Renata Dessordi*
Department of Internal Medicine, Faculty of Medicine of RibeiraoPreto, University of São Paulo-FMRP/USP
*Address for Correspondence: Renata Dessordi. Endereco: Avenue Bandeirantes, 3900. Monte Alegre, 14049-900. Ribeirao Preto, Sao Paulo, Brazil, Tel: +55-16-3602 2466; Fax: +55-16-3602 2466; E-mail: re_dessordi@hotmail. com
Dates: Submitted: 08 March 2017; Approved: 01 May 2017; Published: 04 May 2017
Citation this article: Dessordi R. Biochemical Assessment of Bone Biomarkers in Diabetic Rats. J Res Diabetes Metab. 2017;3(1): 017-023.
Copyright: © 2017 Dessordi R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Diabetes; Supplementation; Bone Metabolism
Abstract
Diabetes Mellitus is a chronic disease characterized by a disorder of the metabolism of carbohydrates, lipids, and protein. Studies have shown that this illness deregulate the activity of osteoblasts that play a role in osteoporosis. Thus the objective this study was to evaluate the influence of diet supplemented with calcium, phosphorus, vitamin E and zinc in bone metabolism related biochemical parameters for 30 days. We evaluate the possible bone changes during the development of complications generated by diabetes for histological analysis and biochemical parameters, bone alkaline phosphatase, tartrate resistant acid phosphatase, total calcium, ionized calcium and phosphorus in the serum of diabetic rats. The study consisted of 30 animals divided into groups, being a diabetic group fed with rations without supplementation (DN; n = 10), another group fed with ration supplemented (DS; n = 10), and a third made up of control animals (CN; n = 10). From the analysis, it observed that there was no significant difference in phosphorus concentrations, total calcium, ionized calcium and magnesium in the serum of animals for all groups (p > 0.05). The alkaline phosphatase enzyme showed significantly higher levels for DN (390 U/I) and DS (592 U/I) animals compared with the CN group (p = 0,001). There was no significant difference in the enzyme tartrate-resistant acid phosphatase enzyme tartrate-resistant acid phosphatase for all groups (p = 2,41). The histological analysis showed that was not observed significant changes microscopically for the period of 5 days, and regarding the period of 30 days. Thus, the results suggest that supplementation with minerals in combination with vitamin E has not been able to improve the biochemical profile related to bone health and was not sufficient to preserve the integrity of the hematopoietic tissue.
Abbreviations
DN: Diabetic Animal Non-supplemented, CN: Control Animal, DS: Diabetic Animals Supplemented, DM: Diabetes Mellitus, AIN: American Institute of Nutrition, FAO: Bone Alkaline Phosphatase, TRAP: Tartrate Resistant Acid Phosphatase
Introduction
The role of nutrition in bone tissue development has been the focus of many studies to investigate the dietary components necessary for the proper maintenance of bone functions, as well as its proper development. The nutrients such as calcium, phosphorus, magnesium, vitamin D, fluoride, zinc, copper and boron are known to promote normal development of bone functions, improving the gain mass and strength throughout the development. Inadequate consumption of these nutrients or changes in your metabolism, increase their excretion, absorption losses due to the presence of disease, can lead impaired on bone structure and consequently the development of bone-related diseases like osteoporosis [1].
Osteoporosis is a systemic skeletal disease characterized by an increase in susceptibility to fractures. Most cases associated with post menopause or aging, but this disease can also develop as a result of any pathological situation [2]. Osteoporosis is most prevalent in postmenopausal women than in men, and approximately 200 million people around the world feature this disease.
Diabetes Mellitus (DM) is an example of a disease that can lead to the development of osteoporosis [3]. It characterized by a breakdown in the metabolism of carbohydrates, proteins and lipids, the high incidence and prevalence in the population, translating into a challenge for health systems [4].
The DM can negatively affect various parts of the body, such as the bones, muscles, retina, kidneys and the cardiovascular system. The effects of this disease in the bone cells are very complex, and several studies are currently being conducted to try to exploit the exact mechanisms through which the DM induces osteoporosis and consequently the increase in the index of bone fractures [5].
Hyperglycemia, a condition that involves various complications of diabetes, negatively regulates the proper operation of osteoblastic cells responsible for bone formation and positively controls the activity of osteoclastic cells that related to bone resorption. This condition that would facilitate the development process of osteoporosis and moreover can still cause dysfunction and failure of various organs [6-8].
