Simplified Method for Isthmus Block Assessment in Atrial Flutter Ablation: Results of a Single Center Experience?
Mathieu Granier*, Xavier Brunet, Guillaume Cayla and Pierre-Francois Winum
Cardiolgy Department Caremeau University Hospital Nimes, France
*Address for Correspondence: Granier Mathieu, Cardiology service CHU Caremeau 1 pl R. Debre, 30900 Nimes Cedex, France, E-mail: [email protected]
Submitted: 19 August 2017; Approved: 14 September 2017; Published: 19 September 2017
Citation this article: Granier M, Brunet X, Cayla G, Winum PF. Simplified Method for Isthmus Block Assessment in Atrial Flutter Ablation: Results of a Single Center Experience. Int J Cardiovasc Dis Diagn. 2017;2(2): 043-048.
Copyright: © 2017 Granier M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Atrial Flutter; Radiofrequency Ablation; Cavotricuspid Isthmus Block; Atrial Flutter Ablation; Cavotricupid Isthmus Ablation
Download Fulltext PDF
Background: Typical Atrial Flutter (AFl) is standardly cured by Radiofrequency (RF) ablation with the goal of Cavo-Tricuspid Isthmus (CTI) block. Here we describe our method to assess CTI block and its result on clinical recurrence.
Methods: CTI ablations performed at our center were analyzed. We used two catheters: a quadripolar in the coronary sinus (Cs) and the RF catheter. The endpoint of ablation was the assessment of isthmus block if S-A2 interval was > 140 msec, measured from the Cs ostium (S) to the RF catheter placed lateral to the CTI (A2). We aimed to study the rate of recurrence after a maximal follow up of 24 months.
Results: 74 patients were included (age 69.8 ±11). Post procedural S-A2 interval was 162 ± 26 msec. S-A2>140 msec was achieved in 66 patients (89%). Eight patients (11%) exhibited a S-A2 <140 msec but >130 msec. One patient exhibited a S-A2=110 msec and flutter recurrence after 13 months.
During follow-up (14.1 ± 6.8 months), 4 patients were lost. Flutter recurred in 3 (4%). AFl-free and arrhythmia-free survival probabilities were respectively 84% [CI 0.71-0.99] and 54 % [CI 0.36-0.80]. Fifteen patients (21.5%) developed AF.Conclusion: In our cohort, assessment of CTI block by a S-A2 interval >140 msec is feasible with a rate of recurrence of 4%. This cut-off value seems relevant but would deserve a comparative analysis. This simplified approach is safe and effective with clinical results comparable to the literature.
Abbreviations
AF: Atrial Fibrillation; AFI: Atrial Flutter; CL: Cycle Length; Cs: Coronary Sinus; CTI: Cavo-Tricuspid Isthmus; EP: Electrophysiological; FU: Follow Up; IVC: Inferior Vena Cava; LAO: Left Anterior Oblique; PPI: Post Pacing Interval; RF: Radiofrequency; SR: Sinus Rhythm; SVT: Supra Ventricular Tachycardia
Introduction
Typical Atrial Flutter (AFl) is a frequent macro-reentrant right atrial arrhythmia [1], whose Radio Frequency (RF) ablation of the Cavo Tricuspid Isthmus (CTI) demonstrated excellent acute and long term success rates [2,3]. Despite the widespread use of this curative approach, there is no specific guideline about the method and material required to achieve CTI ablation. Numerous techniques have been published most of which have been described using a duo-decapolar catheter to record atrial depolarization and test CTI block during RF ablation [4-7]. Obtaining CTI block, on top of sinus rhythm restoration as an endpoint for acute procedural success, demonstrated best clinical results in long term follow up [8].
Here we describe a simplified method to achieve RF of the CTI and test CTI block, by using only the RF catheter and a deflectable quadripolar catheter introduced in the Coronary Sinus (Cs). We hypothesized that a unidirectional measurement of conduction delay between Cs ostium and the lateral part of the isthmus line > 140 msec is a clinically relevant marker of bidirectional isthmus block, and can demonstrate clinical results comparable to the literature.
Patients and Methods
Study design
Single center retrospective observational study. Local ethics committee of our institution approved the protocol.
Patients
All consecutive patients referred to our center for RF ablation of typical atrial flutter were screened.
Criteria for patients’ selection were: age >18, documented common atrial flutter on 12 leads ECG, RF ablation of the CTI after validation of the isthmus dependence of the flutter. Patients were excluded if previous RF of the CTI, left atrial tachycardia documented in the EP lab, or Atrial Fibrillation (AF) at the time of the procedure occurred.
