Intracoronary Imaging Isolates Type 3 Spontaneous Coronary Artery Dissection of the Left Main?
Naddi Marah1*, Muhammad Khan2 and Kumudha Ramasubbu2
1Department of Medicine, New York Presbyterian Brooklyn Methodist Hospital, Brooklyn, NY, 11215
2Division of Cardiology, New York Presbyterian Brooklyn Methodist Hospital, Brooklyn, NY, 11215
*Address for Correspondence: Naddi Marah, Department of Medicine, New York Presbyterian Brooklyn, Methodist Hospital, Brooklyn, NY, 11215; Tel: 718-780-5246; E-mail: [email protected]
Submitted: 06 May 2017; Approved: 28 May 2017; Published: 02 June 2017
Citation this article: Marah N, Khan M, Ramasubbu K. Intracoronary Imaging Isolates Type 3 Spontaneous Coronary Artery Dissection of the Left Main. Int J Cardiovasc Dis Diagn. 2017;2(1): 011-014.
Copyright: © 2017 Marah N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
Keywords
Spontaneous Coronary Artery Dissection (SCAD); Intramural Hematoma; Intravascular ultrasound (IVUS); Optical Coherence Tomography (OCT); Post-Partum; Fibromuscular Dysplasia (FMD)
Introduction
Spontaneous Coronary Artery Dissection (SCAD) is an often under diagnosed condition given its spectrum of clinical presentation, thus posing a significant diagnostic dilemma for clinicians. Pathologically, it occurs as a consequence of non-traumatic, non-iatrogenic separation of arterial walls, creating a false lumen with intramural hematoma (IMH) formation, which may compromise anterograde blood flow and cause ischemia [1]. First defined in 1931 via a post-mortem study, and arbitrarily labeled as a predominantly idiopathic condition, the recent advent and utilization of intracoronary imaging has increased the diagnostic yield of SCAD, highlighted multiple associated predisposing risk factors, and defined the disease subtypes.
Case Series
A 37-year-old woman 2 weeks postpartum, complicated by preeclampsia and gestational diabetes, who initially complained of substernal chest pain and dizziness, was found by the emergency medical services immediately after losing consciousness. On cardiac monitoring, she was in Ventricular Fibrillation (VF), and was successfully defibrillated with 200 Joules.
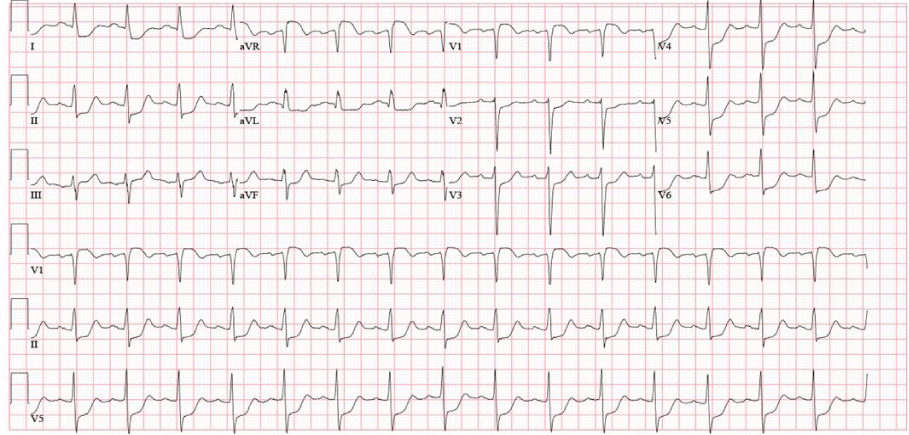
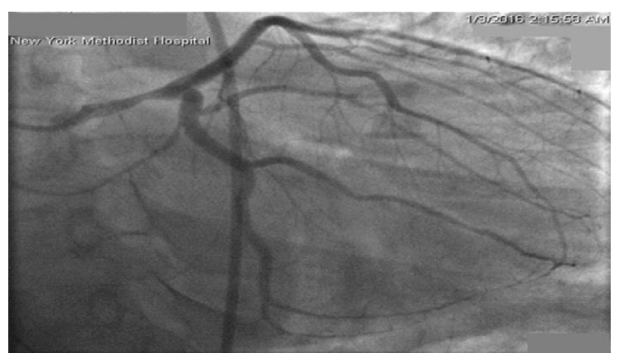
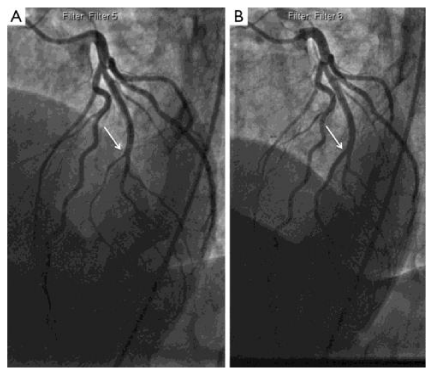
In the emergency room, she was hemodynamically stable with complaints of persistent substernal chest pain. Her initial electrocardiogram (ECG) showed sinus tachycardia without active ischemic changes. She developed progressive shortness of breath, with coarse crackles at bilateral lower lung fields, and significant jugular venous distension. She was subsequently intubated for acute hypoxemic respiratory failure due to cardiogenic pulmonary edema. A repeat ECG now showed ST segment elevations in leads V1andaVR, with extensive ST depression in the inferior and anterolateral leads (Figure 1). Emergent coronary angiogram showed a right dominant circulation, with 40% stenosis of the mid left main coronary artery (LM). Remaining coronary circulation shows no significant atherosclerotic disease and an Ejection Fraction (EF) was estimated at 25%, with a left ventricular end diastolic pressure of 32mmHg (Figure 2).
