Case Report
Unexpected Origin of Infection in a Patient with Diabetic Ketoacidosis: Candida Esophagitis
Eray Eroglu, Zafer Parlak, Serkan Fatih Yegen, Davut Sanli, Ozgur Kilic, Ali Sonmezand Ismail Kocyigit
Eray Eroglu1,6, Zafer Parlak2, Serkan Fatih Yegen3, Davut Sanli4, Ozgur Kilic1, Ali Sonmez5and Ismail Kocyigit6
1Department of Internal Medicine, Elbistan State Hospital, Kahramanmaras, Turkey
2Department of Infectious Disease and Clinical Microbiology, Elbistan State Hospital, Kahramanmaras, Turkey
3Department of General Surgery, Elbistan State Hospital, Kahramanmaras, Turkey
4Department of Radiology, Elbistan State Hospital, Kahramanmaras, Turkey
5Department of Neurology, Elbistan State Hospital, Kahramanmaras, Turkey
6Division of Nephrology, Department of Internal Medicine, Erciyes University Medical School, Kayseri, Turkey
*Address for Correspondence: Eray Eroglu, Division of Nephrology, Department of Internal Medicine, Erciyes University Medical School, 38039, Kayseri, Turkey
Dates: Submitted: 24 November 2016; Approved: 28 December 2016; Published: 30 December 2016
Citation this article: Eroglu E, Parlak Z, Yegen SF, Sanli D, Kilic O, et al. Unexpected Origin of Infection in a Patient with Diabetic Ketoacidosis: Candida Esophagitis. J Res Diabetes Metab. 2016;1(1): 001-003
Copyright: © 2016 Eroglu E, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Diabetic Ketoacidosis; Candida Albicans; Esophagitis
Introduction
Diabetic Ketoacidosis (DKA) is an acute complication of Diabetes Mellitus (DM) that can be life-threatening. The basic underlying mechanism for DKA is insulin deficiency coupled with elevated levels of counter-regulatory hormones, such as glucagon, cortisol, catecholamine's, and growth hormone. The most common precipitating factor in the development of DKA is infection [1]. Other precipitating factors include discontinuation of insulin therapy or inadequate insulin therapy, myocardial infarction, cerebrovascular accident, pancreatitis and drugs [2,3]. The management of DKA includes: correction of dehydration, hyperglycemia and electrolyte imbalances, identification of comorbid precipitating events, and frequent patient monitoring [2].
Esophagitis is predominantly caused by noninfectious conditions, mainly gastroesophageal reflux disease [4]. Generally, infectious esophagitis is strongly associated with immunodeficiency including human immunedeficiency virus infection, malignancies, chemotherapy and malnutrition. The mostly seen pathogen is Candida among infectious esophagitis. Candida esophagitis is being increasingly recognized in the practice of clinical gastroenterology with the frequent use of endoscopy for the evaluation of esophageal symptoms. The main clinical features of Candida esophagitis are odynophagia and dysphagia. However, gastrointestinal bleeding may rarely be the only presenting symptom [5,6].
The high frequency of Candida infections in patients with diabetes mellitus has been well identified for many years. Especially, patients with poorly controlled diabetes are susceptible to the colonization of Candida species [7]. Since both neutrophil chemotaxis and phagocytosis decreased in patients with DM, uncontrolled DM may be considered to be immunocompromised hosts [8,9].
Herein, we present a poorly controlled diabetic patient presenting with diabetic ketoacidosis and resistance to insulin therapy with nausea, vomiting and dysphagia due to Candida esophagitis without any other infections.