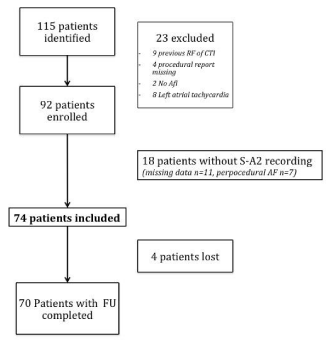
Among the 115 patients identified, 23 were excluded (nine patients with previous RF of the CTI, four patients without procedural report, two patients didn’t presented atrial flutter, eight patients presented left atrial tachycardia). From the remaining 92 patients, 18 could not be included for missing data or AF conversion during ablation. Finally 74 patients were included in the study. Four patients were lost at follow up (figure 1).
Electrophysiological (EP) procedure
Patients were under conscious sedation. Midazolam and Sufentanyl were injected before the beginning of the procedure (usually 1 mg and 2.5 µg) and then titrated to avoid pain and discomfort. A quadripolar deflectable catheter (Xtrem , Sorin™) and a RF catheter 8 mm non irrigated tip (Blazer II Boston Scientific™ ) or 4 mm irrigated tip (Coolflex, St Jude medical™) were introduced via femoral vein. The quadripolar catheter was positioned in the coronary sinus. Cycle length (CL) of the tachycardia and proximo-distal depolarization were assessed. Typical 12-lead ECG and proximo-distal depolarization were sufficient to consider the flutter as isthmus-dependent and begin CTI ablation. If necessary, Post Pacing Intervals (PPI) were measured throughout right atrium and coronary sinus to assess isthmus dependence of the circuit [9]
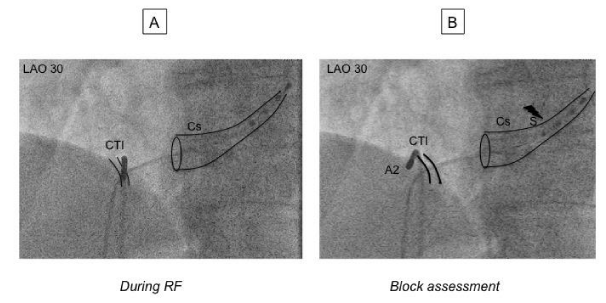
Ablation: RF energy was delivered with a IBI-1500T9-CP generator (St Jude Medical, St-Paul, MN). For non irrigated catheter, maximum power output was 50 Watts and temperature limited to 65°C, for irrigated catheter power output was 30 Watts and temperature limited to 45°C. CTI ablation was performed point-by-point or by dragging under fluoroscopic and electrical guidance: in 30° Left Anterior Oblique view (LAO 30), RF catheter was positioned and banded at 6 hours until a ventricular electrogram was obtained. The RF catheter was then pulled down until appearance of an atrial electrogram. RF was then delivered, pulling down the catheter to the Inferior Vena Cava (IVC). Applications were repeated along the CTI line until achievement of electrical endpoints.
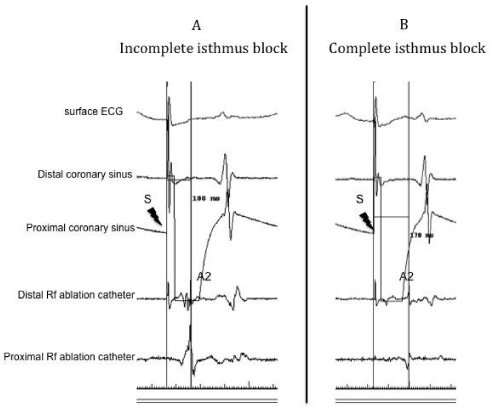
Acute procedural success and Electrical endpoints: Objectives of the procedure were to restore sinus rhythm and to achieve line of block on the CTI. We hypothesized that a S-A2 interval >140 msec was a strong marker of bi-directional isthmus block (figure 2). This interval was defined as the time separating the spike of stimulation (pacing at 600 msec CL) on the proximal bipole of the quadripolar deflectable catheter (located at proximal part of the coronary sinus) and the tip of the RF catheter that has been moved to the lateral side of the CTI. On the bipolar signal, caliper was adjusted to the maximum dV/dt of the A2 signal (figure 3). The cut off value of 140 msec was decided after considering the result of previous studies (7,10) (see discussion section for details) A waiting period of at least ten minutes was then observed until the end of the procedure.
Clinical follow up: Patients were followed up to a maximum of 24 months after the procedure. Medical investigation was performed by phone call to the patient’s general practitioner or cardiologist or to the patient himself. If a history of palpitation recurrence or hospitalization for arrhythmia or any cardiovascular cause was found, investigation was continued to confirm ECG documented flutter recurrence or any other arrhythmia.
