Involvement of Protein-Protein Interactions of eNOS and Genetic Polymorphisms in Coronary Artery Disease?
Isabele Pereira Tannous1, Thaynara Gonzaga Santos1, Juliana Santana de Curcio1, Livia do Carmo Silva1, Amanda Alves de Oliveira1, Andreia Marcelino Barbosa2, Raisa Melo Lima1 and Kleber Santiago Freitas e Silva1*
1Biological Sciences Institute, Federal University of Goiás, Brazil
2Replicon, Pontifical Catholic University of Goiás, Brazil
*Address for Correspondence: Kleber Santiago Freitas e Silva, Institute of Biological Sciences, Federal University of Goiás, 74710-310, Goiania, Goias, Brazil, Tel/Fax: +556-232-820-744; ORCID ID: orcid.org/0000-0001-5350-7313; E-mail: [email protected]
Submitted: 13 October 2018; Approved: 27 October 2018; Published: 29 October 2018
Citation this article: Tannous IP, Santos TG, de Curcio JS, do Carmo Silva L, E Silva KSF, et al. Involvement of Protein-Protein Interactions of eNOS and Genetic Polymorphisms in Coronary Artery Disease. Int J Clin Cardiol Res. 2018;2(3): 066-071.
Copyright: © 2018 Tannous IP, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: eNOS; NOSTRIN; cdc37; Calmodulin 1; PPIs; Hot spots; Polymorphism
Download Fulltext PDF
Coronary Artery Disease (CAD) features anomalies such as angina, infarction and cardiac death, among others. Several risk factors are related to CAD onset and progression, the main ones are diabetes, sedentary lifestyle, high blood pressure, obesity, alcohol consumption, smoking and cholesterol. CAD is a globally serious health problem, affecting over 110 million people worldwide and leading to 9 million deaths each year. Endothelial dysfunction may be associated with genetic polymorphisms that take place in genes and proteins with essential roles in maintaining endothelial homeostasis. Among these genes, eNOS stands out, being responsible for the synthesis of nitric oxide, which is a lipophilic substance highly active in a great variety of physiological processes. NOSTRIN, cdc37 and calmodulin interact with eNOS and modulate its activity within endothelial cells in order to maintain vascular integrity and proper homeostasis. Dysfunction affecting any of those proteins, their structures or the PPI patterns they establish may increase CAD susceptibility. Here, we hypothesize that PPIs could be one of the explanations for CAD susceptibility. We have shown an in silico approach of interaction between eNOS and the CAD-related proteins NOSTRIN, cdc37 and calmodulin 1 through the identification of hot spots within the interface of interaction between eNOS and target proteins. We also pointed that several SNPs identified in clinical practice and deposited on dbSNP were related to the predicted hot spots and could increase susceptibility to CAD significantly due to alteration of eNOS and partner proteins conformation or loss of function due to impairment of the PPIs they perform. Further studies need to be performed and through the hot spots presented here, eNOS, NOSTRIN, cdc37 and calmodulin 1 could be used as molecular markers and peptides PPI modulator could be design and tested as alternative therapies against CAD progression.
Introduction
Coronary Artery Disease (CAD) comprises a series of anomalies, which includes angina, infarction and cardiac death, among others [1]. The main risk factors related to CADs include diabetes [2-5], sedentary lifestyle [6], high blood pressure [7], obesity [4], alcohol consumption [8], smoking [3] and cholesterol [5]. The onset of CADs occurs because the blood with oxygen and nutrients are unable to reach heart muscles due to fatty deposits inside the arterial walls [9]. CAD is a health problem worldwide, affecting over 110 million people around the globe and leading to 9 million deaths each year [10,11].
Some of the risk factors for CADS, such as, high blood cholesterol, hypertension, diabetes and smoking may lead to endothelial dysfunction, and consequently to a pro-inflammatory and a pro-thrombotic endothelial state [12,13]. Endothelial dysfunction may be associated with genetic polymorphisms that take place in genes and proteins with essential roles in maintaining endothelial homeostasis [14-17]. Among these genes, eNOS (endothelial Nitric Oxide Synthase) is responsible for the synthesis of nitric oxide, which is a lipophilic substance highly active in a great variety of physiological processes [18-21]. The nitric oxide produced by eNOS protein activity regulates the vascular tone [22], cell cycle progression [23,24], immune system cell adhesion [25,26] and platelet aggregation [27,28].
