Bilharziasis and Haemorrhoidectomy: Case Report?
Delgadillo Xavier* and Wuthrich Philippe
Centre Medico Chirurgical Volta, La Chaux‑de‑Fonds, Switzerland
*Address for Correspondence: Delgadillo Xavier, Centre Medico Chirurgical Volta, La Chaux‑de‑Fonds, Switzerland, ORCid : 0000-0003-4236-6310 ; E-mail: [email protected]
Submitted: 24 August 2020; Approved: 03 September 2020; Published: 04 September 2020
Citation this article: Xavier D, Philippe W. Bilharziasis and Haemorrhoidectomy: Case Report. Int J Case Rep Short Rev. 2020; 6(9): 051-055. https://dx.doi.org/10.37871/ijcrsr.id83
Copyright: © 2020 Xavier D, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Bilharziasis; Schistosoma Mansoni; Milligan-Morgan’s haemorrhoidectomy
Download Fulltext PDF
Schistosoma Mansoni is a fairly common disease in subtropical Africa and Middle East. However it is unknown in Europe and it has not been reported for haemorrhoid cases. Nevertheless, it is secondary to the actual migration phenomena but therefore possibly can be intensified by the lack of hygiene facilities during refugee exodus. We report the case of an Eritrean woman, with a grade-IV haemorrhoidal disease. LigaSure Precise-Triad Milligan-Morgan’s haemorrhoidectomy was performed. Pathology reported SM parasites phagocyted by giant cells within 3 haemorrhoidal cushions without malignancy. Screening of SM parasites was positive in faeces examination and specific treatment was administrated. As we know, SM is retained in the recto-anal mucosa/sub-mucosa inciting a severe inflammatory reaction with infiltration and granuloma formation within a severe haemorrhoidal disease. The diagnosis is often a phenomenon of unexpected finding.
Introduction
Schistosoma Mansoni (SM) is a fairly common cause of diseases in subtropical Africa and in the Middle East. However it is unknown in Europe and it has not been reported for haemorrhoid cases [1]. Nevertheless, secondary to the actual migration phenomena and therefore very possibly to be intensified by the lack of hygiene facilities during refugee migration by one hand [2]. By the other hand, patients suffering of bilharzia or SM, can be asymptomatic porters for more than 40 years, this concept demonstrates the longevity of Schistosoma as some authors had well reported in North America [1,2].
Schistosomiasis, also known as bilharziasis, in honour to who identified and described initially the disease, Theodor Bilharz, a German parasitologist trained at the University of Tubingen. He described a parasitic pathology caused by trematodes belonging to the Schistosoma genus. After malaria and the disease caused by intestinal helminths, it is the third most frequent tropical inducing fibrosis parasitic pathology actually. It is considered an important source of morbidity and mortality in developing countries (Africa, Latin America, the Caribbean, the Middle East and Asia). [1- 3].
The life Schistosoma’s cycle is limited to those tropical and subtropical endemic areas. Active media of diseases are identified in freshwater streams and lakes of medium size, these concept play a role as artificial reservoirs. Certain systems of artificial irrigation are also fully involved in the origin of bilharziasis, as in some underdeveloped countries. In fact, the geographical distribution of parasitic diseases has expanded due to the problems associated with socio-cultural migration resulting from armed conflicts in infected populations [1-4].
Intestinal bilharziasis caused by S. mansoni, a trematode parasite found in our patient, has been described in more than 50 countries, including the Caribbean (Santa Lucia, Antigua, Montserrat, Martinique, Guadeloupe, Dominican Republic and Puerto Rico), countries of the Eastern Mediterranean, South America’s northern countries (Brazil, Venezuela and Surinam) and in a vast majority of countries in Africa [5].
It has been estimated that more than 207 million people in more than 75 countries worldwide have an active bilharziasis condition. In this population universe, close to 60% of patients are symptomatic and manifest symptoms of the disease, including specific visceral problems and conditions related to chronic anaemia and malnutrition secondary to parasitizes. The prevalence of parasitizes is irregular and very heterogeneous in many vulnerable sites and has a tendency to worsen in areas with low environmental sanitation associated with the abuse of irrigation with fresh water in conjunction with the daily drinking of water[1,2,5-7].
According to the publications of the World Health Organization (WHO), the worldwide distribution of bilharziasis has been modified due to its recent eradication in Japan and the islands of the Lesser Antilles. Antiparasitic programs have caused schistosomiasis to halt its transmission in Tunisia, finally there are reports that indicate low transmission in Morocco, Saudi Arabia, Venezuela and Puerto Rico. [2,3,5,7,8].
