Usefulness of Moderate Intensity Daily Physical Activity As a Predictor of Left Ventricular Reverse Remodeling After ST-Elevation Myocardial Infarction?
Akihiro Makino1, Minako Yamaoka-Tojo2*, Shuhei Yamamoto1, Ryo Kameda3, Shinichi Obara4, Atsuhiko Matsunaga2 and Junya Ako3
1Department of Rehabilitation Sciences, Kitasato University Graduate School of Medical Sciences, Sagamihara, Japan
2Department of Rehabilitation, Kitasato University School of Allied Health Sciences, Sagamihara, Japan
3Department of Cardiovascular Medicine, Kitasato University School of Medicine, Sagamihara, Japan
4Department of Rehabilitation, Kitasato University East Hospital, Sagamihara, Japan
*Address for Correspondence: Minako Yamaoka-Tojo, Department of Rehabilitation, Kitasato University School of Allied Health Sciences. 1-15-1 Kitasato, Minami-ku, Sagamihara, 252-0373 Japan, Tel/Fax: +81-42-778-9696; E-mail: [email protected]
Submitted: 02 August 2017; Approved: 23 August 2017; Published: 26 August 2017
Citation this article: Makino A, Yamaoka-Tojo M, Yamamoto S, Kameda R, Obara S, et al. Usefulness of Moderate Intensity Daily Physical Activity As a Predictor of Left Ventricular Reverse Remodeling After ST-Elevation Myocardial Infarction. Int J Cardiovasc Dis Diagn. 2017;2(1): 022-028.
Copyright: © 2017 Yamaoka-Tojo M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Physical Activity; Reverse Remodeling; STEMI; Heart Failure Prevention
Download Fulltext PDF
Left Ventricular (LV) reverse remodeling after ST-Segment Elevation Myocardial Infraction (STEMI) reduces the incidence of re-hospitalization or death from heart failure or cardiovascular disease. The present study aimed to clarify whether Physical Activity (PA) level may serve as a predictor of LV reverse remodeling in post-STEMI out patients. Subjects were stable 113 patients with old myocardial infarction who had successful reperfusion following STEMI. PA was assessed by an accelerometer, which measured average step count and light-moderate and vigorous-intensity activity time per day for 1 week at baseline. Single Photon Emission Computed Tomography (SPECT) was used to measure myocardial perfusion and LV function at baseline and at 12 months. LV reverse remodeling was defined as a >10% reduction in LV end-systolic volume at 12 months relative to baseline SPECT. Patients were divided into LV reverse remodeling group (n = 57) and no-LV reverse remodeling group (n = 56). After adjusting for age, PA index, and baseline LV function, logistic regression analysis showed that moderate-intensity activity time was a significant and independent predictor of LV reverse remodeling (odds ratio, 2.091; 95% Confidence Interval [CI]: 1.476-2.967; p = 0.0001). Receiver operating characteristic curve analysis identified a cut-off value of moderate-intensity activity for 29 minutes per day as a predictor of LV reverse remodeling, with a sensitivity of 74% and specificity of 89% (area under the curve, 0.860; 95% CI: 0.789-0.931; p = 0.0001). In conclusion, time spent performing moderate-intensity activity as measured by accelerometer was found to be an independent predictor of LV reverse remodeling in post-STEMI patients with maintenance phase.
Introduction
Progression of Left Ventricular (LV) remodeling after Myocardial Infarction (MI) may lead to higher mortality rates and likelihood of developing Heart Failure (HF) [1,2]. In order to reduce rates of re-hospitalization and death due to HF after hospital discharge, ongoing disease management guidance should be provided to patients with Coronary Artery Disease (CAD). For this purpose, Physical Activity (PA) management serves as a cornerstone of secondary prevention and an integral part of a multifaceted, evidence-based approach to post-MI risk reduction. In particular, meta-analysis and randomized controlled trials have found that aerobic training is beneficial in post-MI or HF patients with LV remodeling [3-5]. Therefore, We hypothesized that daily habitual PA would effectively contribute to LV reverse remodeling in patients with post-MI. If specific target values could be set for PA levels to enable patients to achieve LV reverse remodeling, this would represent an extremely effective clinical educational method that could be used to maintain and improve patient motivation and adherence to disease management. Several studies have assessed predictors and cut-off values for LV reverse remodeling. However, to the best of our knowledge, no one has collected much insight on LV reverse remodeling within the chronic clinical settings. The present study was performed to evaluate whether or not PA level could serve as a predictor of LV reverse remodeling in post-MI patients.