She was transferred to the cardiac ICU for further management. An ECG within an hour after catheterization showed resolution of the ST segment deviations. She was started on intravenous diuretics with improvement in hypoxemia and eventually extubated. Initial troponin I was 0.019 with a peak of 68 (normal range 0–0.05 ug/l) and peak creatinine phosphokinase of 313U/I (normal range 24–170 U/l). A follow up trans-thoracic echocardiogram within 24 hours showed an EF of 49%, moderate diffuse hypokinesia, with mildly increased wall thickness. Her clinical status improved significantly with guideline-directed heart failure therapy.
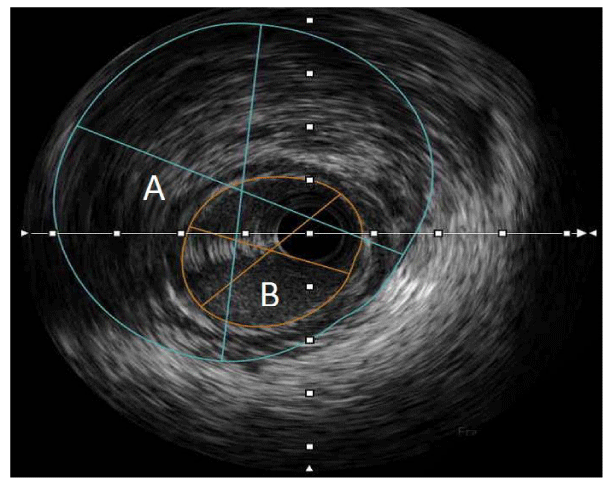
A Cardiac MRI revealed normalization of her EF to 65%, however with antero-septal hyper-enhancement consistent with a small, subendocardial infarct. With a newfound suspicion for a true ischemic event as the etiology of her cardiac arrest, a repeat coronary angiogram coupled with IVUS was performed, which revealed a plaque with IMH compromising the lumen of the mid segment of the LM, with a minimal luminal area of 6mm2 (Figure 3). Interestingly, no dissection plane was identified and the coronary circulation continued to show non-significant atherosclerotic disease. Based on these findings, a diagnosis of LM Type 3 SCAD with IMH formation was made. A consideration for Implantable Cardioverter Defibrillator (ICD) implantation for secondary prevention of Sudden Cardiac Death (SCD) was made, given her unique presentation with VF. She was discharged with a life vest as she deferred subcutaneous ICD placement. On post subsequent post -hospitalization follow up, she has remained asymptomatic and clinically euvolemic. A repeat TTE six months after presentation showed normal left and right ventricular function, no regional wall motion abnormalities, normal diastolic function, and no valvular disease. She is scheduled to undergo a nuclear stress test for surveillance of LM disease.
Discussion
SCAD could mimic an acute myocardial infarction with similar presenting symptoms, electrocardiographic findings, and serological elevation of cardiac biomarkers. In contrast to ACS, SCAD is notably a non-atherosclerotic entity, induced either by a tear in the intimal layer of the arterial wall or by rupture of the supporting vasa vasorum, with a subsequent separation between intima and media, or media and adventitia [2]. An IMH forms as blood accumulates extraluminally, compressing the vessel, and consequentially reducing anterograde blood flow. As a result, patients could develop myocardial ischemia, infarction, ventricular arrhythmias, or sudden cardiac death [3]. In the University of British Columbia (UBC) series, the largest SCAD registry to date, all cases presented with an elevation of troponin, 26% with STEMI, and a smaller percentage (3.6%) with ventricular arrhythmias [4].
Previously defined as an idiopathic condition of young women and identified primarily on post-mortem studies, advances in intracoronary imaging has enabled better understanding of the disease pathophysiology. The reported prevalence of SCAD may be as high as 8.7% in women presenting with ACS and10.8% in a subgroup presenting with ST segment elevation [5]. In the UBC series, the average age was 52.1+/- 9.2 years, with 58% presenting at age 50 years or older. In the same study, angiographic review identified SCAD in as high as 24% of women < 50 years of age who had an MI [4].