Case report
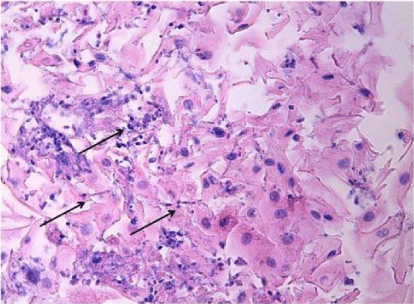
A 54-year-old man from a rural area was admitted to the emergency department of our state hospital with nausea, vomiting, dysphasia, abdominal pain, weakness and drowsiness. He had a history of diabetes mellitus for ten years and he had been under insulin therapy for the last two years. He reported that he was not in compliance with adequate insulin therapy. He was fully oriented but tended to fall asleep. Clinical examination revealed that a dehydrated and cachectic male with a heart rate of 114 beats/ min, temperature of 37�C and a blood pressure of 90/50 mmHg. He was tachypneic and dyspneic with a respiratory rate of 28 breaths/ minute. The patient's skin was hot and dry, pupils were isochoric and he had no focal neurological deficit. Lung auscultation was normal and the abdomen was soft and non-tender; bowel sounds were present in 2 of the 4 quadrants. His body mass index was 17.6 kg/ m2. Laboratory investigation revealed a metabolic acidosis with arterial blood pH of 7.29 and bicarbonate of 16.6 mmol/ L. Arterial pO2 and pCO2 levels were 92 mm Hg and 30 mm Hg, respectively. Plasma glucose was 1140 mg/ dL. 2+ ketones and 4+ glucose were present in the dipstick urine test. There were no erythrocyte and leukocyte casts in the microscopic evaluation of the urine. Blood urea level was 178 mg/ dL and serum creatinine level was 2.37 mg/ dL. Serum sodium level was 133 mmol/ L and potassium was 4.2 mmol/ L, cardiac enzymes, serum amylase and lipase level was normal. Liver function tests were within normal range. Complete blood count showed an elevated white blood cell count of 16.400 mm3/ �L with a 90% rate of neutrophil. High-sensitive C-reactive protein (hs-CRP) was 7.11 mg/ dL. Both urinary and blood cultures were taken. Abdomen ultrasonography was reported to be normal findings. There were no any sign of pneumonia and urinary infection. Serum hemoglobin A1c level was 12.1%. The patient was diagnosed as diabetic ketoacidosis with acute kidney failure. Intravenous fluid and insulin treatment were started promptly. On the third day, metabolic acidosis was resolved, urinary ketone was negative and the renal function was normal with the creatinine level of 0.9 mg/ dl. However, nausea, vomiting and dysphagia have been continued and insulin requirement was still at high levels with 120 unit/ day to control the blood glucose level. Serum hs-CRP level was still high with a level of 6.99 mg/ dL. Both urinary and blood cultures were found as negative. Hepatitis and HIV tests were all negative. Upper gastrointestinal endoscopy performed to evaluate the resistant nausea, vomiting and dysphagia. The endoscopy was showed the thrush lesions in the distal esophagus (Figure 1). The biopsy was taken for the diagnosis. The gram staining showed yeasts. Treatment with fluconazole (200mg/12hours) was started intravenously. The culture reported the Candida albicans and the pathologic examination of the biopsy specimen demonstrated the Candida esophagitis in the light microscopy with hematoxylin and eosin staining (Figure 2). On the 3rd day of fluconazole treatment, nausea, vomiting and dysphasia were ceased. His glucose level was normalized between 100-140 mg/ dL with total 36 unit/ day (3*8 pre-meal, 1*12 basal) insulin dosages. On the 7th day of the fluconazole treatment the patient was discharged from hospital with oral fluconazole (200 mg / 12hours) for seven days. 14th day of the fluconazole treatment hs-CRP level was 0.3 mg/ dL and there was no complaint, thus fluconazole treatment was stopped. Three months later his hemoglobinA1c level was 7.5%.