The eNOS protein comprises four main domains (Table 1) [29]. The N-terminal oxygenase domain is featured by heme-thiolate proteins and has a role in the activity of the protein. This family of hemoprotein contains a thiolate anion as the axial ligand to the heme group [30]. The flavodoxin-like domain has a Flavin Mononucleotide (FMN)-binding site, which is related to electron transfer reactions. This domain is important to enable the nitric oxide production, which has a role as a messenger molecule within the metabolism [31]. The FAD binding domain regulates the exchange of reducing equivalents between electron owners [32] and the NAD binding domain regulates the electron flux for ATP synthase and reducing power of cells undergoing active glycolysis [33].
Several other proteins influence endothelial metabolism and they interact with eNOS in order to complement its function and guarantee vascular stability. NOSTRIN (Nitric Oxide Synthase Trafficking) [34], cdc37 (cell division cycle 37) [35], calmodulin [36-39], GCDH (Glutaryl-CoA Dehydrogenase) [35] and SIRT 1 (sirtuin 1) [40] interact with eNOs in order to complement its function and those Protein-Protein Interactions (PPIs) regulate several vascular functions. Dysfunction affecting any of those proteins, their structures or the PPI patterns they establish may increase CAD susceptibility [41-44].
Here, we show the involvement of protein-protein interactions of eNOS and genetic polymorphisms in coronary artery disease by an in silico approach. We highlight hot spots residues on the interaction interface between eNOS and the proteins NOSTRIN, cdc37 and calmodulin 1 and polymorphisms that may alter structure, function and PPI patterns between them. The complex and dynamic metabolic processes engaged by those proteins may influence CADs onset, progression and prognosis.
Materials and Methods
Briefly, we used the I-TASSER server in order to model the eNOS protein. A series of templates were used in order to find homologous proteins available in the PDB (protein databank) and to build up a 3-D structure that represents a stable conformation of the protein. The most common eNOs, NOSTRIN, cdc37 and calmodulin 1 polymorphisms related to CAD were retrieved from dbSNP (the single nucleotide polymorphism database). The interaction interface was determined by the ClusPro server and all the 3-D structures were visualized and analyzed by PyMol (https://pymol.org/2/). Amino acid residues were classified into hot spots through the KFC2 server [45].
Results and Discussion
The interaction between eNOS and NOSTRIN
The main function of NOSTRIN is the intracellular trafficking of eNOS between different compartments. NOSTRIN catalyzes the detachment of eNOS from the plasma membrane and transport the protein to several cellular compartments [46]. eNOS trafficking is essential to maintain normal levels of nitric oxide within intracellular compartments. The stimulation of endothelial cells leads to the transportation of eNOS in vesicles via NOSTRIN activity [47] and by endocytosis [34].
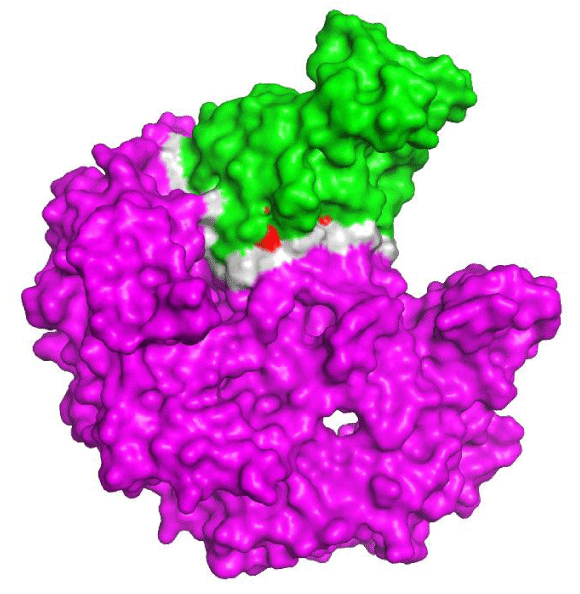
Figure 1 shows the interaction interface between eNOS and NOSTRIN through an in silico approach. The 3-D structure of NOSTRIN in figure 1 shows only the SH3 domain, which is the part of the protein that interacts with eNOS. Figure 1 also shows the hot spots predicted in the interaction interface. We found eight hot spots residues (Table 2) that contribute to the interaction between the proteins and also to the stabilization of the complex, as they are energetically favored residues [45]. Among those, five are polymorphic residues and may alter slightly the protein structure, the efficiency of interaction and finally disrupt eNOS transportation to intracellular compartments, reducing the availability of nitric oxide for the cell signaling and general metabolism. Several polymorphism linked to eNOs have been pointed as an increase to CAD susceptibility [15,48-50].