Main objective of the study was freedom from common flutter recurrence at the end of follow up.
Statistics: Statistical analyses were performed with biostaTGV online software (http://marne.u707.jussieu.fr/biostatgv/). Kaplan-meier method was used to calculate event-free survival curve. For comparisons of groups, continuous variables are expressed as means ± standard deviation and were compared with Student t-test or Wilcoxon-Mann Whitney if distribution was not normal. Qualitative variables are expressed as percentage of the effective, and were compared with Chi-square test or exact Fischer test depending of the effective (expected effective n>5 for chi-square validation).
Results
Seventy-four patients met inclusion criteria. Patient’s characteristics are resumed in (table 1).
Ablation procedure
Most of the procedures were performed with non-irrigated catheter. At the time of RF ablation, 19 % (n=14) of the patients were in Sinus Rhythm (SR).
Immediate ablation results
Among the patients in AFl during the procedure (n=60), restoration of SR was achieved in all. Of note, seven patients presented AF conversion during ablation and have not been enrolled in the study as mentioned in method section (Figure 1). Among the 14 patients in SR during the procedure, the endpoint defined as S-A2 >140 msec was obtained in 11 (79%). Mean Fluoroscopy time was 9.8 ± 10.4 min.
Isthmus block assessment
S-A2 interval at the end of procedure has been recorded all patients. 65 patients (89%) exhibited a S-A2 interval >140 msec.
Overall, S-A2 interval was 162 ± 26 msec. Eight patients (11%) exhibited a S-A2 interval < 140 msec and are described in (table 2). Three of them were in sinus rhythm and five in AFl at the time of the procedure. All but one exhibited a S-A2 interval <140 msec but > 130 msec. One patient presented an acute termination of the flutter with S-A2 interval of 110 msec at the end of the procedure. AFl recurred thirteen months later, requiring a second procedure with a S-A2 interval of 210 msec at the end of the second procedure without any recurrence after 18 months. Among the group of patient with incomplete isthmus block (S-A2 < 140 msec), three presented AF during the follow up.
Clinical follow up (FU)
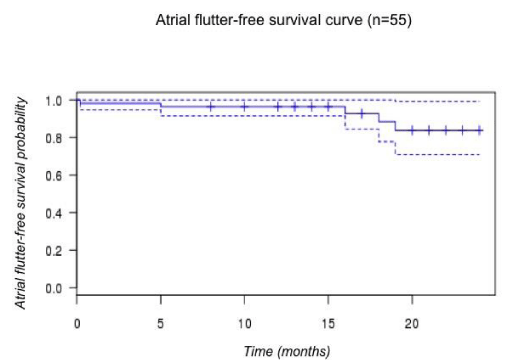
Mean FU was 14.1±6.8 months, four patients were lost. Among the 70 patients with follow up completed, three presented common atrial flutter recurrence (4%) and are described in (table 3). For one patient AFl recurred 24 hours after the index procedure, and block was obtained after a second procedure (S-A2 187 msec). For a second patient, AFl recured after five month despite a S-A2 interval of 150 msec at the end of the index procedure. Atrial flutter-free survival probability at the end of FU was 84% [CI 0.71-0.99] (figure 4).
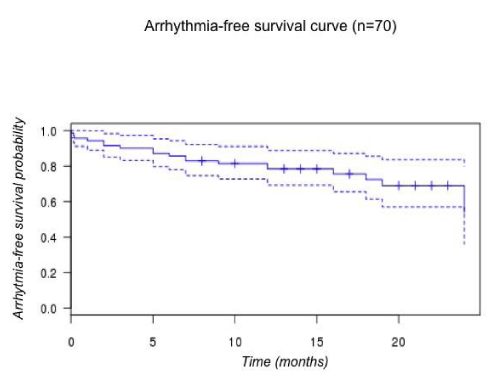
Fifteen (21.5%) patients developed AF during follow up. Overall, arrhythmia-free survival probability at FU was 54 % [CI 0.36-0.80] in our cohort (figure 5).
Complications
Neither groin hematoma requiring surgery nor serious cardiovascular complications were recorded during FU.
Two patients died during FU. One patient died from non-cardiac cause seven months after the procedure, the second presented unexplained nocturnal sudden death six days after discharge.