The main effect in the pathogenesis of bone abnormalities in insulin deficiency is related to the bone formation. In experimental models of type 1, DM was found a decrease in the number of osteoblasts in bone remodeling, accompanied by a reduction in mineral content [6,9].
Diabetic patients are likely to have reduced levels of minerals that play important roles in bone metabolism, and moreover, due to complications caused by hyperglycemia can suffer a loss of bone health. Calcium and phosphorus are the primary components of bone and related to the maintenance of bone health. A suitable concentration of calcium and phosphorous is necessary to maintain skeletal integrity in humans and animals. A low intake of calcium is the most important nutritional factors involving osteoporosis and phosphorus are a regulator of bone formation and resorption inhibitor [10,11]. Zinc and magnesium are minerals that directly related to bone health being essential in the mineralization and bone formation and maintenance of normal parathyroid gland function and metabolism of vitamin D, respectively. Vitamin E performs better influences on the quality of bone health in older animals, because it is a potent biological antioxidant, protect against bone loss [12].
Thus, supplementation with essential nutrients for bone metabolisms such as calcium, phosphorus, magnesium, zinc and vitamin E may be an important factor in preserving the bone mass, suggesting have a nutritional and pharmacological function in the prevention and adjunct treatment of diabetic osteoporosis. Based on the importance that the nutrients discussed exercise on bone health, this study aimed to evaluate the influence of diet supplemented with calcium, phosphorus, vitamin E and zinc in bone metabolism related biochemical parameters.
Materials and Methods
Animals
Male Wistar rats, weighing between 200-220 g, kept in Animal testing lab at the University of Ribeirao Preto (UNAERP), received water ad libitum and 20 g of feed unsupplemented or 20 g dietary supplementation manufactured with 250% of calcium and phosphate, twenty times of vitamin E and 150 mg of zinc per kg feed, for a period of 30 days [12].
Animals were divided into three groups: a group of control animals fed with rations not supplemented (CN, n = 10) and two groups of experimental diabetic animals, one group received rations supplemented (DS, n = 10) and the other group received no rations supplemented (DN, n = 10), (Figure 1). The animals used in the study were 8 weeks old and during the experiment ten animals not survive.
 Figure 1: Division of experimental groups of animals treated with non-supplemented and supplemented ration. Diabetic animals (DN and DS) and control (CN).
Figure 1: Division of experimental groups of animals treated with non-supplemented and supplemented ration. Diabetic animals (DN and DS) and control (CN).
The animals treated and followed according to the recommendations of the Commission on Ethics in the use of Animals, according to the precepts of the law 11.794/2008 Resolution no 879/2008. The project approved by the Committee of ethical conduct in research Animal of UNAERP, registered under number 066/09.
Waste generated during procedures were discarded according to recommendations of ANVISA (RDC 306/2004), being the material forwarded to autoclaving and subsequently packaged in milky white bag with two-thirds of its capacity, identified with the inscription "anatomical parts of animals" and sent to final disposal.
Experiment
After fasting not water, ad libitum, for approximately 24 hours the animals received via the penile vein, streptozotocin (Sigma®) dissolved in sodium citrate tampon 0.01 M, pH 4.5, at a ratio of 40 mg/ kg.
The tenth day after the induction was considered day zero, this period being necessary for the installation of chronic experimental diabetes. We are found to be experimental diabetic animals who have blood glucose levels equal to or greater than 250 mg/ dl, associated with polyphagia, polydipsia and weight loss. The selected animals were housed in metabolic cages and the feed offered was weighed daily, a total of 20g of feed for each animal. The evaluation of the amount of supplement per day was performed according to AIN-93 [13].
Diabetics and control animals treated with special diets balanced and formulated in the laboratory following the rules established by the American Institute of Nutrition (AIN) in 1993, specifically for rodents maintenance [13].
The formulations developed as ingredients: cornflour, casein, soybean oil, salt mixture, vitamin mixture, choline and supplements: calcium, phosphorus, vitamin E and zinc. The feed components purchased from the supplier Rhoster® Commerce and Industry Ltd a/SP.
Only one of the formulations supplemented with 250% calcium and phosphorus, plus 20 times of vitamin E and 150 mg of zinc per kg of feed. Table 1 shows the formulation of the supplemented and non-supplemented feed ration.
