Research Article
Time Trends in Complications of Prematurity and Respiratory Support 2002-2010 in Norway: Use of a National Patient Registry
Anne Lee Solevåg2*and Inger Cathrine Kann1
1The Health Services Research Centre H⊘KH, Norway
2The Department of Pediatric and Adolescent Medicine, Akershus University Hospital, Norway
*Address for Correspondence: Anne Lee Solevåg, The Department of Pediatric and Adolescent Medicine, Akershus University Hospital, 1478 LØrenskog, Norway
Dates: Submitted: 05 August 2016; Approved: 12 December 2016; Published: 03 January 2017
Citation this article: Solevåg AL, Kann IC. Time Trends in Complications of Prematurity and Respiratory Support 2002-2010 in Norway: Use of a National Patient Registry. Open J Pediatr Neonatal Care. 2016;2(1): 001-007
Copyright: © 2017 Solevåg AL, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Aim: To describe time trends in complications and respiratory support in Norwegian preterm infants 2002-2010. To discuss strengths and limitations of using a national patient registry in epidemiological research.
Methods: A total population study using data from The Norwegian national patient registry (NPR) 2002-2010. Temporal changes in Respiratory Distress Syndrome (RDS), Bronchopulmonary Dysplasia (BPD), Retinopathy of Prematurity (ROP), Intraventricular Hemorrhage (IVH), Necrotizing Enterocolitis (NEC), in-hospital mortality and respiratory support were measured in multivariate logistic regressions using 2002 as reference year and adjusting for potential confounders.
Results: The odds ratio (OR) of RDS increased 65% from 2002-2010 (p<0.001), whereas the OR of BPD decreased 52% (p<0.001). The OR of ROP and IVH decreased and then increased again. NEC and in-hospital mortality did not change. Use of mechanical ventilation decreased and continuous positive airway pressure increased slightly.
Conclusion: The time trends in RDS and BPD cannot be explained by changes in birth weight, gestational age and multiple birth. Changes in registration practices might be an explanation and the results should be interpreted with caution. The total population character with a high number of patients represents a strength of our study. NPR data can be used for generation of hypotheses to be further explored.
Abbreviations
RDS: Respiratory Distress Syndrome ; NPR: The Norwegian Patient Registry; GA: Gestational Age; CPAP: Continuous Positive Airway Pressure; ROP: Retinopathy of Prematurity; NICU: Neonatal Intensive Care Unit; SGA: Small for Gestational Age; BPD: Bronchopulmonary Dysplasia; IVH: Intraventricular Hemorrhage; NEC: Necrotizing Enterocolitis; PDA: Patent Ductus Arteriosus
Introduction
Preterm birth, i.e. prior to 37 completed weeks of gestation is the second leading cause of infant mortality in the industrialized world after congenital anomalies [1]. Improved prenatal and obstetric care has together with advances in the care of the newborn infant contributed to increased survival rates the last decades, particularly for infants born at the threshold of viability [2,3]. However, in the 1990s increased survival was associated with an increase in neurological and mental complications [4,5]. This trend seems to have changed after the turn of the century. However, still a large proportion of preterm infants suffer from significant long-term sequelae [4,6,7] with major morbidity in 68% of survivors born <27 weeks of gestation [7].
Many industrialized countries spend substantial resources on health surveillance through national patient registries, but the utility of such registries in epidemiological research varies [8,9].
The primary aim of this study was to describe the temporal changes 2002-2010 in complications of prematurity and respiratory support in preterm infants. The secondary aim was to explore and discuss strengths and limitations of using a national patient registry in epidemiological studies of the newborn population.
METHODS
The study was retrospective using individual register data from the Norwegian Patient Registry (NPR).
Setting and Participants
In Norway Gestational Age (GA) is based on routine ultrasound screening of pregnancies at 17-19 weeks of gestation. Birth weight is defined as appropriate for gestational age or not according to centiles developed by Skjaerven et al. [10]. A prospective national registry of neonatal quality of care was introduced in Norway in 2006 with complete registrations from 2008.
We defined an infant as being <1 year of age and born in the year of observation and identified 557,790 infants admitted to hospital 2002-2010 in the NPR [Table 1]. Of these, 33,882 were preterm, i.e., had a gestational age <37 weeks at birth.
Data Collection and Definitions
Our unit of observation was preterm infants born the same year as they were admitted to hospital. All observations were censored at the end of the year or when the infant was transferred to a different hospital. For 15% of the observations we did not have birth date, only birth year. For these observations we used the first admission date in the year of birth as a proxy for birth date.