Methods
We conducted a single-center, retrospective observational study, enrolling a total of 851 post-MI outpatients who underwent comprehensive risk management for the secondary prevention of Cardiovascular Disease (CVD) in as part of the Kitasato Registry for Cardiovascular Disease Prevention (KRCDP) from December 2002 to March 2014. Patients who have (1) any previous coronary artery bypass surgery, (2) severe congestive Heart failure (3) hemodialysis (4) severe peripheral artery disease, and (5) limited walking ability (i.e., dementia, low vision or blindness, orthopedic abnormalities, and paralysis due to stroke) were excluded. At the time of registration in the KRCDP program and at every annual visit, vital signs, body weight, blood sample analyses, Ankle Brachial Index (ABI), and Brachial Ankle Pulse Wave Velocity (baPWV) were examined in CAD patients. We also provided comprehensive guidance concerning exercise and diet based on these results, and stored those data in the database for KRCDP programs. Our study protocol required an investigation of clinical background factors including blood pressure, Heart Rate (HR), ABI, baPWV, blood sampling, and PA level at baseline. Myocardial perfusion Single-Photon Emission Computed Tomography (SPECT) was used to measure myocardial perfusion and LV function at baseline and 12 months later (follow-up). All patients gave informed consent before participating in the study. The present study design was approved by the Ethics Committee of Kitasato University Medical Ethics Organization.
We assessed clinical characteristics including age, sex, Body Mass Index (BMI), peak creatine kinase, peak creatine kinase-MB, coronary risk factors (hypertension, diabetes mellitus, and dyslipidemia), and medications. Hypertension was defined as Systolic Blood Pressure (SBP) ≥ 140 mmHg, Diastolic Blood Pressure (DBP) ≥ 90 mmHg, or current use of antihypertensive medications [6]. Diabetes mellitus was defined as fasting plasma glucose ≥ 126 mg/dL, hemoglobin A1c (HbA1c) ≥ 6.5%, or current use of medications for diabetes mellitus [7]. Dyslipidemia was defined as low-density lipoprotein cholesterol (LDL-C) ≥ 140 mg/dL, high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL, or current use of medication for dyslipidemia [8]. Obesity was defined as BMI ≥ 25 kg/m2, according to the criteria of the Japan Society for the study of Obesity [9]. Chronic Kidney Disease (CKD) was defined either as a kidney disorder (proteinuria, etc.) or as decreased kidney function with glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 lasting for 3 months or longer [10]. Estimated GFR (eGFR) is calculated using the following formula: eGFR (mL/min/1.73 m2) = 194×Cr-1.094 × Age-0.287 (multiply by an additional 0.739 for women).
PA index was measured with a Kens Lifecorder uniaxial accelerometer (LC; SUZUKEN CO., LTD., Nagoya, Japan). We used average daily number of steps, as well as the times for light-intensity, moderate-intensity, and vigorous-intensity activities performed per day over 7 days as the PA index [11,12]. Based on body weight and acceleration pattern, this device determines the time spent performing PA (min/day) of light (< 3 METs), moderate (3-6 METs), and vigorous (> 6 METs) intensity, as well as the step count (steps/day) [13]. All patients wore the accelerometer on a belt at waist level just above either leg for 24 hours a day for 8 days except while bathing and sleeping.