Throughout the trial was held a biweekly monitoring of blood glucose levels and the body weight evolution of controls and diabetic animals. Blood glucose measured through blood glucose Monitor ADVANTAGE-BOEHRINGER MANNHEIM®, wherein 40 seconds is determined the level of glucose in mg/ dl and the weight on the scale Filizola® Star precision of 0,5g.
After the experimental period of 30 days, the animals were allowed in non-fasting water ad libtum between ten and twelve hours. After this period the animal was anesthetized by peritoneal route with Ketamine Agener 10% - Agener Union (0.1 ml / 0.1 kg / animal weight) more Dopaser - Realpet (0.05 mL / 0.1 kg / animal weight) and subjected to a laparotomy to collect approximately 4 mL of blood through the vein. The sample collected in a tube containing anticoagulant and centrifuged in a centrifuge Otma/ Presvac (RVT Model DCS-16, Brazil, 1996) at 2,500 rpm for ten minutes. The serum obtained used for the realization of biochemical measurements from which was determined the levels of phosphate, calcium, alkaline phosphatase and tartrate-resistant acid phosphatase. All measurements performed to ensure reproducibility of the results in triplicate.
Dosages of phosphorus and calcium
Determination of phosphorus concentration was conducted in serum samples from control and experimental groups of diabetic animals a volume of 10 µL. The method based on the reaction of phosphate present in the sample with the molybdate in an acid medium, with formation of the phosphomolybdate complex measured by spectrophotometry by reading the absorbance at 340 nm, RA50 in a biochemical analyzer (Bayer Diagnostics, Ireland, 1998). The white reaction was 1 ml of the reagent solution and as standard mixing 1 ml of the reagent solution and 10 πL of a phosphate standard solution at 5 mg/ dL [14,15]. The phosphate concentration in mg/dL obtained from the ratio of sample absorbance and standard multiplied by ten. The method for calcium measurement based on the reaction of the calcium present in the sample with arsenazo III, with the formation of a colored compound, is measured spectrophotometrically by reading the absorbance at 650 nm, RA50 in a biochemical analyzer (Bayer Diagnostics Ireland, 1998). ). The white reaction was 1 ml of the reagent solution, and as a standard and the mixture 1.0 ml of the reagent solution and 10 µL standard calcium solutions at 10 mg/ dl [16]. Calcium concentration in mg/ dl obtained from the ratio of sample absorbance and standard multiplied by ten.
Determination of enzyme activity
Bone alkaline phosphatase (FAO) present in the sample in an alkaline catalyzed transfer of the phosphate group to the 4-nitrophenyl 2-amino-2-methyl-1-propanol (AMP), releasing 4-nitrophenol. The catalyst concentration was determined from the rate of formation of 4-nitrophenol, whereas that the initial absorbance was determined at 650 nm using a biochemical analyzer RA50 (Bayer Diagnostics, Ireland, 1998). The reaction occurred after mixing 1 ml of the reagent solution containing 0.35 mol / L 2-amino-2-methyl-1-propanol, 2 mmol / L magnesium acetate, 1 mmol / L, zinc sulfate, 2mmol/L N-hydroxyethyl ethylenediamine triacetic acid, pH 10.4, 12 mmol / L 4-Nitrofenildphosphate and 20 µL of the sample, followed by agitation and insertion into a stirred and thermostatted cuvette port at 37°C. The initial absorbance was determined at 650 nm one Biochemical Analyzer RA50 (Bayer Diagnostics, Ireland, 1998), being simultaneously triggered the stopwatch and made new readings every minute for three minutes. One unit of enzyme defined as the amount of alkaline phosphatase which hydrolyses 1.0 mmol of 4-nitrophenyl phosphate per minute at 37°C.
For the determination of the enzyme tartrate resistant acid phosphatase it was added to the corresponding volume 200 µL of serum which was incubated at 37°C for 30 min with 0.8 ml p-nitrophenyl phosphate substrate (35 mg p-nitrophenylphosphate, cyclohexylamine salt). Four drops of pH 4.8 buffer / Tartaric Acid (0.25 M trisodium citrate /0.4 M tartaric acid, pH 4.8) added for the determination of acid phosphatase not inhibited by Tartaric Acid [18]. After the incubation period it was added to each tube, 5 ml of sodium hydroxide 0.1 M. The absorbance was read at a wavelength of 410 nm, RA50 in a biochemical analyzer (Bayer Diagnostics, Ireland, 1998). The phosphatase activity is proportional to the amount of p-nitrophenol newly formed. The white reaction was 0.8 ml p-nitrophenyl phosphate substrate (35 mg p-nitrophenylphosphate, cyclohexylamine salt) and 4 drops of pH 4.8 tampon solution (0.25 M trisodium citrate, pH 4.8) incubation at 37°C for thirty minutes, adding 5 ml of Sodium Hydroxide 0.1 M posteriorly.