Case Report
We report a unique European case of a 45 year old Eritrean woman, refugee in Switzerland after 2 years, with a 5 year history of anal bleeding and pruritus admitted in our specialized Unit. Abdominal examination was irrelevant. Grade-IV haemorrhoidal disease was diagnosed after an exhaustive proctologic examination. A complete coloscopy prior to surgery was negative to the presence of any inflammatory reaction or any polyps. After a standard preparation and under general anaesthesia a LigaSure Precise Triad Milligan-Morgan’s haemorrhoidectomy was performed. Post-operative follow-up was uneventful.
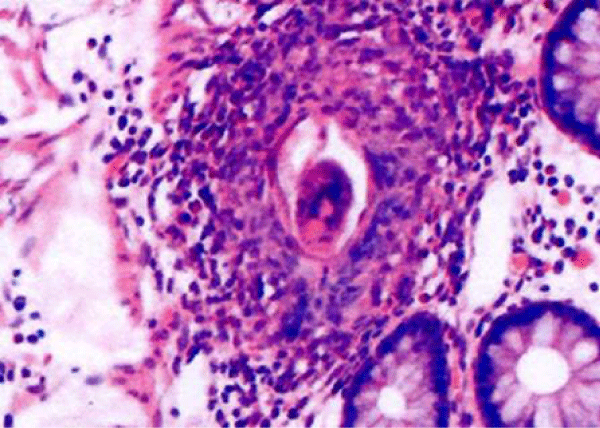
The excised haemorrhoid’s histopathology report demonstrated the presence of SM parasites phagocyted by giant cells within 3 haemorrhoidal cushions with no signs of malignancy (Figure 1).
Screening of SM parasites was positive in faeces examination and a treatment of Prazyquantel 600 mg/day/dose was administrated. Later on, a faecal parasites study showed no evidence of SM. Unfortunately the patient was lost of view, result of a forced return to her background country.
Literature Review
The term “haemorrhoid” is derived from the Greek: haima - blood and rhoos - flowing. The word “pile” is derived from the Latin pi/a, meaning a pill or ball. Humans have complained of haemorrhoids since at least biblical times [9]. The evidence for the aetiology and pathogenesis is surprisingly sparse, especially in light of increased interest in the surgical treatment of haemorrhoids.
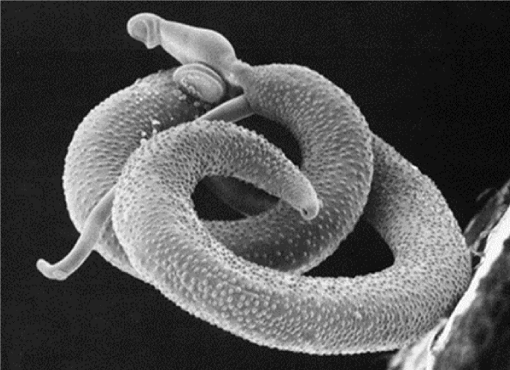
Pathophysiology of haemorrhoids associated to aetiological factors and postulated theories of pathogenesis associated with bilharzia are fairly few known. Bilharzia is a tropical disease caused by worms of the genus Schistosoma. Specific cycle of transmission of the disease requires contamination of water by infested faeces, specific freshwater snails as intermediate hosts, and human water contact [3,4,7,10]. The main disease-causing species are S. haematobium, S. mansoni, (Figure 2) and S. japonicum. Schistosomiasis is characterised by focal epidemiology and over dispersed population distribution, with higher infection rates in children than in adults. Complex immune mechanisms lead to the slow acquisition of immune resistance, though innate factors also play a part [3,6,11].
Acute schistosomiasis, a feverish syndrome, is mostly seen in travellers after primary infection. Chronic schistosomal disease affects mainly individuals with long-standing infections in poor rural areas. Immunopathological reactions against schistosoma eggs trapped in the tissues lead to inflammatory and obstructive disease in the urinary system (S. haematobium) or intestinal disease, hepatosplenic inflammation, and liver fibrosis (S. mansoni, S. japonicum), bilharzia with haemorrhoidal piles S mansoni) [6,11,12].