Discussion
Diabetic ketoacidosis is an acute and potentially mortal complication of diabetes mellitus, sometimes it could be the first manifestation of the disease [1]. Although it has been repeatedly shown that infection is a common precipitating event in DKA [1,2], the omission of insulin or inadequate treatment with insulin may be the most important precipitating factor [9,10]. Moreover, acute medical illnesses involving the cardiovascular system (myocardial infarction, stroke) and gastrointestinal tract (bleeding, pancreatitis), diseases of the endocrine axis (acromegaly, Cushing's syndrome), and stress of recent surgical procedures may contribute to the development of DKA by an increase in insulin counter-regulatory hormones, causing dehydration, and worsening of peripheral insulin resistance. Other factors that can contribute to DKA include psychological problems, eating disorders and illegal sub-stance use [11,12]. The initial management of DKA consists of fluid and electrolyte therapy, insulin therapy, treatment of precipitating causes, the monitoring of therapy and complications.
Urinary tract infection and pneumonia are the most common infections in patients with DKA [1]. Otherwise, fungal infection is commonly seen in patients with DM. These infections involve skin, respiratory system and also genitourinary system [13]. Additionally, some specific types of fungal agents such as zygomycosis have been reported in many diabetic cases [14]. Piziak, et al. [15] reported a case with Candida sepsis due to urinary colonization manifested by DKA. Dooley, et al. [16] have reported a diabetic patient with Candida albicans sinusitis as a potential reason for DKA. Cox, et al. [17] reported oral candidiasis in a patient with DKA. Additionally, there are few case reports in the literature with Candida osephagitis and diabetic ketoacidosis [18-20].
Infectious esophagitis is associated with in patients with impaired immunity such as AIDS, malignancy and chronic steroid therapy. The mostly seen pathogen is Candida among infectious esophagitis [4,5]. Uncontrolled DM may be considered to be immunocompromised hosts [9,15]. Granulocyte chemotaxis and phagocytosis are decreased, and granulocytes reportedly have a decreased ability to kill Candida [8]. It has been shown that candida colonization is increased in patients with DM [9]. Our patient had uncontrolled DM and he was from rural area which made him vulnerable to fungal infection. The development of DKA in this patient can be explained by his non-compliance in insulin therapy. Candida esophagitis may simply occur because of his poorly controlled diabetes mellitus.
The salient clinical features of Candida esophagitis include odynophagia and dysphagia, although gastrointestinal bleeding may occasionally be the sole presenting symptom [5]. The diagnosis of Candida esophagitis is usually made when white mucosal plaque-like lesions are seen on endoscopy. Confirmatory biopsy shows the presence of yeast and pseudohyphae with invasion of mucosal cells. This kind of esophagitis requires systemic antifungal therapy [21]. Intravenous therapy may be required initially in patients with severe disease if the patients could not take oral therapy. In our patient, dysphasia was resolved, and serum glucose level was normalized on the 3rd day of the systemic fluconazole therapy.
In conclusion, continuation of the high serum glucose level with nausea, vomiting and dysphagia despite the resolution of metabolic acidosis could be a clue for the esophagitis in patients with DKA. Physicians should be alert to fungal infections in patients with DKA because a delay in diagnosis may be fatal.
Figure 1
Upper gastrointestinal endoscopy images illustrate the white colored plaques in distal Esophagus of the patient.
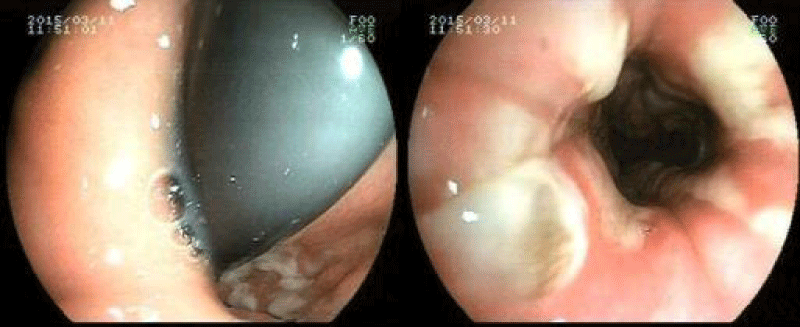
Figure 2
Biopsy specimen of the distal esophagus indicates the Candida hyphae and spores in the light microscopy (Hematoxylin and Eosin stain *400).