Histological analysis of the femur
The left femur of control and experimental diabetic animals was extracted, and after free of all adherent soft tissue, was immersed immediately in 10% buffered formalin fixing solution, for a minimum period of 48 hours. After fixation, decalcification was performed through a 10% trichloroacetic solution for 24 hours. The specimens were then washed with tap water to remove acidic wastes and placed in a 4% sodium sulfate solution for one hour. After this procedure, the dehydration and diaphanization process was carried out using a Leica TP 1010® automatic tissue processor (Leica Instruments GmbH, Nussloch, Germany) containing twelve stations, ten Baker cups and two thermal paraffin bath mugs. The samples were bathed for a period of 12 hours in the following solutions: three alcohols (70%, 80% and 90%) for dehydration, three bouts of absolute alcohol, one alcohol / xylol bath (1:1). Then, the diaphanization process was started, bathing the samples three times in xylol and finally the inclusion of two baths of molten paraffin.
The fragment was then included in a paraffin block and sectioned on a Leica RM 2145® rotary microtome (Leica Instruments GmbH, Nussloch, Germany) in serial sections five micrometers thick, which were subsequently stretched in a histological bath at a temperature of 32°C and placed on glass slides. The slides were taken to the drying oven overnight at 60°C to remove excess paraffin, then stained with Hematoxylin-Eosin (HE) and a battery of successive baths (twenty-five steps) for staining with alcohols, xylol, water, hematoxylin-eosin.
Once the dehydration, diaphenization and coloring processes were completed, the coverslips were assembled with the permount resin and morphometry using a Nikon optical microscope, eclipse 600 using a 4x/0.06 eyepiece and a 10x/25 magnification objective, obtaining a final increase of forty times.
Histological analyzes of the femurs were performed with the collaboration of the Laboratory of Pathology of the University of Ribeirao Preto - UNAERP.
Estatistic
Exploratory analysis of data for an overview of the features of the variables was obtained, describing them through tables with descriptive measures. Median, minimum, and maximum statistics were calculated with a reliability rate of 95%. Statistical tests were performed to test the equality between groups.
We used the SPSS Statistics software version 22.0 for statistical analysis For a comparison between all groups, we used the two-way nonparametric ANOVA test with Tukey as a post hoc test. The statistical significance was defined as p ≤ 0.05.
Results
The main characteristic of Diabetes Mellitus is chronic hyperglycemia. Excess glucose can glycate proteins such as albumin, thus producing Reactive Oxygen Species (ROS). These species are capable of damaging cells in the body, such as osteoblasts and osteoclasts, which are cells involved in bone metabolism and are capable of altering bone mineral density. The dosage of glucose measurements shown in table 2. The analysis revealed differences in weight of the CN and DN animals compared to the DS group in the second measurement period, indicating significant weight loss of supplemented diabetic animals. During the last period of weight measurement, the difference was observed in the weight of animals DN and DS groups compared to CN, indicating a significant loss, frequent in chronic diabetic animals (p < 0.05). The glucose measurements revealed significant differences in all analysis period for DN and DS groups compared to the CN group.
The increase in the production of EROs the osteoblasts are attacked and large amount of the enzyme alkaline phosphatase (FAO) is released. The results found in this study point to an increase in FAO in the blood of diabetic animals fed with supplemented feed and in diabetic animals fed with non-supplemented ration, in contrast to non-diabetic animals, suggesting that diabetes is causing the production of reactive species causing injury to osteoblasts and freeing the FAO in circulation.
As bone cells act in equilibrium, if there is a decrease in the number of functional osteoblasts, the process of bone formation will be compromised, resulting in a more pronounced bone resorption by the osteoclasts.
Osteoclasts during their activity release the enzyme acid phosphatase tartrate resistant (TRAP), used as a marker of bone resorption. The increase in TRAP found in the diabetic group in the 30 day period, in contrast to the control animals suggest that there was a greater bone resorption in the diabetic groups.