The pathological description of chronic schistosomiasis is based on the frequency of its presentation, which is much more common than the acute form, and this due to the infectious response induced by the cercarial eggs. The formation of granulomas and the associated fibrotic changes are secondary descriptions. Although adult cercariae are poorly immunogenic, eggs on the other hand induce very intense local immune and blood responses. (It is possible that the eggs require intense immune responses to help themselves in their migration through the body) [6,11,13].
Adult forms can absorb host proteins. If they are not attacked by the immune system, they can live for years in the bloodstream because they are coated with host antigens. Egg retention and granuloma formation in the bowel wall (usually in case of S. mansoni) may cause bloody liquid stools, cramps and, in some cases inflammatory reactive colonic polyps [6,11,13,14].
Patients severely affected at the intestinal wall, have a higher rate of inter-current infection with Salmonella sp, blood cultures are generally positive, while stool cultures are negative. Forms of chronic intestinal schistosomiasis that present as acute complications of appendicitis, [15,16] perforation of the distal ileum, and low gastrointestinal bleeding after a long exposure related to the initial trip (endemic) have been described [17].
There are some authors’ descriptions in which the bilharzia eggs penetrate the intestine adjacent to the mesenteric vessels where finally adult Schistosomas are found. Those eggs that are dragged into the portal circulation, lodge in the venous endothelium and from there induce granulomatous reactions throughout the portal tree [13,16].
Severe hepatic clinic manifestations has also been described, inducing important peri-portal fibrosis in a characteristic pipe-stem pattern. Despite severity of the peri-portal damage, the hepatocellular function is spared, and an isolated portal hypertension with the usual potential sequelae, including ascites, oesophageal variceal bleeding and development of porto-systemic collaterals. It is through these collaterals or directly from the inferior vena cava, in the case of bladder wall schistosomiasis, that eggs can reach the pulmonary circulation. The resulting pulmonary granulomatosis and fibrosis can lead to pulmonary hypertension (19%) and frank cor pulmonale with a high mortality rate.
The diagnostic standard is microscopic demonstration of eggs in faeces. Praziquantel is the drug treatment of choice. Vaccines are not yet available. Great advances have been made in the control of the disease through population-based chemotherapy but these required political commitment and strong health systems [1-3,14].
Discussion
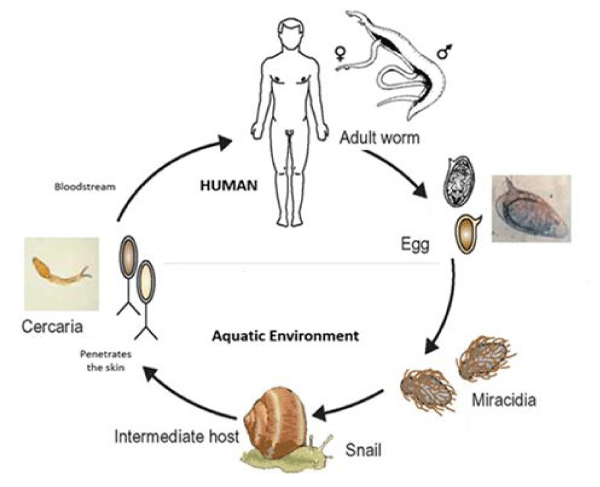
SM parasite, a digenetic blood-dwelling fluke of the flatworm variety, possessing a definitive mammalian host and an intermediate snail host [2,3]. SM adult worms are found in pairs in the mesenteric vessels, where they lay their eggs. Ovaries penetrate the intestinal wall in which they shed, following their host in faeces. Upon contact with fresh water, the eggs hatch and release miracidia which then after infects the appropriate snail host, multiplying asexually into cercarial larvae [2]. After the penetration of human skin, the cercarial larvae transforms into a SM and undergoes its development in the portal vein. Mature female and male worms mate and inhabit the mesenteric venules. Female worms release eggs, which pass through the intestinal wall to reach faeces and then renew the cycle [1-3,5,7,10] (Figure 3).
Cercariae can survive up to 48 hours in water but are most infectious to humans in the first few hours after release from the snail. Cercariae pass via the lung and the liver into the portal venous and then mature into adult worms and unite. Pairs of worms then migrate to the superior mesenteric veins (in the case of S. mansoni), the inferior mesenteric and superior haemorrhoidal veins (in the case of S. japonicum), or the vesical plexus and veins draining the ureters and the urinary bladder (in the case of S. haematobium).
Schistosomiasis is the infection of humans by trematodes (a class of helminths). Three major species of schistosomes are described: S. haematobium, S. japonicum, and S. mansoni [14].