Based on electrocardiography-gated SPECT by 99mTc-tetrofosmin, LV function was analyzed using common nuclear cardiology software. The Quantitative Gated SPECT (QGS) algorithm was used to measure LV Ejection Fraction (LVEF), LV End-Systolic Volume (LVESV), and LV End-Diastolic Volume (LVEDV). LV reverse remodeling was defined as a >10% reduction in LVESV at follow-up compared with baseline SPECT [14].
Blood samples were collected after an overnight fast. Biochemical markers such as Hemoglobin (Hb), Creatinine (Cr), eGFR, Triglycerides (TG), LDL-C, HDL-C, HbA1c, and Brain Natriuretic Peptide (BNP) levels were measured.
Data are expressed as means ± standard deviation or as proportions. Student’s t test was used to compare continuous variables, and the Chi square test or Fisher’s exact test was used for categorical variables between groups at baseline. Furthermore, all follow-up data were analyzed by comparing them to baseline data. Differences between the groups and changes over time within each group (time effect), as well as any interaction (different trends over time between groups) and LV function were assessed by repeated measures 2-way ANOVA. To test the hypothesis that PA level serves as a predictor of LV reverse remodeling, all variables with a univariate association of P < 0.10 were included in the model for the multivariate analysis. Receiver operating characteristic (ROC) curves were constructed by plotting sensitivity and specificity to determine the best cut-off value for differences in these values between LV reverse remodeling and no LV reverse remodeling groups. Area Under The Curve (AUC) and 95% Confidence Intervals (CI) were also calculated. The cut-off value according to the ROC curves also allowed for a comparison of the rate of change in LVESV between the groups. All statistical analyses were performed using SPSS version 21.0 (SPSS, Japan, Tokyo, Japan). P< 0.05 was considered statistically significant.
Results
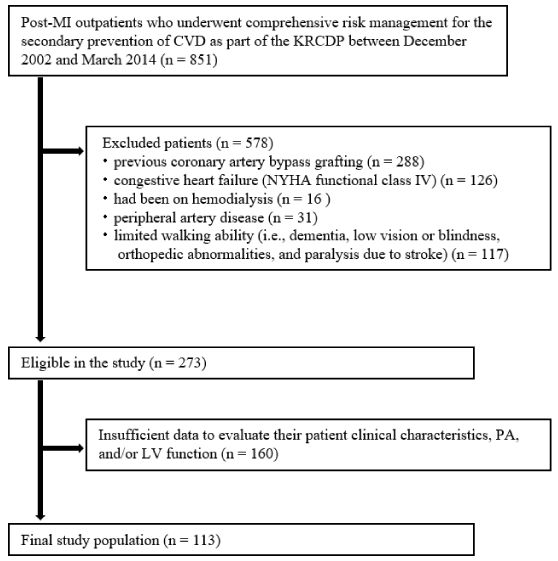
As shown in [Figure 1], among 851 patients, 578 patients fulfilled the excluded criteria. During the analysis, 273 patients were excluded because of insufficient data to evaluate clinical characteristics, PA, and/or LV function. Finally, 113 patients were included in the study.
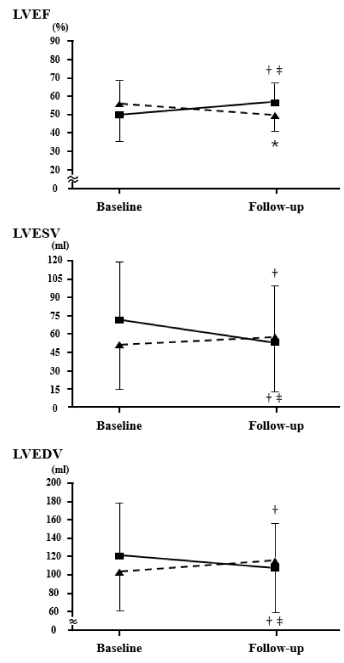
Clinical characteristics of the study population are shown in [Table 1]. Patients were categorized into 2 groups: LV reverse remodeling group (n = 57) and no LV reverse remodeling group (n = 56). The patients in the LV reverse remodeling group were significantly younger than those in the no LV reverse remodeling group. PA data are shown in [Table 2]. Step counts and times spent performing moderate- and vigorous-intensity PA were higher in the LV reverse remodeling group than tin those of the no LV reverse remodeling group. [Figure 2], shows changes in LV function by SPECT. At baseline, LVEF, LVESV, and LVEDV were similar in the LV reverse remodeling and no-LV reverse remodeling groups. At the point of the 1-year follow-up, LVEF was significantly higher in the LV reverse remodeling group than in the no LV reverse remodeling group. Both LVESV and LVEDV were lower in the LV reverse remodeling group than in those of the no LV reverse remodeling group.