The interaction between eNOS and calmodulin 1
Calmodulin 1 belongs to the calcium-modulated protein family. Their localization is mainly in the cytosol or attached to membranes and they are regulated the levels of calcium in cells and tissues, taking part in processes such as cell cycle progression, growth, movement, and signal pathways. Calmodulin 1 also regulates eNOS activity in endothelial cells in the presence of cellular stressors. In addition, eNOS is more expressed if calcium is available, then the protein dissociates from membranes and is phosphorylated in the cytosol [51], leading to local vasodilatation [52]. The interaction between eNOS and calmodulin can be modulated by polypeptides, drugs and other small molecules [53]. This is important regarding CAD therapeutics and prognostic, once alterations in the binding energy between those proteins can increase susceptibility to atherosclerosis [54], for example.
Figure 2 shows the interaction between eNOS and calmodulin 1 binding motif. This interaction plays an important role in regulating eNOS activity as a response to cellular stress. Anomalies may rise when alterations within the coding sequence of any of the mentioned genes and proteins leading to onset of vascular diseases. We found seven hot spot residues (Table 3) in the interaction interface of eNOS and calmodulin. Many of those residues are polymorphic and susceptible to mutation; on the other hand, several of those possible SNPs (single nucleotide polymorphisms) are synonymous and have no effect in the coding sequence of eNOS or calmodulin 1. Figure 2 also shows a couple of hot spot residues on the interaction interface of eNOS and calmodulin. The design of modulator peptides is an alternative for treatment of CAD and the development of molecular markers to identify susceptible patients at earlier stages of the disease.
The interaction between eNOS and cdc37
The protein cdc37 is a chaperone with a role in signal transduction; it interacts with protein partners in order to stabilize their structure so they can perform their activities properly. This is essential for eNOS to be active and disruption or reduction of the interaction efficiency may lead to several diseases, including CAD [55,56], diabetes [57] and cancer [58]. Chaperones and cdc family proteins have been implicated in diseases related to inflammation (atheromatous plaques, for example) and immune response [59]. Genes coding for proteins related to inflammatory processes, such as eNOS and cdc37, are potentially candidates for biomarkers in order to assess the risk of CADs onset and progression. In addition, interactions of such genes could be modulated by synthetic or natural small molecules for therapeutics purposes [60-62].
We found ten hot spot residues (Table 4) on the interaction interface of eNOS and cdc37 (Figure 3) and among those residues, four are likely to carry SNPs and influence the susceptibility to CADs. The cdc37 protein regulates eNOS activity and prevent promiscuous eNOS activity [63]. Table 4 highlights the predicted hot spots and SNPs that may take place within this region, such alterations in either proteins, could lead to irregular binding or no binding at all and increase the predisposition to vascular diseases.
Concluding Remarks
CAD is costly and a complex public health problem around the globe. It is the disease with the highest rates of death worldwide. Family history of CADs is an indication for genetic counseling and genetic testing. Several genes such eNOS, NOSTRIN, cdc37 and calmodulin are potential candidates as genetic markers of vascular diseases. Knowledge on SNPs that increase susceptibility to CADs may shed new light on methods of prevention, diagnosis, treatment, genetic counseling and prognosis of CADs. PPIs are present in every compartment of a cell and they are responsible for a proper function of proteins, regulating cell cycle, cell homeostasis and disease onset and progression. Here, we hypothesize that PPIs could be one of the explanations for CAD susceptibility. We have shown an in silico approach of interaction between eNOS and the CAD-related proteins NOSTRIN, cdc37 and calmodulin 1 through the identification of hot spots within the interface of interaction between eNOS and target proteins. We also pointed that several SNPs identified in clinical practice and deposited on dbSNP were related to the predicted hot spots and could increase susceptibility to CAD significantly due to alteration of eNOS and partner proteins conformation or loss of function due to impairment of the PPIs they perform. Further studies need to be performed and through the hot spots presented here, eNOS, NOSTRIN, cdc37 and calmodulin 1 could be used as molecular markers and peptides PPI modulator could be design and tested as alternative therapies against CAD progression.