S. haematobium infection is acquired predominantly in North Africa, sub-Saharan Africa, the Middle East, Turkey, and India. The majority of S. mansoni infections are found in sub-Saharan Africa. S. japonicum infection still occurs in China, Indonesia, and the Philippines [1,3].
Morphologically, S. haematobium ova have a spine in the apical position, whereas the spine of S. mansoni ova is on the lateral aspect, and S. japonicum ova have no spine [3]. S. haematobium is the organism most often identified in females genitalia. [1, 4, 6]. In most cases, the lesions are composed of epitheloid histiocytes and a lot of eosinophils and plasma cells terminal spines characteristic of S. haematobium [9].
Live schistosome eggs are excreted in the feces (in the case of S. mansoni and S. japonicum) or urine (in the case of S. haematobium). As we know, SM is retained in the recto-anal mucosa/sub-mucosa inciting a severe inflammatory reaction with infiltration and granuloma formation (ulcers, abscess, polypes, cancer) within the severe haemorrhoidal disease.
Direct retrograde spread of the adult worms from their usual sites into the venous system supplying skin leads to deposition of ova in the skin and subsequent formation of lesions, as in our patient. The life cycle is completed when the eggs hatch, releasing miracidia that, in turn, infect specific freshwater snails [1,3,14]. The adult worms remain in the blood vessels for life and survive for five to seven years and can even persist for up to 40 years [4].
Physiopathologically, 2 to 3 cm adult worms may cause venous obstruction where they reside, but the disease is more commonly caused by the daily deposition of numerous eggs by the female (hundreds to thousands eggs per day, depending on the species), which invades local tissues, where they release toxins and enzymes and provoke a TH-2-mediated immune response [4, 7,14].
Inflammation and granuloma formation occur around deposited eggs, which can lead to extensive tissue damage (fibrosis and scarring) [5,14]. Most patients infected with schistosomiases§ of all species are asymptomatic. Acute symptoms tend to be more common in nonimmune individuals, such as travellers, due to a more intense immune response to exposure [16].
By contrast, chronic complications require a higher burden of infection and, thus, are mainly seen in individuals from endemic areas [10]. The clinical picture of anal schistosomiasis and the pathological findings vary according to the organ affected. The clinical manifestations are nonspecific except the presence of sandy patches (areas of roughened mucosa surrounding egg deposits) on the anal verge [12,13]. It can include irregular bleeding or itching [3].
The lesions are polypoid or papillomatoses tumour like lesions start with an irritation of the skin, followed by oedema and hyperaemia. Later small nodules developed under the haemorrhoidal piles and papillomatoses lesions appeared forming masses resembling big piles [3,8]. In travellers returning from the tropics, due to the unspecific nature of proctologycal findings, delays of more than 24 months in diagnosis have been reported, the lesions being misdiagnosed as, for example, anal warts [8,18].
The standard of care for treatment is a single dose of oral praziquantel, a pyrazinoisoquinoline derivative, at a dose of 40 mg/kg [1,3,11,14]. The drug’s precise action on adult worms is unknown. Although praziquantel is not considered to be teratogenic or mutagenic, the drug is not recommended in pregnancy or in lactating women [8]. Re-examination of faeces or urine one month after treatment is recommended in order to assess efficacy [1]. In our patient, this control has showed negative results before the treatment.
Meltzer et al. and some other authors proposes to screen and treat asymptomatic travellers with history of freshwater exposure in endemic countries [15,16].
Conclusion
Our final considerations and ending points concerning haemorrhoidal schistosomiasis defined this situation as a rare entity in the western world.
Our patient illustrates an unusual presentation of schistosomiasis in Europe, via the localisation of the lesion and the patient’s backgrounds. It should be keep in mind as a cause of granulomatous inflammation on the anal verge and must enter into the differential diagnosis of symptomatic anal itching and localized lesions when the clinical history indicates travel in endemic areas or endemic origins.
Definitive examination of eggs in faeces samples is mandatory, and frequently buds can be identified by cytology or histologic methods.
An appropriate and specific treatment can be initiated administrating Praziquantel, as the drug of choice for the treatment of all forms of schistosomiasis. It is important to retain that a major weakness of praziquantel is its relative inefficacy against recent infections, a factor that may occasionally result in low cure rates in hyperendemic areas.
Recent recommendations for Bilharzia isolates with a decreased sensitivity to praziquantel discussed in the broader context of a possible emergence of drug resistance.