Discussion
Clinical results
In this study we investigated if a simplified method for RF ablation of typical AFl, using two catheters and a simple isthmus block criteria could achieve a good clinical success rate. In a meta-analysis involving 10719 patients and 158 studies, Perez et al [8]. Reported an immediate procedural success rate of 91.1% and a rate of recurrence of 6.7%, which is similar to our results (89% and 4% respectively). Atrial fibrillation incidence during follow up was 33.6% with a mean follow up of 15.2 months, as we reported an incidence of 21.5% after 14.1 months.
Simplified approach
Several authors already reported simplified methods with the use of two catheters [11-13], however none of them used the S-A2 interval as a bidirectional isthmus block criterion. Klug et al. demonstrated excellent results in a limited number of patients (n=30), with an isthmus block criterion, using surface ECG P wave analysis, which however is not widely used. Also, Maury et al. demonstrated a relatively high rate of recurrences at 15 months (13%) in a series of 255 patients, using differential pacing to assess clockwise and counterclockwise isthmus block. Liew et al. demonstrated in a randomized study aggregating all supra ventricular tachycardia (SVT) (n=200) the cost reduction of using a minimal number of catheter. In the two catheters AFl ablation group (n=29), using differential pacing for isthmus block assessment and a FU of six weeks, only one recurrence occurred.
S-A2> 140 msec as a marker of bi-directional isthmus block
Use of S-A2> 140 msec was based on the results of Oral et al. and Chen et al. studies [7,10]. Indeed, in a series of patients with bi-directional isthmus block criteria, Chen et al. reported a S-A2 minimum value of 140 msec [10], whereas Oral et al. reported a mean value of 195 ± 31 msec (and so a minimal value of 164 msec) [7]. Our result of 162 ± 26 msec is consistent with these previous findings and the minimal value of 140 msec has been achieved in the vast majority of our patients (89%). However, this is an empiric cut-off that should theoretically be adapted to each patient, based on the size of right atrium, the distance between Cs ostium and RF ablation catheter, and the possibility of a posterior crista terminalis conduction as previously reported by Scaglionne et al. [14].
Our study was underpowered to demonstrate a significant difference in the rate of recurrences between the nine patients with a S-A2<140 msec and the rest of the cohort. This is a limitation of the study, but our objective was to describe our clinical practice and confront our results to the literature, and not to compare both group. The only patient that presented recurrence in this group exhibited a S-A2 of 110 msec at the end of the index procedure, and of 210 msec after the second procedure. In this particular case it is obvious that CTI block was not achieved in the first procedure. For the other three patients that exhibited a S-A2 between 130 and 140 msec, as there was no recurrence, isthmus block remains unclear and at least some of them might have a posterior crista terminalis conduction that make S-A2 >140 msec impossible to obtain.
Uni- or bi-directional isthmus block?
As far as Chen et al. mentioned in their study that “a unidirectional conduction block in the CTI was never observed”[10], we assumed, in order to simplify the procedure, that uni-directional conduction delay measurement was sufficient to assess bi-directional isthmus block and thus long term clinical result. Following this hypothesis, we’ve tested the clockwise block with the S-A2 interval, but never checked the counter clockwise block.
Procedural outcome
We didn’t have any major complication, especially groin hematoma and one could expect that reducing the number of catheters would favor the safety of the procedure regarding this frequently reported complication [15].
In our retrospective cohort, the total fluoroscopy time was 9.8 ± 10.4 min. In a recent meta-analysis comparing results of cryo vs RF catheter ablation, mean fluoroscopy duration for RF group was 27.5 min [16]. We did not recorded total procedural time, this is a limitation of the study, however, we may hypothesize that reducing the number of catheter and using a quadripolar catheter, easier to manipulate, would reduce the total time of catheter positioning and fluoroscopy duration.
Finally, reducing the number of catheter with a comparable clinical result should reduce the cost of the procedure. In our institution, considering the cost of a duodecapolar catheter, reducing the number of catheter needed saved 355 euros per procedure.
As no specific guideline about the material to be used for this procedure is available to date, a randomized comparison would be of interest to help electrophysiologists in the choice of the best technique.
Conclusion
In this single center retrospective cohort, we’ve found that our simplified method for radiofrequency of Atrial Flutter using two catheters and a simple criterion of isthmus block was safe and could provide clinical results comparable those of the literature.
A S-A2 interval > 140 msec seems an adequate goal to assess isthmus block but this remains to be demonstrated in a comparative study. This approach could be time and cost saving compared to conventional ablation using multipolar catheter.
Aknowledgement
We thank Dr Lionel Beck and Mrs Mariella Lomma and Sarah Kabani for their active collaboration and precious help.