- Wong ND. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol. 2014; 11: 276-289. https://goo.gl/X9PQJm
- Aronson D, Edelman ER. Coronary artery disease and diabetes mellitus. Cardiol Clin. 2014; 32: 439-455. https://goo.gl/DNQUQX
- Ma X, Zhang J, Deng R, Ding S, Gu N, Guo X. Synergistic effect of smoking with genetic variants in the AMPKα1 gene on the risk of coronary artery disease in type 2 diabetes. Diabetes Metab Res Rev. 2014; 30: 483-488. https://goo.gl/z51Bbo
- Strissel KJ, Denis GV, Nikolajczyk BS. Immune regulators of inflammation in obesity-associated type 2 diabetes and coronary artery disease. Curr Opin Endocrinol Diabetes Obes. 2014; 21: 330-338. https://goo.gl/KGrUvS
- Hong L F, Yan X N, Lu Z H, Fan Y, Ye F, Wu Q, et al. Predictive value of non-fasting remnant cholesterol for short-term outcome of diabetics with new-onset stable coronary artery disease. Lipids Health Dis. 2017; 16: 7. https://goo.gl/fxX3Fn
- Lian X Q, Zhao D, Zhu M, Wang Z M, Gao W, Zhao H, et al. The influence of regular walking at different times of day on blood lipids and inflammatory markers in sedentary patients with coronary artery disease. Prev Med. 2014; 58: 64-69. https://goo.gl/jeeUx6
- Lieb W, Jansen H, Loley C, Pencina MJ, Nelson CP, Newton Cheh C, et al. Genetic predisposition to higher blood pressure increases coronary artery disease risk. Hypertension. 2013; 61: 995-1001. https://goo.gl/FYDeo8
- Matsumoto C, Miedema MD, Ofman P, Gaziano JM, Sesso HD. An expanding knowledge of the mechanisms and effects of alcohol consumption on cardiovascular disease. J Cardiopulm Rehabil Prev. 2014; 34: 159-171. https://goo.gl/mfV5Sz
- Lala A, Desai AS. The role of coronary artery disease in heart failure. Heart Fail Clin. 2014; 10: 353-365. https://goo.gl/R8BHgW
- GBD 2015 disease and injury incidence and prevalence collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016; 388: 1545-1602. https://goo.gl/aXMU85
- GBD 2015 Mortality and causes of death collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016; 388: 1459-1544. https://goo.gl/JGv7FT
- Sanchis Gomar F, Mayolas Pi C, Garatachea N. Inflammation and coronary artery disease: The exercise paradox. Cytokine. 2018; 111: 371-372. https://goo.gl/W5XHw1
- Voudris KV, Chanin J, Feldman DN, Charitakis K. Novel inflammatory biomarkers in coronary artery disease: potential therapeutic approaches. Curr Med Chem. 2015; 22: 2680-2689. https://goo.gl/sZ7QVK
- Jaafar NI, Vasudevan R, Ismail P, Abdul Aziz AF, Mohamad NA, Kandavello G, et al. Analysis of angiotensin converting enzyme, endothelial nitric oxide synthase & serotonin gene polymorphisms among atrial septal defect subjects with and without pulmonary arterial hypertension. J Cardiovasc Dev Dis. 2018; 5: 48. https://goo.gl/oVjJNN
- Barbosa AM, Silva KSF, Lagares MH, Rodrigues DA, da Costa IR, Morais MP, et al. Atherosclerosis: analysis of the eNOS (T786C) gene polymorphism. Genet Mol Res. 2017; 16. https://goo.gl/7GrtRH
- Shen Z, She Q. Association between the deletion allele of Ins/Del polymorphism (Rs145204276) in the promoter region of GAS5 with the risk of atherosclerosis. Cell Physiol Biochem. 2018; 49: 1431-1443. https://goo.gl/Jk996t
- Solim LA, Gencan IA, Çelik B, Ataacar A, Koç U, Buyukoren B, et al. Endothelial lipase gene polymorphism (584 c/t) in coronary artery patients among a turkish population. In Vivo. 2018; 32: 1105-1109. https://goo.gl/QaHpuo
