [37].
CCK: Cholecystokinin (CCK) is an intestinal peptide produced by cells I of the duodenojejunal mucosa, with a plasma half-life of few minutes [2]. It is secreted in response to intraluminal nutrients, particularly lipids and proteins. It is capable of inducing meal termination, reducing its size and duration. In addition, the CCK satiating effect is attributed to the ability of inhibiting gastric emptying, increasing the stomach mechanoreceptors stimulation [2]. In addition to its distribution in the G.I. tract, CCK is widely distributed in the hypothalamus, mainly at the level of the ventromedial nucleus. Two types of receptors it interacts with have been identified: CCKA and CCKB. CCKA (alimentary) is primarily expressed at the level of the G.I. tract, liver, pancreas and vagal afferences [38], while CCKB (brain) is the predominant form in the CNS [39]. The satiating effect of CCK, in part mediated by the CCK-A receptors in the digestive tract, proves the role of the hormone of peripheral production in regulating eating habits.
GLP-1: “Incretins” are intestinal peptide hormones released for the presence of nutrients in the digestive tract (fat and carbohydrates induce its secretion, directly by intraluminal contact, indirectly for neurohumoral mechanisms in the duodenum) and capable of enhancing the post-prandial insulin secretion through the activation of the entero-insular axis. In humans and animals, glucagon-like peptide-1 is the most powerful incretin secreted at the end of a meal (GLP-1) [40]. Within a few minutes after its delivery into the bloodstream, plasma GLP-1 levels are drastically reduced due to the degradation of the dipeptidyl-peptidase IV (DPP-IV) enzyme. GLP-1 results to be the most powerful peptidergic stimulus for the synthesis and release of insulin by the pancreatic beta cells. Its effect is glucose-dependent: the higher the plasma glucose levels, the greater the insulin production [41]. In the G.I. tract, GLP-1 inhibits the acid secretion of the stomach and slows gastric emptying. The destruction of GLP-1R does not seem to alter the eating habits of either transgenic rodents or genetically obese subjects [42]. Therefore, GLP-1 is not essential either for the appetite regulation or for the long-term control of body weight.
OXM: Oxyntomodulin (OXM) is a 37-amino acid peptide originating from the post-translational transformation of proglucagon in the intestinal cells. It was called this way after its inhibitory action on the stomach oxyntic glands [43]. OXM is released into the blood in response to food ingestion and is proportional to the meal calorie content. Its effect is to reduce food intake [44].
PYY: Peptide Tyrosine Tyrosine (PYY) is a member of the Pancreatic Polypeptide family (PP), which includes neuropeptide Y (NPY) and PP, and is synthesized by the same L-cells of the gastrointestinal tract co-expressing GLP-1. It is secreted in response to the nutrients ingested with two other intestinal hormones, GLP-1 and OXM, and is proportional to the calorie load, especially the one related to lipids [45]. It is reduced in obese, thus decreasing stimulation of satiety [46].
Amylin: Amylin or Islet Amyloid Polypeptide (IAPP) is a 37-amino acid peptide, synthesized by the islets of Langerhans beta cells as pre-pro-amylin undergoing proteolysis to finally locate in the secretory granules [47]. It is released in response to the stimuli leading to insulin secretion. IAPP plays an adaptive role in glucose metabolism and homeostasis. It helps control gastric emptying, suppress the release of glucagon and regulate satiety [48].