Postulated risk factors include fibromuscular dysplasia (FMD), connective tissue disorders, systemic inflammatory diseases, as well as pregnancy [6]. Of the 168 patients with SCAD in the UBC series, 72% were found to have FMD [4]. Given the infrequency of both conditions, a causal implication was suggested as the pathophysiological changes associated with FMD could cause weakened arterial walls, predisposing coronary arterial segments to dissection. Similarly, a retrospective study conducted at the Mayo Clinic that involved 200 patients, also found iliac FMD in 50% of femoral angiograms performed on SCAD patients [7].
Peripartum SCAD has gained significant interest in the recent years. The UBC series reported a rate of 3-8% of pregnancy related SCAD, with the highest frequency noted during the first post-partum month, with a peak in the second week [5]. The hormonal and hemodynamic changes that occur during pregnancy have been implicated in creating a weakened media due to impaired collagen synthesis, generating a hypercoagulable or prothrombic milieu, increasing the shear stress from augmented cardiac output and increased circulatory volume, with a cumulative risk of false lumen creation and thrombosis [8,9].
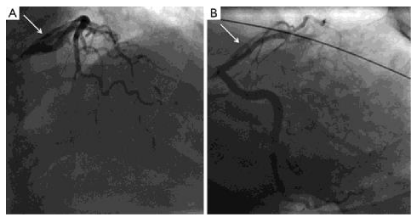
SCAD may involve any coronary artery, however it has been shown that the proximal, mid, and distal LAD are more frequently affected. In the Mayo series, the frequency of LM involvement as compared to other coronary branches was only1.2% [7]. With the emergence of intracoronary imaging, three distinct angiographic patterns of SCAD have been described. Type 1 illustrates the pathognomonic angiographic appearance of SCAD with contrast staining of both the arterial wall and false lumen of the dissected lesion (Figure 4). Type 2 involves stenosis of varying severity and usually affects the mid to distal segments of coronary arteries (Figure 5). Type 3, also known as angiographically silent SCAD, is notoriously the most challenging to differentiate from atherosclerotic disease and requires intracoronary imaging for definitive diagnosis. Angiographic features include the lack of atherosclerotic changes in other coronary arteries, longer lesions between 11-20 mm, hazy, and linear stenosis [6]. Interestingly, the UBC series reported that only 3.9% had Type 3 SCAD whereas an overwhelming majority (67%) had Type 2 [4]. It was also noted in this series that recurrent dissection occurred in 13.1% of cases, compared to the Mayo Clinic study which observed a rate of 17% [6].
Requiring a high index of suspicion for SCAD, angiographers must consider intracoronary imaging for definitive diagnosis. Conventional, gold standard angiographyis a 2-dimensional luminogram that can effectively depict luminal narrowing in Type 1 disease, but has been shown to be inferior for assessing arterial wall structure pathologies that predominates SCAD Types 2 and 3. Intracoronary imaging modalities include intravascular ultrasound (IVUS) and optical coherence tomography (OCT), which differ in their spatial resolution, and depth of penetration. IVUS has a lower spatial resolution but penetrates deeper, thus allowing for full vessel visualization. With this imaging modality, IMH appears as a homogenous collection behind the intima-media membrane. OCT, on the contrary, has a higher resolution with the capability to visualize true and false lumens, and intimal tears, but at the expense of poor penetration. In comparative studies, OCT has been shown to be more sensitive and better at detecting SCAD than IVUS, though both are able to identify IMH equally [10,11].
The proposed approaches to the management of SCAD have been met with significant controversy, given the scarcity of supportive data to serve as precedence. The choice between medical versus revascularization therapies depends on the clinical status of the patient and affected coronary vessel. In the Mayo series, all 79 patients were treated conservatively and demonstrated spontaneous angiographic healing at > 4 weeks following their event [7]. In the UBC study, an excellent clinical outcome was obtained after a series of 50 patients were treated with conservative management. There was no demonstrable in hospital mortality and only 4.8% of patients suffered a recurrent MI. Theoretically, reducing prothrombotic and shear stress burden on coronary vessels should confer a protective effect. Beta-blockers have been routinely administered for both acute and long-term management of SCAD. Extrapolating from the established benefits of dual anti-platelet agents in secondary prevention of CAD, aspirin and clopidogrel have been promoted in the acute phase of SCAD, with aspirin encouraged for life-long therapy. As the pathophysiology of SCAD entails dissection and IMH formation, the role of anti-coagulation and anti-thrombotic therapy has been debated for its risk of dissection extension. A retrospective study by Shamloo et al (2010) [12], looked at 440 cases of SCAD and reported that of the patients who received thrombolytic therapy, 60% consequently required percutaneous coronary intervention or Coronary Artery Bypass Grafting (CABG) to prevent progression of dissection [12]. PCI is preferred for patients with significant hemodynamic instability, dissected left main or proximal LAD segments.