- Yu L, Liu H. Perillaldehyde prevents the formations of atherosclerotic plaques through recoupling endothelial nitric oxide synthase. J Cell Biochem. 2018. https://goo.gl/4ZYNej
- Wan X, Liu P, Jin X, Xin X, Li P, Yuan J, et al. Electrospun PCL/keratin/AuNPs mats with the catalytic generation of nitric oxide for potential of vascular tissue engineering. J Biomed Mater Res A. 2018. https://goo.gl/YgwkyB
- Lipphardt M, Dihazi H, Muller GA, Goligorsky MS. Fibrogenic secretome of sirtuin 1-deficient endothelial cells: wnt, notch and glycocalyx rheostat. Front Physiol. 2018; 9: 1325. https://goo.gl/EVzuA9
- Hou H T, Wang J, Zhang X, Wang Z Q, Chen T N, Zhang J L, et al. Endothelial nitric oxide synthase enhancer AVE3085 reverses endothelial dysfunction induced by homocysteine in human internal mammary arteries. Nitric Oxide. 6 de outubro de 2018; 81: 21-27. https://goo.gl/usW55o
- Wu D, Hu Q, Zhu D. An update on hydrogen sulfide and nitric oxide interactions in the cardiovascular system. Oxid Med Cell Longev. 2018; 2018: 4579140. https://goo.gl/wch52Y
- Liao Q, Huang Y-M, Fan W, Li C, Yang H. Endothelial nitric oxide synthase deficiency influences normal cell cycle progression and apoptosis in trabecular meshwork cells. Int J Ophthalmol. 2016; 9: 799-803. https://goo.gl/g1vvgR
- Lo H W, Hung M C. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer. 2006; 94: 184-188. https://goo.gl/kFNyQm
- Kaminski A, Pohl CB, Sponholz C, Ma N, Stamm C, Vollmar B, et al. Up-regulation of endothelial nitric oxide synthase inhibits pulmonary leukocyte migration following lung ischemia-reperfusion in mice. Am J Pathol. 2004; 164: 2241-2249. https://goo.gl/Dm434j
- Santizo RA, Xu H-L, Galea E, Muyskens S, Baughman VL, Pelligrino DA. Combined endothelial nitric oxide synthase upregulation and caveolin-1 downregulation decrease leukocytse adhesion in pial venules of ovariectomized female rats. Stroke. 2002; 33: 613-616. https://goo.gl/kfLHq9
- Kader KN, Akella R, Ziats NP, Lakey LA, Harasaki H, Ranieri JP, et al. eNOS-overexpressing endothelial cells inhibit platelet aggregation and smooth muscle cell proliferation in vitro. Tissue Eng. 2000; 6: 241-251. https://goo.gl/JjEDDB
- Randriamboavonjy V, Fleming I. Endothelial Nitric Oxide Synthase (eNOS) in platelets: how is it regulated and what is it doing there? Pharmacol Rep. 2005; 57: 59-65. https://goo.gl/PThsjA
- Finn RD, Attwood TK, Babbitt PC, Bateman A, Bork P, Bridge AJ, et al. InterPro in 2017-beyond protein family and domain annotations. Nucleic Acids Res. 2017; 45: 190-199. https://goo.gl/BHJc1f
- Omura T. Heme-thiolate proteins. Biochem Biophys Res Commun. 2005; 338: 404-409. https://goo.gl/L5ZUbu
- Grandori R, Carey J. Six new candidate members of the alpha/beta twisted open-sheet family detected by sequence similarity to flavodoxin. Protein Sci. 1994; 3: 2185-2193. https://goo.gl/q5RJJA
- Karplus PA, Bruns CM. Structure-function relations for ferredoxin reductase. J Bioenerg Biomembr. 1994; 26: 89-99. https://goo.gl/ARw7y2
- Hyde GE, Crawford NM, Campbell WH. The sequence of squash NADH:nitrate reductase and its relationship to the sequences of other flavoprotein oxidoreductases. A family of flavoprotein pyridine nucleotide cytochrome reductases. J Biol Chem. 1991; 266: 23542-23547. https://goo.gl/6z9MHs