References
- Eledrisi MS, Alshanti MS, Shah MF, Brolosy B, Jaha N. Overview of the diagnosis and management of diabetic ketoacidosis. Am J Med Sci. 2006; 331: 243-51.
- Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2006; 29: 2739-2748.
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009; 32:1335-43.
- Wilcox CM. Overview of infectious esophagitis. Gastroenterol Hepatol (N Y). 2013; 9: 517-9.
- Mathieson R, Dutta SK. Candida esophagitis. Dig Dis Sci. 1982; 28: 365-70.
- Baehr PH, McDonald GB. Esophageal infections: risk factors, presentation, diagnosis, and treatment. Gastroenterology. 1994; 106: 509-32.
- Chandler PT, Chandler SD. Pathogenic carrier rate in diabetes mellitus. Am J Med Sci. 1977; 273: 259-65.
- Raith L, Csat� M, Dobozy A. Decreased Candida albicans killing activity of granulocytes from patients with diabetes mellitus. Mykosen. 1983; 26: 557-64.
- Wilson RM, Reeves WG. Neutrophil phagocytosis and killing in insulin-dependent diabetes. Clin Exp Immunol. 1986; 63: 478-84.
- Umpierrez GE, Kelly JP, Navarrete JE, Casals MMC, Kitabchi AE. Hyperglycemic crises in urban blacks. Arch Intern Med. 1997; 157: 669-675.
- Musey VC, Lee JK, Crawford R, Klatka MA, McAdams D, Phillips LS. Diabetes in urban African Americans: cessation of insulin therapy is the major precipitating cause of diabetic ketoacidosis. Diabetes Care. 1995; 18: 483-489.
- Kitabchi AE, Nyenwe EA. Hyperglycemic crises in diabetes mellitus: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinol Metab Clin North Am. 2006; 35: 725-751.
- Poradzka A, Jasik M, Karnafel W, Fiedor P. Clinical aspects of fungal infections in diabetes. Acta Pol Pharm. 2013; 70: 587-96.
- di Coste A, Costantino F, Tarani L, Savastano V, Di Biasi C, Schiavi L, et al. Rhinocerebral zygomycosis with pansinusitis in a 14-year-old girl with type 1 diabetes: a case report and review of the literature. Ital J Pediatr. 2013; 10: 77.
- Piziak VK, Carpentier W. Candida sepsis manifested by recurrent diabetic ketoacidosis. Diabetes Care. 1987; 10: 784-5.
- Dooley DP, McAllister CK. Candidal sinusitis and diabetic ketoacidosis. A brief report. Arch Intern Med. 1989; 149: 962-4.
- Cox DT, Allen CM, Plouffe JF. Locally invasive oral candidiasis mimicking zygomycosis in a patient with diabetic ketoacidosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.1996; 81: 70-3.
- Kim YH, Choi SY. Black esophagus with concomitant candidiasis developed after diabetic ketoacidosis. World J Gastroenterol. 2007;13: 5662-3.
- Takasawa H, Takahashi Y, Abe M, Osame K, Watanabe S, Hisatake T, et al. An elderly case of type 2 diabetes which developed in association with oral and esophageal candidiasis. Intern Med. 2007; 6: 387-90.
- Gupta A, Attar BM, Kotwal V. Hematemesis in a patient with diabetic ketoacidosis and chronic HCV infection. Gastroenterology. 2013; 145: 292, 491.
- Darouiche RO. Oropharyngeal and esophageal candidiasis in immunocompromised patients: treatment issues. Clin Infect Dis. 1998; 26: 259-72.
Authors submit all Proposals and manuscripts via Electronic Form!




