Biochemical mineral dosages revealed no differences in any of the study groups. The dosage FAO showed significant differences between DN and DS groups compared to the CN group. No difference between groups for the determination of TRAP enzyme. The data presented in table 3.
The microscopic analysis of the left femurs of both periods had as objective to evaluate the epiphysis of the bone (trabecula and medullary space), the diaphysis (trabecula and medullary space) and the growth cartilage. The femurs of all the rats of the three groups and periods were analyzed. This analysis was based on an evaluation of the morphofunctional integrity, comparing the characteristics observed in the non-diabetic group of both periods with the non-supplemented and supplemented diabetics.
The group of non-diabetic animals from the 5-day period. (Figure 2) presented preserved medullary spaces (75%) and preserved growth cartilage (75%).
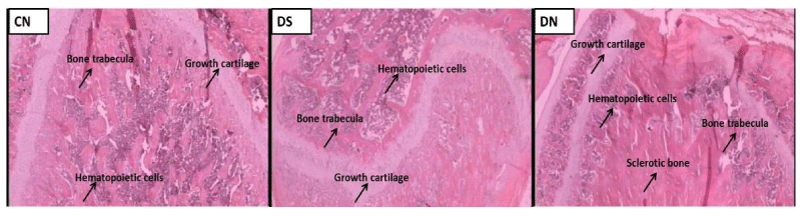 Figure 2: Microscopic image of the left femur of control animals - CN (n = 10), supplemented diabetics - DS (n = 10) and diabetics not supplemented - DN (n = 10) in the experimental period of 5 days. Dye: hematoxylin - eosin. 2x magnification
Figure 2: Microscopic image of the left femur of control animals - CN (n = 10), supplemented diabetics - DS (n = 10) and diabetics not supplemented - DN (n = 10) in the experimental period of 5 days. Dye: hematoxylin - eosin. 2x magnification
The non-supplemented diabetic group (Figure 2), corresponding to the 5-day period, showed that 55.5% had irregular growth cartilage, 55.5% presented sclerotic bone in the medullary space and 66.6% had irregular articular surface. In relation to the supplemented diabetic group (Figure 2), referring to the same period, it was observed that 54.5% was with growth cartilage with preservation characteristics similar to those of the control animals and in 54.5% of the animals there was a lack of trabecula well defined.
Regarding the 30-day period, non - diabetic animals presented preserved growth cartilage (87.5%) and well defined medullar spaces (87.5%) (Figure 3).
 Figure 3: Microscopic image of the left femur of control animals - CN (n = 10), supplemented diabetics - DS (n = 10) and diabetics not supplemented - DN (n = 10) in the experimental period of 30 days. Dye: hematoxylin - eosin. 2x magnification.
Figure 3: Microscopic image of the left femur of control animals - CN (n = 10), supplemented diabetics - DS (n = 10) and diabetics not supplemented - DN (n = 10) in the experimental period of 30 days. Dye: hematoxylin - eosin. 2x magnification.
The group of non-supplemented diabetic animals (Figure 3) presented lack preservation of growth cartilage (57.1%), presence of sclerotic bone in the medullary space (71.4%) and presence of adipose tissue (85.7%). Supplemented diabetic animals (Figure 3) showed a decrease in growth cartilage thickness (55.5%), but 88.8% had regular structure, 55.5% had increased trabecula and 66.6% had adipose tissue into the medullary spaces.
Discussion
This study investigated possible changes in the concentrations of trace minerals and enzymes bone alkaline phosphatase and tartrate-resistant acid phosphatase in control animals and supplemented and non-supplemented diabetic.
According to the literature, osteoporosis can develop from a pre-existing condition like DM. This disease leads to deterioration of bone microarchitecture, leading to insufficient strength and increased susceptibility to fractures [3,18]. Biweekly weight measurement and glucose enabled the monitoring of the evolution of the disease in diabetic animals. It observed that the animals of the diabetic group non-supplemented showed a reduction in blood glucose levels and the same was ascertained for DS group at a lower progression. The literature indicates that the induction of DM via streptozotocin leads to hypoinsulinemia and hyperglycemia. However, once it occurs a partial destruction of pancreatic β cells, the remaining cells can produce insulin, reducing blood glucose and consequently, this process can lead to cell regeneration [19]. The lower glucose levels in DN group resulted in less weight loss compared to the DS group in the second moment of blood glucose measurement.