Blood pressure, vascular function, and biochemical marker data are shown in [Table 3]. At the follow-up visit, SBP, DBP, HbA1c, HR, baPWV, and BNP were significantly lower in the LV reverse remodeling group than in those of the no LV reverse remodeling group.
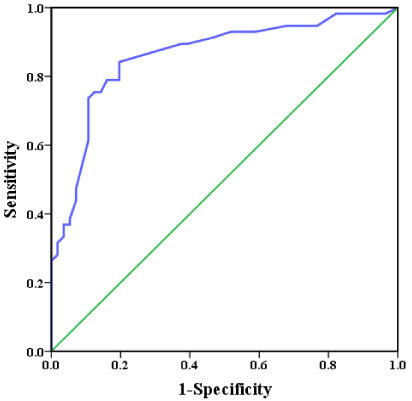
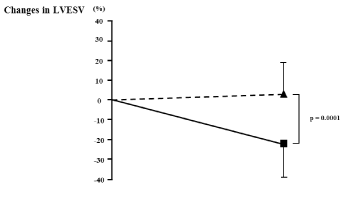
[Table 4] shows the results of the multivariate logistic regression analysis for LV reverse remodeling. Univariate predictors of LV reverse remodeling were age, step count, moderate- and vigorous-intensity activity times, and baseline LVEF, LVESV, and LVEDV. The multivariate model that included these variables identified moderate-intensity activity time as the strongest predictor of LV reverse remodeling. The ROC curve for moderate-intensity activity time as a predictor of LV reverse remodeling is shown in [Figure 3]. ROC curve analysis identified a cut-off value of 29 minutes/day as a predictor of LV reverse remodeling (74% sensitivity, 89% specificity; AUC = 0.860; 95% CI: 0.789-0.931; p = 0.0001). Rate of change in LVESV divided by the cut-off value is shown in [Figure 4]. Patients who achieved the targeted level of moderate-intensity activity time (29 minutes/day) had significantly lower rates of change in LVESV.Discussion
The major finding of the present study was that moderate-intensity activity time was a strong independent predictor of LV reverse remodeling among post-STEMI patients with chronic stable phase undergoing total risk management for secondary prevention of CVD.
Other studies have found that PA was an independent predictor of HF and CVD death [15,16]. High levels of PA associated with lower morbidity and mortality from CVD [17,18]. Therefore, PA management strategies can serve as a cornerstone for secondary prevention and an integral part of a multifaceted, evidence-based approach to reduce post-MI risks. It is also known that HF is caused by ventricular remodeling. However, few reports have investigated the association between PA and LV remodeling after MI. To the best of our knowledge, the present study was the first to show that PA at one time could be a predictor of 1-year later LV reverse remodeling in patients with post-STEMI in choronic phase of stable conditions.
The potential mechanism underlying the association between PA and LV reverse remodeling in post-STEMI patients remains unclear. Several studies have found that moderate-intensity aerobic training can effectively promote LV reverse remodeling after MI through distinct mechanisms, including improvements in coronary and peripheral vascular endothelial function, myocardial contractility, reduced systemic vascular resistance, reduced preload, adjustments in autonomic system function, reduction in HR and blood pressure at rest and in submaximal loads, and reduction in the LV systolic and diastolic wall stress [19-21]. Furthermore, moderate-intensity physical activity is also associated with increased vascular endothelial function and decreased blood pressure. Indeed, baPWV, SBP and HR were decreased at 1-year follow-up only in the LV reverse remodeling group. These phenomena favorably affect LV wall tension and may limit the deleterious effects on LV structure and function over time. This seems to be supported by the fact that BNP had declined at the time of follow-up only in the LV reverse remodeling group. We think that drug-related effects can be excluded, because drug treatment did not change in any of the patients during the study period.