Extrahypothalamic Control
Recent observations have proved a complex network (used to modulate hunger/ satiety system, metabolic-energetic homeostasis and eating habits), which links the arcuate nucleus and hypothalamic nuclei to the prefrontal cortex, amygdala, hippocampus and thalamus. In addition to the action of brain neuromediators (e.g., serotonin, dopamine), each of these areas responds to sensory stimuli [49] and peripheral circulating signals of energy availability (leptin, insulin, G.I. hormones) [50]. These multiple paths and signals ensure that food is consumed when needed to maintain the energy homeostasis. However, the compulsive consumption of so-called “palatable” foods (rich in fat and simple sugars) results in a compensatory desensitization of satiety signals both in humans and animals, thus causing a nutritional overweight and obesity conditions. Hence the description of obesity as “Food Addiction”. By the striatal dopamine increase mediated by the action of endogenous opioids, palatable food can in fact activate the mechanism of food reward, triggering addictive behaviours in laboratory animals. Food reward involves the interaction among the limbic system, cortex, and basal ganglia, responsible for learning, memory, motivation functions and for the hedonic response [51]. The cascade of neural reward begins with the hypothalamus in the midbrain (mesolimbic system), where serotonin activates enkephalins for the opioid mu receptors stimulation in the substantia nigra (GABA). Substantia nigra is projected into the ventral tegmental area (VTA), where dopaminergic neurons produce “effective” amounts of dopamine released by the nucleo accumbens (NAc): the site of reward. DA D2R receptors “turn on” the cortical circuit [52]. Recent evidence suggests that the dysregulation of this system plays an important role in the excessive consumption of food and in the development of obesity [53]. In obese patients, neuroimaging studies showed a reduced expression of dopamine D2 receptors at the striatal level. For some authors, the increased food intake would be a compensatory attempt to the reduced dopaminergic signalling. Others interpret the reduced density of D2 receptors as the result of a repeated hyperstimulation of the system [51]. An alternative hypothesis considers the repeated consumption of palatable food as cause of the increased dopamine release, overexpression of the transporter, and modification of the expression of D1 and D2 receptors in the nucleus accumbens [54]. According to this interpretation, this causes a behavioural addiction especially to palatable food, called “craving”, and to withdrawal symptoms. Lastly, non-palatable food may also interfere with food reward: environmental stimuli, such as the sight and smell of food, can lead to the craving of unpalatable food [55]. Paradigmatic examples of this complex mechanism of control have been indirectly demonstrated by experimental evidence of some phytotherapeutic extracts, such as Griffonia simplicifolia, Rhodiola rosea and Theobroma Cacao. Griffonia simplicifolia, with its similar serotoninergic actions, is useful in reducing the craving due to the sense of satiety induced by increased leptin levels. Similarly, Rhodiola rosea acts by inhibiting the serotonin degradation (catechol-O-methyltransferase inhibition, COMT), stimulating the transport of 5-HTP and increasing dopamine levels. This latter action is attributable to the glycosides of the root [56,57]. Both phototherapeutic extracts also have a lipase activity. Instead, Theobroma Cacao seeds, rich in methylxanthine (theobromine and caffeine) and tetrahydroisoquinoline (salsolinol, salsoline), give sense of satiety through the inhibition of MAOs, tyrosine hydroxylase and catecholamine uptake [58,59].