Diagnosis of proctological bilharzia in patients welcomed in developed countries, result of a recent migratory tragedy and usually is a matter of an incidental findings.
Conflict of Interests
We certify that there is no actual or any kind of potential conflict of interest in relation to this article. We have no financial interest or arrangements with any organizations that could be perceived as a real or apparent. No conflict of interest in the context of the subject of this article can be mentioned.
The case presented in this article, has been reported as the Swiss Visceral Surgery Poster Communications, during the Swiss Gastroenterology Meeting, Interlaken, Switzerland, September 2018.
- Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006; 368: 1106‐1118. DOI:10.1016/S0140-6736(06)69440-3
- Ross AG, Bartley PB, Sleigh AC, G Richard Olds, Yuesheng Li, Gail M Williams, et al. Schistosomiasis. N Engl J Med. 2002; 346: 1212‐1220. DOI:10.1056/NEJMra012396
- Delgadillo X, Ott V, Wuthrich Ph. Surprising haemorrhoidectomy. Swiss Med Weekly. 2018; 7: 148.
- Berberian DA, Paquin HO Jr, Fantauzzi A. Longevity of schistosoma hematobium and schistosoma mansoni: Observations based on a case. J Parasitol. 1953; 39: 517‐519. https://bit.ly/3jGZc0j
- Blanchard TJ. Schistosomiasis. Travel Med Infect Dis. 2004; 2: 5‐11. DOI:10.1016/j.tmaid.2004.02.011
- Van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007; 212: 475‐490. DOI:10.1016/j.imbio.2007.03.009
- Chitsulo L, Loverde P, Engels D. Schistosomiasis. Nat Rev Microbiol. 2004; 2: 12‐13. DOI:10.1038/nrmicro801
- King C, Mahmoud A. Schistosomiasis. Tropical infectious diseases: Principles, pathogens and practice. Guerrant R, Walker DH, Weller PF, editors. Churchill Livingstone. USA: 1999. pp.1031-1038.
- Dirckx JH. The biblical plague of "hemorrhoids". An outbreak of bilharziasis. Am J Dermatopathol. 1985; 7: 341‐346. https://bit.ly/3bqlY9B
- Arnon R. Life span of parasite in schistosomiasis patients. Isr J Med Sci. 1990; 26: 404‐405. https://bit.ly/3lIhMXA
- Cheever AW, Hoffmann KF, Wynn TA. Immunopathology of schistosomiasis mansoni in mice and men. Immunol Today. 2000; 21: 465‐466. DOI:10.1016/s0167-5699(00)01626-1
- Ahmed ME, Himaida TI. Haemorrhoids in Khartoum. East Afr Med J. 1990; 67: 48‐50. https://bit.ly/2QQ89rP
- Coutinho HM, Acosta LP, Wu HW, McGarvey ST, Su L, Langdon GC, et al. Th2 cytokines are associated with persistent hepatic fibrosis in human Schistosoma japonicum infection. J Infect Dis. 2007; 195: 288‐295. DOI:10.1086/510313
- H Salim OE, Hamid HK, Mekki SO, Suleiman SH, Ibrahim SZ. Colorectal carcinoma associated with schistosomiasis: A possible causal relationship. World J Surg Oncol. 2010; 8: 68. DOI:10.1186/1477-7819-8-68
- Grobusch MP, Muhlberger N, Jelinek T, Z Bisoffi, M Corachan, G Harms, et al. Imported schistosomiasis in Europe: Sentinel surveillance data from TropNetEurop. J Travel Med. 2003; 10: 164‐169. DOI:10.2310/7060.2003.35759
- Weerakoon KG, Gobert GN, Cai P, McManus DP. Advances in the diagnosis of human schistosomiasis. Clin Microbiol Rev. 2015; 28: 939‐967. DOI:10.1128/CMR.00137-14
- Warren KS, Mahmoud AA, Cummings P, Murphy DJ, Houser HB. Schistosomiasis mansoni in yemeni in California: Duration of infection, presence of disease, therapeutic management. Am J Trop Med Hyg. 1974; 23: 902‐909. DOI:10.4269/ajtmh.1974.23.902
- Meltzer E, Artom G, Marva E, Assous MV, Rahav G, Schwartzt E. Schistosomiasis among travelers: New aspects of an old disease. Emerg Infect Dis. 2006; 12: 1696‐1700. DOI:10.3201/eid1211.060340

Sign up for Article Alerts