Several studies of PA management have targeted HF patients and secondary prevention of CVD [15,16,22]. However, the target value of LV reverse remodeling for HF prevention was not clear. In the present study, ROC analysis identified a cut-off value of 29 minutes as the predictor of LV reverse remodeling post-STEMI. A meta-analysis and randomized controlled trials have found that aerobic training helped to prevent LV remodeling in post-MI and HF patients [3-5]. In those studies, subjects performed approximately 30 minutes of moderate-intensity aerobic training. Our study results are consistent with those from previous studies on both exercise intensity and time.
The American College of Sports Medicine and the European Society of Cardiology have stated that adults must perform moderate-intensity exercise for at least 30 minutes per day, 5 days per week in order to maintain optimal health.23,24 The physical activity intensity and time for achieving the LV reverse remodeling found by this study are considered to be safe as they comply with the prescribed exercise regimen specified in the guidelines. Furthermore, the PA index for the present study was measured with a uniaxial accelerometer. The pedometer with an accelerometer we used is easy to use and has a high reliability [25-27]. Therefore, the cut-off value determined here can be considered appropriate as a management target value to achieve LV reverse remodeling.
The present study has some limitations. First, because of the small sample size and retrospective observational study design, we were unable to match the patient backgrounds completely. Second, we could not investigate moderate-intensity activity time at 1-year follow-up, and thus were unable to examine the relationship between moderate-intensity activity time and LV reverse remodeling.
In conclusion, time spent performing moderate-intensity activity as measured by accelerometer was found to be an independent predictor of LV reverse remodeling in post-STEMI patients with chronic stable phase.
- Hutchins GM, Bulkley BH. Infarct expansion versus extension: two different complications of acute myocardial infarction. Am J Cardiol 1978; 41: 1127-1132. https://goo.gl/DV6Z8P
- St John Sutton M, Pfeffer MA, Moye L, Plappert T, Rouleau JL, Lamas G, et al. Cardiovascular death and left ventricular remodeling two years after myocardial infarction: baseline predictors and impact of long-term use of captopril: information from the Survival and Ventricular Enlargement (SAVE) trial. Circulation 1997; 96: 3294-3299. https://goo.gl/8WdKKu
- Haykowsky M, Scott J, Esch B, Schopflocher D, Myers J, Paterson I, et al. A meta-analysis of the effects of exercise training on left ventricular remodeling following myocardial infarction: start early and go longer for greatest exercise benefits on remodeling. Trials 2011; 12: 92. https://goo.gl/WLkt9y
- Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol 2007; 49: 2329-2336. https://goo.gl/NgN3Vw
- Chen YM, Li ZB, Zhu M, Cao YM. Effects of exercise training on left ventricular remodelling in heart failure patients: an updated meta-analysis of randomised controlled trials. Int J Clin Pract 2012; 66: 782-791. https://goo.gl/9ug52K
- Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009). Hypertension research: official journal of the Japanese Society of Hypertension 2009; 32: 3-107. https://goo.gl/489vP2
- Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Journal of diabetes investigation 2010; 1: 212-228. https://goo.gl/VmyW43
- Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, et al. Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan -2012 version. Journal of atherosclerosis and thrombosis 2013; 20: 517-523. https://goo.gl/RqCFcs