Recently it was found that ghrelin causes a powerful dopaminergic modulation of reward and plays an important role in motivating and reinforcing the effects of sucrose at the level of neural circuits of reward. Indeed, it is released from the stomach during fasting and activates the meso-cortico-limbic dopaminergic system in the NAc, stimulating the hyperpalatable over standard food intake. This effect is perceived as reward or pleasure, thus increasing not only food but also alcohol and cocaine consumption. Consequently, food limiting can promote the introduction of additional food to compensate for the reward reduction determined by calorie restriction. In recent years, research has also focused on the Endocannabinoid System (ES), as endocannabinoids have effects on food intake and weight gain very similar to ghrelin. It is assumed that they share common pathways, such as the activation of hypothalamic adenosine 5’-monophosphate-activated protein kinase [60]. Through the interaction with specific Cb-1 receptors (cannabinoid receptor 1), ECs modulate in the mesolimbic system the gratification and pleasure related to food, alcohol, nicotine and some drugs intake. In the hypothalamus, they modulate the secretion of anorexigenic and orexigenic neuropeptides [61]. Therefore, ECs are pivotal in eating habits as they play a key role in controlling appetite, that is in motivation, gratification and stimulation to food intake as well as in the regulation of energy balance [62,63]. The system is activated during fasting (even short, for example skipping a meal) by increasing the sense of hunger (leptin tends to decrease) and unconsciously addressing towards highly “palatable” food [64]. Short periods of fasting reduce leptin levels and excite hypothalamic neurons controlling hunger. Among these, orexinergic neurons containing the neuropeptide orexin-A (OX) generate signals that push towards food intake and to searching for food-related exploratory behaviours. Once food is reintroduced, the system is turned off and hunger calms down. This does not happen in many obese. As well as smoking and alcohol intake, obesity is linked to the system hyperstimulation as in the obese it receives continuous stimuli and remains therefore very active [65]. On one hand, stressing the system with repeated intake of fatty and caloric food leads to an increase in endogenous cannabinoids and a higher desire for hypercaloric foods. On the other, it is also influenced by plasma concentrations of hormones associated with metabolism and nutrition (insulin, leptin and adiponectin). For instance, an increase of leptin levels reduces the ECs concentrations in the hypothalamus and consequently inhibits food intake [66]. The interaction site between leptin and EC is located at the lateral area of the hypothalamus, which represents a junction of information linking periphery to the superior centers responsible for food control. In this area, by the Cb-1 receptors, ECs raise the excitability of neurons producing MCH by further promoting food intake, whereas leptin inhibits the release of ECs and the excitability of MCH neurons is reduced with a final effect that is anorexigenic [67].
Conclusions
Hypothalamic nuclei receive peripheral metabolic (glucose) and hormonal (leptin and gastrointestinal hormones) signals acting directly and indirectly, via vagal afferences, through the solitary tract nucleus in the brainstem. The plasma concentrations of nutrients and hormones activate orexigenic (NPY/ AgRP) and anorexigenic (POMC/ CART) pathways in the brain, modulating the hunger/ satiety system and the growth of metabolically active hormones at the hypothalamic-pituitary level. Through the interconnection with other phylogenetically older CNS areas - such as the amygdala and the hippocampus (memory and emotions), the thalamus (sensory processes), and more “recent” cortical areas orbitofrontal cortex), responsible for decision and behavioural processes - these nuclei play a key role in regulating metabolism and structuring food behaviour. However, in environments with high availability of food, individual eating habits respond to the energy homeostasis mechanisms as well as to other interrelated factors. They are sensory (food palatability), hedonic (reward components, partly modulated by neurosensory aspects), neurobiological (stress and circadian rhythms), cognitive (information, expectations, motivations). They are modulated by specific brain environments. In this regard, important progress has been made to delineate the mechanisms of “exceeding” intake as an immediate response to the display of rewarding food. Despite clear improvements in physiological and adaptive neurobiological knowledge, the understanding of the short-term neuronal adaptations to food reward is, however, still incomplete.
Acknowledgement
The authors would like to thank Marco Tinghino for his kind support and reliable cooperation and Paola Mastrorilli for her valuable commitment in helping translate our article.
- Gali Ramamoorthy T, Begum G, Harno E, White A. Developmental programming of hypothalamic neuronal circuits: impact on energy balance control. Front Neurosci. 2015; 9: 126. https://goo.gl/CvRkF2
- Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010; 57: 359-372. https://goo.gl/RSBWAU
- Blundell JE. Regulation of energy intake: appetite control and the potential for weight gain, in Angel A. Anderson H, Bouchard C, Lau D, Leiter L, Mendelson R, editors. Progress in obesity research, London: John Libbey & Company; 1996. p. 215-22.