- Puech P, Latour H, Grolleau R. [Flutter and his limits]. Arch Mal Coeur Vaiss 1970; 63: 116-44. https://goo.gl/xAdUMh
- Granada J, Uribe W, Chyou PH, Maassen K, Vierkant R, Smith PN, et al. Incidence and predictors of atrial flutter in the general population. J Am Coll Cardiol 2000; 36: 2242–6. https://goo.gl/VEbg5N
- Blomstrom-Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary. A report of the American college of cardiology/American heart association task force on practice guidelines and the European society of cardiology committee for practice guidelines (writing committee to develop guidelines for the management of patients with supraventricular arrhythmias) developed in collaboration with NASPE-Heart Rhythm Society. J Am Coll Cardiol 2003; 42: 1493–531. https://goo.gl/nZ386X
- Anselme F, Savouré A, Cribier A, Saoudi N. Catheter ablation of typical atrial flutter: a randomized comparison of 2 methods for determining complete bidirectional isthmus block. Circulation 2001; 103: 1434–9. https://goo.gl/zXyu6a
- Andronache M, de Chillou C, Miljoen H, Magnin-Poull I, Messier M, Dotto P, et al. Correlation between electrogram morphology and standard criteria to validate bidirectional cavotricuspid block in common atrial flutter ablation. Europace 2003; 5: 335–41. https://goo.gl/6e3wEg
- Tada H, Oral H, Sticherling C, Chough SP, Baker RL, Wasmer K, et al. Double potentials along the ablation line as a guide to radiofrequency ablation of typical atrial flutter. Journal of the American College of Cardiology 2001; 38: 750–5. https://goo.gl/6KPTHj
- Oral H, Sticherling C, Tada H, Chough SP, Baker RL, Wasmer K, et al. Role of trans isthmus conduction intervals in predicting bidirectional block after ablation of typical atrial flutter. J Cardiovasc Electrophysiol 2001; 12: 169–74. https://goo.gl/4SqvXE
- Perez FJ, Schubert CM, Parvez B, Pathak V, Ellenbogen KA, Wood MA. Long-Term Outcomes after Catheter Ablation of Cavo-Tricuspid Isthmus Dependent Atrial Flutter: A Meta-Analysis. Circulation: Arrhythmia and Electrophysiology 2009; 2: 393–401. doi:10.1161/CIRCEP.109.871665. https://goo.gl/yg7aYn
- Deo R, Berger R. The clinical utility of entrainment pacing. J Cardiovasc Electrophysiol 2009; 20: 466–70. https://goo.gl/STEC2A
- Chen J, de Chillou C, Basiouny T, Sadoul N, Filho JD, Magnin-Poull I, et al. Cavotricuspid isthmus mapping to assess bidirectional block during common atrial flutter radiofrequency ablation. Circulation 1999; 100: 2507–13. https://goo.gl/HdMnPA
- Liew R, Baker V, Richmond L, Rajappan K, Gupta D, Finlay M, et al. A randomized-controlled trial comparing conventional with minimal catheter approaches for the mapping and ablation of regular supraventricular tachycardias. Europace 2009; 11: 1057–64. https://goo.gl/fV8ukB
- Maury P, Raczka F, Gaty D, Duparc A, Couderc P, Hollington L, et al. Radio-frequency ablation of atrial flutter: long-term results and predictive value of cavo-tricuspid isthmus bidirectional block as determined by a simplified technique. Cardiology 2008; 110: 17–28. https://goo.gl/i5cZ9t
- Klug D, Lacroix D, Marquié C, Mairesse G, Alix D, Dennetière S, et al. Prospective evaluation of a simplified approach for common atrial flutter radio frequency ablation with only two catheters. Europace 2001; 3: 208–15. https://goo.gl/oM2qma
- Scaglione M, Riccardi R, Calò L, Di Donna P, Lamberti F, Caponi D, et al. Typical atrial flutter ablation: conduction across the posterior region of the inferior vena cava orifice may mimic unidirectional isthmus block. J Cardiovasc Electrophysiol 2000; 11: 387–95. https://goo.gl/pymmgk
- Brembilla-Perrot B, Filali ML, Zinzius P-Y, Sellal J-M, Beurrier D, Schwartz J, et al. Is ablation of atrial flutter always safe? Pacing Clin Electrophysiol 2012; 35: 1061–6. https://goo.gl/nrYG2t
- Andrew P, Hamad Y, Jerat S, Montenero A, O’Connor S. Approaching a decade of cryo catheter ablation for type 1 atrial flutter-a meta-analysis and systematic review. J Interv Card Electrophysiol 2011; 32: 17–27. https://goo.gl/Hn7BMG

Sign up for Article Alerts