- Consultation WE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157-163. https://goo.gl/oKjZVi
- Ando Y, Ito S, Uemura O, Kato T, Kimura G, Nakao T, et al. CKD Clinical Practice Guidebook. The essence of treatment for CKD patients. Clinical and experimental nephrology 2009; 13: 191-248. https://goo.gl/mY2ZGS
- Matthews CE, Ainsworth BE, Thompson RW, Bassett DJ. Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc 2002; 34: 1376-1381. https://goo.gl/MnNCkL
- Clemes SA, Griffiths PL. How many days of pedometer monitoring predict monthly ambulatory activity in adults? Med Sci Sports Exerc 2008; 40: 1589-1595. https://goo.gl/L2u3sG
- Kumahara H, Schutz Y, Ayabe M, Yoshioka M, Yoshitake Y, Shindo M, et al. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: a validation study against whole-body indirect calorimetry. Br J Nutr 2004; 91: 235-243. https://goo.gl/U4vkGj
- Yu CM, Bleeker GB, Fung JW, Schalij MJ, Zhang Q, van der Wall EE, et al. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation 2005; 112: 1580-1586. https://goo.gl/yC7gfe
- Izawa KP, Watanabe S, Oka K, Hiraki K, Morio Y, Kasahara Y, et al. Usefulness of step counts to predict mortality in Japanese patients with heart failure. Am J Cardiol 2013; 111: 1767-1771. https://goo.gl/9CMJgV
- Walsh JT, Charlesworth A, Andrews R, Hawkins M, Cowley AJ. Relation of daily activity levels in patients with chronic heart failure to long-term prognosis. Am J Cardiol 1997; 79: 1364-1369. https://goo.gl/BxwdQL
- Hamer M, Chida Y. Walking and primary prevention: a meta-analysis of prospective cohort studies. British journal of sports medicine 2008; 42: 238-243. https://goo.gl/WxBy4s
- Sofi F, Capalbo A, Cesari F, Abbate R, Gensini GF. Physical activity during leisure time and primary prevention of coronary heart disease: an updated meta-analysis of cohort studies. Eur J Cardiovasc Prev Rehabil 2008; 15: 247-257. https://goo.gl/3UbU6N
- Ehsani AA, Biello DR, Schultz J, Sobel BE, Holloszy JO. Improvement of left ventricular contractile function by exercise training in patients with coronary artery disease. Circulation 1986; 74: 350-358. https://goo.gl/d9YAQo
- Belardinelli R, Georgiou D, Ginzton L, Cianci G, Purcaro A. Effects of moderate exercise training on thallium uptake and contractile response to low-dose dobutamine of dysfunctional myocardium in patients with ischemic cardiomyopathy. Circulation 1998; 97: 553-561. https://goo.gl/e8ZaQ8
- Vona M, Rossi A, Capodaglio P, Rizzo S, Servi P, De Marchi M, et al. Impact of physical training and detraining on endothelium-dependent vasodilation in patients with recent acute myocardial infarction. Am Heart J 2004; 147: 1039-1046. https://goo.gl/84Snta
- Ayabe M, Brubaker PH, Dobrosielski D, Miller HS, Kiyonaga A, Shindo M, et al. Target step count for the secondary prevention of cardiovascular disease. Circ J 2008; 72: 299-303. https://goo.gl/wukuJ3
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007; 116: 1081-1093. https://goo.gl/uNrsMo
- Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012; 33: 1635-1701. https://goo.gl/17rAHd
- Schneider PL, Crouter SE, Lukajic O, Bassett DR, Jr. Accuracy and reliability of 10 pedometers for measuring steps over a 400-m walk. Med Sci Sports Exerc 2003; 35: 1779-1784. https://goo.gl/Y5ygU3
- Schneider PL, Crouter S, Bassett DR. Pedometer measures of free-living physical activity: comparison of 13 models. Med Sci Sports Exerc 2004; 36: 331-335. https://goo.gl/KfJkGj
- Crouter SE, Schneider PL, Karabulut M, Bassett DR, Jr. Validity of 10 electronic pedometers for measuring steps, distance, and energy cost. Med Sci Sports Exerc 2003; 35: 1455-1460. https://goo.gl/tZa7e8

Sign up for Article Alerts