- Schloegl H, Percik R, Horstmann A, Villringer A, Stumvoll M. Peptide hormones regulating appetite-focus on neuroimaging studies in humans. Diabetes Metab Res Rev. 2011; 27: 104. https://goo.gl/Pw6vZg
- Stellar E. The physiology of motivation. Psychol Rev. 1954; 101: 301-11. https://goo.gl/eLnNh4
- Oomura Y. Input-output organization in the hypothalamus relating food intake behaviour. Handbook of the hypothalamus. 1980; Vol. 2: 557-620. https://goo.gl/6qAhkG
- Satya P Kalra, Michael G, Dube, Shuye Pu, Bin Xu, Kalra. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999; 20: 68-100. https://goo.gl/yjHDG6
- Michael W. Schwartz, Stephen C. Woods, Daniel Porte, Jr, Randy J. Seeley, Denis G. Baskin et al. Central nervous system control of food intake. Nature. 2000; 404: 661-671. https://goo.gl/UB2ZDK
- Neglia S. Control of food in non-mammalian vertebrates of veterinary interest: localization and distribution of immunohistochemistry of oresizing and anorexing peptides in the gastrointestinal tract of Pisces and Birds. 2006; University of Naples Federico II. 2014; 19: 23. https://goo.gl/iy5QMT
- Schwartz M.W, Baskin D.G, Kaiyala K.J, Woods SC. Model for the regulation of energy balance and adiposity by the central nervous system. Am J Clin Nutr. 1999;69:584-96. https://goo.gl/PDVKTN
- Mercer JG, Speakman JR. Hypothalamic neuropeptide mechanisms for regulating energy balance: from rodent models to human obesity, Neurosci Biobehav Rev. 2001; 25: 101-106. https://goo.gl/NJhEuW
- Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005; 1: 53-61. https://goo.gl/59fpRJ
- Lau J, Herzog H. CART in the regulation of appetite and energy homeostasis. Front Neurosci. 2014; 8: 313. https://goo.gl/aH3WZs
- Vrang N. Anatomy of hypothalamic CART neurons. Peptides. 2006; 27: 1970-80. https://goo.gl/6eKB35
- Manca I. Caratterizzazione farmacologica di un nuovo composto CB1 antagonista con proprietà anti-obesità. 2011; PhD Thesis: Università degli Studi di Cagliari. https://goo.gl/17aJPJ
- Pournaras D.J, Le Roux C.W. The effect of bariatric surgery on gut hormones that alter appetite. Diabetes Metab. 2009; 35: 508-512. https://goo.gl/RU4pWt
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001; 411: 480-4. https://goo.gl/BU13EE
- Rexford S Ahima, Jeffrey S Flier. Leptin annual review of Physiology. 2000; 62: 414-437. https://goo.gl/ijUitf
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001; 414: 813-20. https://goo.gl/dvVFvX
- Unger R H. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications, Diabetes. 1995; 44: 863-70. https://goo.gl/EbAoM9
- Jeffrey M Friedman. Modern science versus the stigma of obesity. Nat Med. 2004; 10: 563-9. https://goo.gl/hWGTZu
- Philippe Cammisotto, Moise Bendayann. A review on gastric leptin: the exocrine secretion of a gastric hormone. Anat. Cell. Biol. 2012; 45: 1-16. https://goo.gl/135zSR
- Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003; 111: 1409-21. https://goo.gl/zdBNMx
- Paniagua J A. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J Diabetes. 2016; 7: 483-514. https://goo.gl/Gbmij8
- Fain JN, Madan AK, Hiler ML, Cheema P and Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004; 145: 2273-82. https://goo.gl/1TxCJy
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001; 7: 941-6. https://goo.gl/sqjtMK
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003; 423: 762-9. https://goo.gl/xwvP8h
- Berg A H, Combs T P, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002; 13 : 84-9. https://goo.gl/8bnBfV
- Nese Cinar, Alper Gurlek. Association between novel adipocytokines adiponectin, vaspin, visfatin, and thyroid: An experimental and clinical update. Endocr Connect. 2013; 2: R30-38. https://goo.gl/DxxFyZ
- Stengel A, Taché Y. Regulation of Food Intake: The Gastric X/A-like Endocrine Cell in the Spotlight, Regulation of food intake: the gastric X/A-like endocrine cell in the spotlight. Curr Gastroenterol Rep. 2009; 11: 448-454. https://goo.gl/RJb8hF
- Shimizu H, Oh-I S, Okada S, Mori M. Nesfatin-1: an overview and future clinical application. Endocr J. 2009; 56: 537-43. https://goo.gl/6Z6dr4
- Inhoff T, Wiedenmann B, Klapp BF, Mönnikes H, Kobelt P. Is desacyl ghrelin a modulator of food intake?. Peptides. 2009; 30: 991-4. https://goo.gl/Unfx4L
- Goebel M, Stengel A, Taché Y. Continued controversy on obestatin as a gut hormone influencing food intake and gastrointestinal motility. Obes Metab. 2008; 4: 143-148.
- Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, et al, Identification of nesfatin-1 as a satiety molecule in the hypothalamus, Nature. 2006; 12: 443: 709-12. https://goo.gl/78scPW
- Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, et al., Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology. 2009; 150: 232-8. https://goo.gl/A59Qek
- Lacquaniti A, Donato V, Chirico V, Buemi A, Buemi M, et al. Obestatin: an interesting but controversial gut hormone. Ann Nutr Metab. 2011; 59: 193-9. https://goo.gl/VjXAoG
- Hill DR, Campbell NJ, Shaw TM, Woodruff GN. Autoradiographic localization and biochemical characterization of peripheral type CCK receptors in rat CNS using highly selective nonpeptide CCK antagonists, J Neurosci. 1987; 7: 2967-76. https://goo.gl/Va6ceQ
- Liddle RA, Green GM, Conrad CK, Williams JA, et al. Proteins but not amino acids, carbohydrates, or fats stimulate cholecystokinin secretion in the rat. Am J Physiol. 1986; 251: G243-G248. https://goo.gl/1irXKW
- Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man, The Lancet. 1987; 8571: 1300-1304. https://goo.gl/EyyBLC
- Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding, Nature. 1996; 379: 69-72. https://goo.gl/Dj8kNL
- McMahon LR. and Wellman PJ. PVN infusion of GLP-1-(7-36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol. 1998; 274: R23-9. https://goo.gl/eEm8a9
- Scrocchi LA, Hill ME, Saleh J, Perkins B, Drucker DJ. Elimination of glucagon-like peptide 1R signaling does not modify weight gain and islet adaptation in mice with combined disruption of leptin and GLP-1 action. Diabetes. 2000; 49: 1552-60. https://goo.gl/srESvs
- Le Quellec A, Kervran A, Blache P, Ciurana AJ, Bataille D. Oxyntomodulin-like immunoreactivity: diurnal profile of a new potential enterogastrone. J Clin Endocrinol Metab. 1992; 74: 1405-9. https://goo.gl/z4RRJq
- Perry B, Wang Y. Appetite regulation and weight control: the role of gut hormones, Nutr. Diabetes. 2012 2:e26.
- Le Roux CW, Batterham RL, Aylwin SJ. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147: 3-8. https://goo.gl/jou7Uq
- Kahn SE, D'Alessio DA, Schwartz MW, Fujimoto WY, Ensinck JW, Taborsky GJ Jr, et al. Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes. 1990; 39: 634-638. https://goo.gl/9TaC34
- Montane J, Klimek-Abercrombie A, Potter KJ, et al. Metabolic stress, IAPP and islet amyloid. Diabetes Obes Metab. 2012;3: 68-77. https://goo.gl/JAvYro
- Rolls ET. Taste, olfactory and food texture reward processing in the brain and obesity, Int J Obes (Lond). 2011; 35: 550-61. https://goo.gl/nTWS31
- Grabenhorst F, Rolls ET, Bilderbeck A. How cognition modulates affective responses to taste and flavor: top-down influences on the orbitofrontal and pregenual cingulate cortices, Cereb Cortex. 2008; 18: 1549-59. https://goo.gl/7EBkcc
- Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. Am J Physiol Regul Integr Comp Physiol. 2011; 300: R1266-77. https://goo.gl/yixfeT
- Melchionda N, Luxardi L, Gravina G, et al. La Centralità della. Food Addiction "in DAO development from the onset of resolution. The Equestrian Circus of Survival of Homo Addictus.