In addition to the bone complications of chronic hyperglycemia caused by diabetes, diabetic patients are at increased risk of concentrations of vitamins and minerals decreased. Studies show that these patients have increased urinary excretion of some minerals such as calcium, phosphorus and zinc [20].
Aiming the improvement in bone complications, the animals of this study were supplemented with calcium, phosphorus, zinc and vitamin E.
The dosages of calcium and phosphorus accomplished in serum showed no significant differences in any study group. In the study of Torabi and cols [21], there was neither significant difference in the prevention of osteopenia nor improvement of growth with calcium and phosphorus supplementation. The circulating calcium remains in dynamic equilibrium, being 50% to 45 in ionic form, 40% to the fraction bound to proteins, mainly albumin and 10 to 15% bound to anions of low molecular weight. Magnesium, an essential mineral for bone health, showed no significant difference in their concentrations for both groups. Studies indicate that low concentrations of magnesium associated with lower bone mineral density, and its homeostasis is critical for the maintenance of bone [22,23].
Hyperglycemia resulting from the DM may predispose to the development of Reactive Oxygen Species (ROS) that can cause cell damage. Therefore, the combination of vitamin E with other minerals was performed aiming to achieve higher antioxidant properties [24]. The increased production of ROS can promote lesion in osteoblastic cells and consequently increase blood levels of FAO. The results of this study indicate an increase in FAO in both diabetic groups, showing injury of osteoblastic cells and, therefore, damaging to bone health. Osteoclasts are cells that have an opposite function in comparison with osteoblasts functions, handling bone resorption. During its activity, osteoclasts release the TRAP enzyme into the bloodstream, being an indicator of bone resorption [25]. The values for the enzyme were higher for the group DN. However, there was no statistically significant difference compared with CN and DS, suggesting that osteoclastic activity was similar for both groups.
The microscopic analyzes performed on the left femur of the animals, literature data suggest that sclerotic bone may appear due to increased osteoblastic and osteoclastic action in a particular bone region. The preservation of metaphyseal cartilage and the lower percentage of diabetic animals supplemented with sclerotic bone in the medullary space may suggest that supplementation based on minerals and vitamin E may have contributed to the preservation of some bone structures.
Another indication of changes in bone structure was the infiltration of adipose tissue indicating that degeneration of hematopoietic tissue is occurring. Therefore, supplementation based on minerals and vitamin E for the period of 30 days was insufficient to maintain the integrity of the hematopoietic cells. Note that the presence of adipose tissue in the medullar space is only observed after a period of experiment higher to 5 days.
From the results obtained it concluded that supplementation of minerals in combination with vitamin E proved ineffective in maintaining the integrity of osteoblastic cells by the values found in the FAO enzyme and the results found in the microscopic analysis of the femur.
Acknowledgements
The CNPq that funded this study.
Conflicts of interest
The authors declare no conflicts of interest.
Financial Support
CNPq.
References
- Ghanizadeh G, Babaei M, Naghii MR, Mofid M, Torkaman G, Hedayati M. The effect of supplementation of calcium, vitamin D, boron, and increased fluoride intake on bone mechanical properties and metabolic hormones in a rat. Toxicol Ind Health. 2014; 30: 211-217. https://goo.gl/sGNixK
- SorianoR, Herrera S, Nogues X. Current and future treatments of secondary osteoporosis. Best Pract Res Clin Endocrinol Metab. 2014; 286: 885-94. https://goo.gl/SUhbR6
- Lampropoulos CE, Papaioannou I, D´Cruz DP. Osteoporosis – a risk factor for cardiovascular disease? Nat. Rev. Rhemautol. 2012; 8: 587-598. https://goo.gl/wTYrGN
- Gao Y, Ordas R, Klein JD, Price SR. Regulation of caspase-3 activity by insulin in skeletal muscle cells involves both PI3- kinase and MEK-1/2. J. Appl. Physiol. 2008; 105: 1772-8. https://goo.gl/3vEoMq
- Jackuliak P, Payer J. Osteoporosis, fractures, and diabetes. Inter. Journ. Of Endocrin. 2014; 2014: 1-10. https://goo.gl/0JeGni
- Szulc P, Kaufman JM, Delmas PD. Biochemical assessment of bone turnover and bone fragility in men. Osteoporos Int. 2007; 18: 1451-1461. https://goo.gl/rZ08B5
- Pan HB, Zhao XL, Zhang X, Zhang KB, Li LC, Li ZY, et al. Strontium borate glass: potential biomaterial for bone regeneration. J. R. Soc. Interface. 2010; 7: 1025-1031.