For the smaller subset of patients presenting with cardiac arrest due to ventricular fibrillation or ventricular tachycardia, the main controversy remains the indication for ICD implantation for secondary prevention of SCD. Based on the 2012 ACCF/AHA/HRS guidelines for device-based therapy of cardiac rhythm abnormalities, in patients resuscitated from cardiac arrest, ICD is associated with clinically and statistically significant reductions in SCD and total mortality, with Class I and Level A evidence of support based on various large clinical trials. However, these accredited recommendations apply to patients with irreversible coronary artery disease, underlying structural heart disease, or severe systolic dysfunction [13]. To date, there is no level of evidence to support any class recommendation for ICD implantation for secondary prevention in patients with SCAD presenting with VF arrest, especially in patients with normalized cardiac function. With an overwhelming majority of patients showing a positive response to conservative medical therapy, Physicians are left to individualize device implantation therapy based on clinical judgment. Without much clinical evidence, the benefit of ICD placement for secondary prevention in VF SCAD remains unknown, and continues to be a topic of significant debate.
Conclusion
Spontaneous coronary artery dissection is an under diagnosed condition with a variable clinical presentation. An increase in disease prevalence is largely attributed to the coupling of conventional coronary angiography with intracoronary imaging including IVUS and OCT.
Retrospective angiographic studies have identified left main coronary disease in only a small percentage of cases. Amongst the subtypes, Type 3 is the rarest and most challenging of allto identify with conventional angiography alone. Intracoronary imaging has alleviated this diagnostic dilemma with a significant uptrend in detection rates. Treatment approaches continue to been heavily debated in the cardiovascular arena. There has been a trend towards conservative pharmacological therapy with dual anti-platelet therapy and beta-blockers, with PCI or CABG reserved only for hemodynamically unstable patients or subsets with left main or proximal LAD involvement. The benefit of ICD implantation remains unanswered.
Our case not only illustrates the unusual presentation of SCAD in a post-partum woman, it also highlights the infrequency of LM involvement, and the rarity of Type 3 disease presenting with left main STEMI, with spontaneous resolution on conservative pharmacological therapy.
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29: 1027-1033. https://goo.gl/WBCIIx
- Alfonso F. Spontaneous coronary artery dissection: new insights from the tip of the iceberg? Circulation 2012; 126: 667-670. https://goo.gl/kGte70
- Alfonso F, Bastante T. Spontaneous coronary artery dissection: novel diagnostic insights from large series of patients. CircCardiovascInterv 2014; 7: 638-641. https://goo.gl/oeF3Kw
- Saw J, Aymong E, Sedlak T, BullerCE, Starovoytov A, Ricci D, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. CircCardiovascInterv 2014; 7: 645-655. https://goo.gl/7qUGcG
- Vanzetto G, Berger-Coz E, Barone-Rochette G, Chavanon O, BouvaistH, Hacini R, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J CardiothoracSurg 2009; 35: 250-254. https://goo.gl/P150Zs
- Yip A, Saw J. Spontaneous coronary artery dissection - a review. CardiovascDiagnTher2015; 5: 37-48. https://goo.gl/JqV0X0
- Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126: 579-588. https://goo.gl/4t0sLp
- Asuncion CM, Hyun J. Dissecting intramural hematoma of the coronary artery in pregnancy and the puerperium. ObstetGynecol 1972; 40: 202-210. https://goo.gl/gxY18x
- Heefner WA. Dissecting hematoma of the coronary artery. A possible complication of oral contraceptive therapy. JAMA 1973; 223: 550-551. https://goo.gl/W8eA5s
- Paulo M, Sandoval J, Lennie V, Dutary J, Medina M, Gonzalo N, et al. Combined use of OCT and IVUS in spontaneous coronary artery dissection. JACC Cardiovasc Imaging 2013; 6: 830-832. https://goo.gl/xYv9Zw
- Auer J, Punzengruber C, Berent R, Weber T, Lamm G, Hartl P, et al. Spontaneous coronary artery dissection involving the left main stem: assessment by intravascular ultrasound. Heart 2004; 90: e39. https://goo.gl/baATW1
- Shamloo BK, Chintala RS, Nasur A, Ghazvini M, Shariat P, Diggs JA, et al. Spontaneous coronary artery dissection, aggressive vs. conservative therapy. J Invasive Cardiol 2010; 22: 222–228. https://goo.gl/rhCCiu
- Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, et al. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am CollCardiol 2013; 61: e6-e75. https://goo.gl/GKOPg6

Sign up for Article Alerts