- Blum K, Chen AL, Giordano J, Borsten J, Chen TJ, Hauser M, et al. The addictive brain: all roads lead to dopamine. J Psychoactive Drugs. 2012; 44: 134-43. https://goo.gl/HYUqRa
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013; 14: 2-18. https://goo.gl/n6iho8
- Avena NM, Rada P, Hoebel. Evidence for sugar addiction: behavioural and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008; 32: 20-39. https://goo.gl/JbE6ok
- Pelchat ML. Food addiction in humans. J Nutr. 2009; 139: 620-2. https://goo.gl/TPCvKy
- Rondanelli M, Opizzi A, Faliva M, Bucci M, Perna S. Relationship between the absorption of 5-hydroxytryptophan from an integrated diet, by means of Griffonia simplicifolia extract, and the effect on satiety in overweight females after oral spray administration. Eat Weight Disord. 2012; 17: e22-8. https://goo.gl/aZFDiC
- Kelly GS. Rhodiola rosea: a possible plant adaptogen. Altern Med Rev. 2001; 6: 293-302. https://goo.gl/31Gz9C
- Rafael F, Oñatibia-Astibia A, Martínez-Pinilla E. Health Benefits of Methylxanthines in Cacao and Chocolate. Nutrients. 2013; 5: 4159-4173. https://goo.gl/QXGf9D
- Melzig MF, Putscher I, Henklein P, Haber H. In vitro pharmacological activity of the tetrahydroisoquinoline salsolinol present in products from Theobroma cacao L. like cocoa and chocolate. J Ethnopharmacol. 2000; 73: 153-9. https://goo.gl/ELNARu
- Schellekens H, Dinan TG, Cryan JF. Lean mean fat-reducing “ghrelin” machine: hypothalamic ghrelin and ghrelin receptors as therapeutic targets in obesity. Neuropharmacology. 2010; 58: 2-16. https://goo.gl/mMzFCZ
- Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006; 27: 73-100. https://goo.gl/kRZEYp
- Balapal S Basavarajappa. Neuropharmacology of the endocannabinoid signaling system-molecular mechanisms. biological actions and synaptic plasticity. Curr Neuropharmacol. 2007; 5: 81-97. https://goo.gl/USgizt
- de Godoy-Matos AF, Guedes EP, de Souza LL, Valério CM, et al. The endocannabinoid system: a new paradigm in the metabolic syndrome treatment. Arq Bras Endocrinol Metabol. 2006; 50: 390-9. https://goo.gl/LDTyKa
- Pagotto U, Vicennati V, Pasquali R. The endocannabinoid system and the treatment of obesity. Rev Med Brux. 2005. 26: S393-405. https://goo.gl/i2eDTV
- Engeli S, Böhnke J, Feldpausch M, Gorzelniak K, Janke J, Bátkai S, et al. Activation of the Peripheral Endocannabinoid System in Human Obesity. Diabetes. 2005; 54: 2838-43. https://goo.gl/XDiXPz
- Pagotto U, Vicennati V, Pasquali R. The endocannabinoid system and energy metabolism control Physiology and pathophysiology. G Ital Cardiol. 9. https://goo.gl/495baH
- Jo YH, Chen YJ, Chua SC Jr, Talmage DA, Role LW. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005; 48: 1055-1066. https://goo.gl/NKUw8P

Sign up for Article Alerts