- Zimmermann K, Opitz N, Dedio J, Renne C, Muller Esterl W, Oess S. NOSTRIN: a protein modulating nitric oxide release and subcellular distribution of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2002; 99: 17167-17172. https://goo.gl/kK9irF
- Soler Lopez M, Zanzoni A, Lluis R, Stelzl U, Aloy P. Interactome mapping suggests new mechanistic details underlying Alzheimer’s disease. Genome Res. 2011; 21: 364-376. https://goo.gl/nKmonU
- Jung SB, Kim CS, Naqvi A, Yamamori T, Mattagajasingh I, Hoffman TA, et al. Histone deacetylase 3 antagonizes aspirin-stimulated endothelial nitric oxide production by reversing aspirin-induced lysine acetylation of endothelial nitric oxide synthase. Circ Res. 2010; 107: 877-887. https://goo.gl/uesgjU
- Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001; 88: 68-75. https://goo.gl/5bBRNW
- Piazza M, Futrega K, Spratt DE, Dieckmann T, Guillemette JG. Structure and dynamics of Calmodulin (CaM) bound to nitric oxide synthase peptides: effects of a phosphomimetic CaM mutation. Biochemistry. 2012; 51: 3651-3661. https://goo.gl/Uvq2S8
- Chen PF, Wu KK. Two synthetic peptides corresponding to the proximal heme-binding domain and CD1 domain of human endothelial nitric-oxide synthase inhibit the oxygenase activity by interacting with CaM. Arch Biochem Biophys. 2009; 486: 132-140. https://goo.gl/rGTi1y
- Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci U S A. 2010; 107: 10268-10273. https://goo.gl/fJVVPM
- Campedelli FL, E Silva KSF, Rodrigues DA, Martins JVM, Costa IR, Lagares MH, et al. Polymorphism of the gene eNOS G894T (Glu298Asp) in symptomatic patients with aterosclerosis. Genet Mol Res. 2017; 16. https://goo.gl/uaAQ55
- Mohler PJ, Hund TJ. Role for CaMKII in cardiovascular health, disease, and arrhythmia. Heart Rhythm. 2011; 8: 142-144. https://goo.gl/8qBydr
- Swaminathan PD, Purohit A, Hund TJ, Anderson ME. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res. 2012; 110: 1661-1677. https://goo.gl/LQzLdQ
- Zinnanti WJ, Lazovic J, Housman C, Antonetti DA, Koeller DM, Connor JR, et al. Mechanism of metabolic stroke and spontaneous cerebral hemorrhage in glutaric aciduria type I. Acta Neuropathol Commun. 2014; 2: 13. https://goo.gl/6X4xJ6
- Darnell SJ, Page D, Mitchell JC. An automated decision-tree approach to predicting protein interaction hot spots. Proteins. 2007; 68: 813-823. https://goo.gl/WkvdfC
- Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol. 2001; 280: 193-206. https://goo.gl/w3iR2K
- Thuringer D, Maulon L, Frelin C. Rapid transactivation of the vascular endothelial growth factor receptor kdr/flk-1 by the bradykinin b2 receptor contributes to endothelial nitric-oxide synthase activation in cardiac capillary endothelial cells. J Biol Chem. 2002; 277: 2028-2032. https://goo.gl/cAZ5uE
- Min BW, Na JY, Juhng SW, Park MS, Park JT, Kim HS. A polymorphism (G894T) in eNOS increases the risk of coronary atherosclerosis rather than intracranial atherosclerosis in Koreans. Acta Neurol Belg. 2010; 110: 255-262. https://goo.gl/b61nsA
- Yilmaz E, Mir S, Berdeli A. Endothelial Nitric Oxide Synthase (eNOS) gene polymorphism in early term chronic allograft nephropathy. Transplant Proc. 2009; 41: 4361-4365. https://goo.gl/zWpfSc