- Gorustovich AA, Steimetz T, Nielsen FH, Guglielmotti MB. A histomorphometric study of alveolar bone modeling and remodeling in mice fed a boron-deficient diet. Arch. Oral Biol.2008; 53: 677-682. https://goo.gl/FFYM2n
- Hakki SS, Dundar N, Kayis AS, Hakki EE, Hamurcu M, Kerimoglu U, et al. Boron enhances strength and alters mineral composition of bone in rab- bits fed a high energy diet. J. Trace Elem. Med. Biol. 2013; 27: 148-153. https://goo.gl/uErFVJ
- Morais GQ, Burgos MGP. Impacto dos Nutrientes na Saude Ossea: Novas Tendencias. Rev. Brás. Ostorp. 2007; 42: 189-94. https://goo.gl/nAORnR
- Arjmanj BH, Juma S, Beharka A, Bapna M, Akhter M, Meydani S. Vitamin E improves bone quality in the aged but not in adult male mice. Journ of Nutr Bioch. 2002; 13: 543-549. https://goo.gl/wFVKds
- Reeves PG, Nielsen FH, George C, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American institute of nutrition ad hoc writing committee on the reformulation of the ain-76a rodent diet. Am Inst of Nutr. 1993: 0022-3166. https://goo.gl/LCEuJj
- Gamst O, Try K, Scand J. Determination of serum-phosphate without deproteinization by ultraviolet spectrophotometry of the phosphomolybdic acid complex. Clin Lab Invest. 1980; 40: 483-486. https://goo.gl/CqSP0Z
- Munoz MA, Balon M, Fernadez C. Direct determination of inorganic phosphorus in serum with a single reagent. Clin. Chem. 1983; 29: 372-374. https://goo.gl/sNRB6f
- Michaylova V, Illkova P. Photometric determination of micro amounts of calcium with Arsenazo III. Anal Chim Acta. 1971: 194-198. https://goo.gl/7unxov
- International federation of clinical chemistry (IFCC). Methods for the measurement of catalytic concentration of enzymes. J. Clin. Chem. 1983; 21: 633-46.
- Bessey OA, Lowry OH, Brock MJJA. Method for the Rapid Determination of Alkaline Phosphatase with Five Cubic Millimeters of Serum. Biol Chem. 1946; 164: 321-9. https://goo.gl/AJFg9B
- Chen H, Deng L, Li J. Prevalence of osteoporosis and its associated factores among older men with type 2 diabetes. Inter J. Endodrin. 2013; 2013: 1-9. https://goo.gl/p10NWo
- Jinzi W, Liang-jun Y. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab Syndr Obes. 2015; 8: 181-8. https://goo.gl/95NFQF
- Palacios C. The role of nutrients in bone health, from A to Z. Crit Rev Food Sci Nutr. 2006; 468: 621-8. https://goo.gl/YnLTQU
- Torabi Z, Moemeni N, Ahmadifshar A, Mazloomzadeh S. The effect of calcium and phosphorus supplementation on metabolic bone in premature infants. J. Pak Med. Assoc. 2014; 64: 635-9. https://goo.gl/oHyEQE
- Orchard TS, Larson TC, Alghothanin N, Bout-tabaku S, Cauley JA, Chen Z, et al. Magnesium intake, bone mineral density, and fracture: results from the Women´s Health Initiative observational study. Am. J. Clin. Nutr. 2014; 99: 926-33. https://goo.gl/T1UJ2s
- Castiglioni S, Cazzaniga A, Albisetti W, Maier JA. Magnesium and osteoporosis: current state of knowledge and future research direction. Nutrients. 2013; 5: 3022-33. https://goo.gl/EZfTKb
- Bhupinder K, Jeyakumar H. Micronutrient Status in Type 2 Diabetes: A Review. Adv Food Nutr Res. 2014; 71: 55-100. https://goo.gl/TqxYQx
- Zaitseva OV, Shandrenko SG, Veliky MM. Biomechal marker of bone collagen type I metabolism. Biochem. J. 2015; 87: 21-32. https://goo.gl/gLzLxi
Authors submit all Proposals and manuscripts via Electronic Form!




