- Spoto B, Benedetto FA, Testa A, Tripepi G, Mallamaci F, Maas R, et al. Atherosclerosis and the Glu298Asp polymorphism of the eNOS gene in white patients with end-stage renal disease. Am J Hypertens. 2005; 18: 1549-1555. https://goo.gl/kBgGJt
- Jeerooburkhan N, Jones LC, Bujac S, Cooper JA, Miller GJ, Vallance P, et al. Genetic and environmental determinants of plasma nitrogen oxides and risk of ischemic heart disease. Hypertension. 2001; 38: 1054-1061. https://goo.gl/zjHb98
- Marsden PA, Schappert KT, Chen HS, Flowers M, Sundell CL, Wilcox JN, et al. Molecular cloning and characterization of human endothelial nitric oxide synthase. FEBS Lett. 1992; 307: 287-293. https://goo.gl/xXaY7A
- Salerno JC, Harris DE, Irizarry K, Patel B, Morales AJ, Smith SM, et al. An autoinhibitory control element defines calcium-regulated isoforms of nitric oxide synthase. J Biol Chem. 1997; 272: 29769-29777. https://goo.gl/Vvyytk
- Rafikov R, Fonseca FV, Kumar S, Pardo D, Darragh C, Elms S, et al. eNOS activation and NO function: Structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. J Endocrinol. 2011; 210: 271-284. https://goo.gl/Hq37hf
- Cengiz M, Yavuzer S, Kılıçkıran Avcı B, Yuruyen M, Yavuzer H, Dikici SA, et al. Circulating miR-21 and eNOS in subclinical atherosclerosis in patients with hypertension. Clin Exp Hypertens. 2015; 37: 643-649. https://goo.gl/b7tCdK
- Rathouska J, Nemeckova I, Zemankova L, Strasky Z, Jezkova K, Varejckova M, et al. Cell adhesion molecules and eNOS expression in aorta of normocholesterolemic mice with different predispositions to atherosclerosis. Heart Vessels. 2015; 30: 241-248. https://goo.gl/Xywi21
- Sheldon RD, Laughlins MH, Rector RS. Reduced hepatic eNOS phosphorylation is associated with NAFLD and type 2 diabetes progression and is prevented by daily exercise in hyperphagic OLETF rats. J Appl Physiol (1985). 2014; 116: 1156-1164. https://goo.gl/iV7S1U
- Smeda M, Kieronska A, Adamski MG, Proniewski B, Sternak M, Mohaissen T, et al. Nitric oxide deficiency and endothelial-mesenchymal transition of pulmonary endothelium in the progression of 4T1 metastatic breast cancer in mice. Breast Cancer Res. 2018; 20: 86. https://goo.gl/5v7hTw
- Sevin M, Girodon F, Garrido C, de Thonel A. HSP90 and HSP70: implication in inflammation processes and therapeutic approaches for myeloproliferative neoplasms. Mediators Inflamm. 2015; 2015: 970242. https://goo.gl/PjwCHs
- Han Y, Zhan Y, Hou G, Li L. Cyclin-dependent kinase 9 may as a novel target in downregulating the atherosclerosis inflammation (Review). Biomed Rep. 2014; 2: 775-779. https://goo.gl/y9D4z3
- Pan H, Palekar RU, Hou KK, Bacon J, Yan H, Springer LE, et al. Anti-JNK2 peptide-siRNA nanostructures improve plaque endothelium and reduce thrombotic risk in atherosclerotic mice. Int J Nanomedicine. 2018; 13: 5187-5205. https://goo.gl/dSLKza
- Guo Y, Yuan W, Yu B, Kuai R, Hu W, Morin EE, et al. Synthetic high-density lipoprotein-mediated targeted delivery of liver x receptors agonist promotes atherosclerosis regression. EBioMedicine. 2018; 28: 225-233. https://goo.gl/S62t3H
- Harris MB, Bartoli M, Sood SG, Matts RL, Venema RC. Direct interaction of the cell division cycle 37 homolog inhibits endothelial nitric oxide synthase activity. Circ Res. 2006; 98: 335-41. https://goo.gl/kJU53h



Sign up for Article Alerts