We used the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) Version for 2010 and the diagnose codes for Respiratory Distress Syndrome (RDS), extremely low birth weight (<500g, 500-749g and 750-999g), very low birth weight (1000-1499g), and low birth weight (1500-1999g and 2000-2499g), Small for Gestational Age (SGA) (birth weight < 2SD below the mean or <10 centile), fetus and newborn affected by multiple pregnancy, Patent Ductus Arteriosus (PDA), extreme immaturity (GA<28 weeks), other preterm infants (GA =28 but <37 weeks), Bronchopulmonary Dysplasia (BPD) originating in the perinatal period, Retinopathy of Prematurity (ROP), Intraventricular Hemorrhage (IVH) and Necrotizing Enterocolitis (NEC) were included.
Definitions according to the Norwegian Association of Pediatricians:
RDS
Criterion A or B:
A. All preterm infants < 32weeks of gestation receiving surfactant based on clinical criteria.
B. Preterm infants < 37 weeks of gestation with respiratory distress lasting > 24 hours requiring supplementary oxygen and respiratory support (mechanical ventilation, continuous positive airway pressure (CPAP), high-flow nasal cannula) to maintain oxygen saturation >90%. Exclusion of other disease such as meconium aspiration, pulmonary hypoplasia or sepsis/pneumonia.
BPD
Pulmonary status should be evaluated at 28 days and 36 weeks postmenstrual age and both criteria should be present: A. Changes on chest x-ray suggestive of BPD B. Prolonged oxygen requirement to keep an oxygen saturation >90% and/or need for respiratory support (mechanical ventilation, CPAP, high-flow nasal cannula).
IVH
Intraventricular non-traumatic hemorrhage in fetus or newborn Papile grade 1
-Isolated subependymal hemorrhage.
Intraventricular non-traumatic hemorrhage in fetus or newborn Papile grade 2
-Subependymal hemorrhage with break-through to the ventricles without dilatation of the ventricles.
Intraventricular non-traumatic hemorrhage in fetus or newborn Papile grade 3-4
-Subependymal hemorrhage with break-through to the ventricles with dilatation of the ventricles. Parenchymal hemorrhage not mandatory.
NEC
One of the following criteria present: i) A + B or ii) C. A. One or more of the clinical signs i) bilious aspirate or vomit, ii) distended abdomen, iii) macroscopic/microscopic (guaiac) stool blood B. One or more of the radiological signs (x-ray/ultrasound) i) pneumatosis, ii) hepatobiliary air, iii) free intraperitoneal gas or iv) fluid suspective of perforation. C. Surgical NEC
Patients with mild to moderate RDS generally start CPAP treatment with a positive end expiratory pressure of 5 cmH2O and this can be increased slightly in the case of difficulties with oxygenation, except in extremely preterm infants with little or no spontaneous breathing efforts at birth. These infants are commonly intubated in the delivery room. Relative CPAP failure criteria leading to intubation and mechanical ventilation include an FiO2>0.4 and/or rising pCO2 above 8 kPa (60 mmHg) or apnea requiring mask ventilation more than twice in an hour.
The NPR assigned patients with a new Identification (ID) number each calendar year and in each hospital the patients were admitted to before 2008. After 2008 the NPR allows for tracking individual patients between years and hospitals. Our definition of an infant secures that data after 2008 is comparable to before 2008.
Outcomes
Number and fraction of infants with RDS, BPD, ROP, IVH, NEC and in-hospital death; as well as mechanical ventilation and CPAP treatment. The NPR does not allow for identifying exact GA below 28 weeks. As outcomes differ widely between GA 24 and 28 weeks, we stratified our results according to birth weight categories as an approximation to stratification by gestational weeks. We calculated Odds Ratio (OR) each year for the different outcomes adjusted for birth weight and other confounders, and unmeasured changes captured in the variable "year". The year variable captures the variation in complications that is not captured by the other predictors included in the regression.
Potential confounders of outcomes of prematurity
Current knowledge suggests that complications of prematurity are associated with birth weight, GA, SGA, male gender, multiple birth and PDA [11,12]. Hence, we controlled for these variables in multiple regression analyses.
Statistics and analytical procedures
Multiple logistic regressions were used to calculate OR for all outcomes using 2002 as reference year. We included the time trend as dummy variable, allowing the trend to both increase and decrease. I.e. it can increase one year, decrease over two years etc. We adjusted for confounders.
Regression results are presented with 95% Confidence Intervals (CI) and figures show the estimated OR with 95% CI. Descriptive statistics are presented as actual observed values without CI since this is a total population material.
All analyses were conducted in Stata version 12 (StataCorp LP, Texas, USA).
The study was performed in accordance with the Helsinki Declaration for medical research (World Medical Association 1964/2002). The Norwegian Data Inspectorate approved the project. The Regional Committee for Medical and Health Research Ethics in Norway decided that the study did not require its approval. All data are presented in a manner that preserves personal anonymity.
Results
Complications
In Table 1 the study population is presented by year and Table 2 shows the fraction of patients with complications and respiratory support by birth weight category.
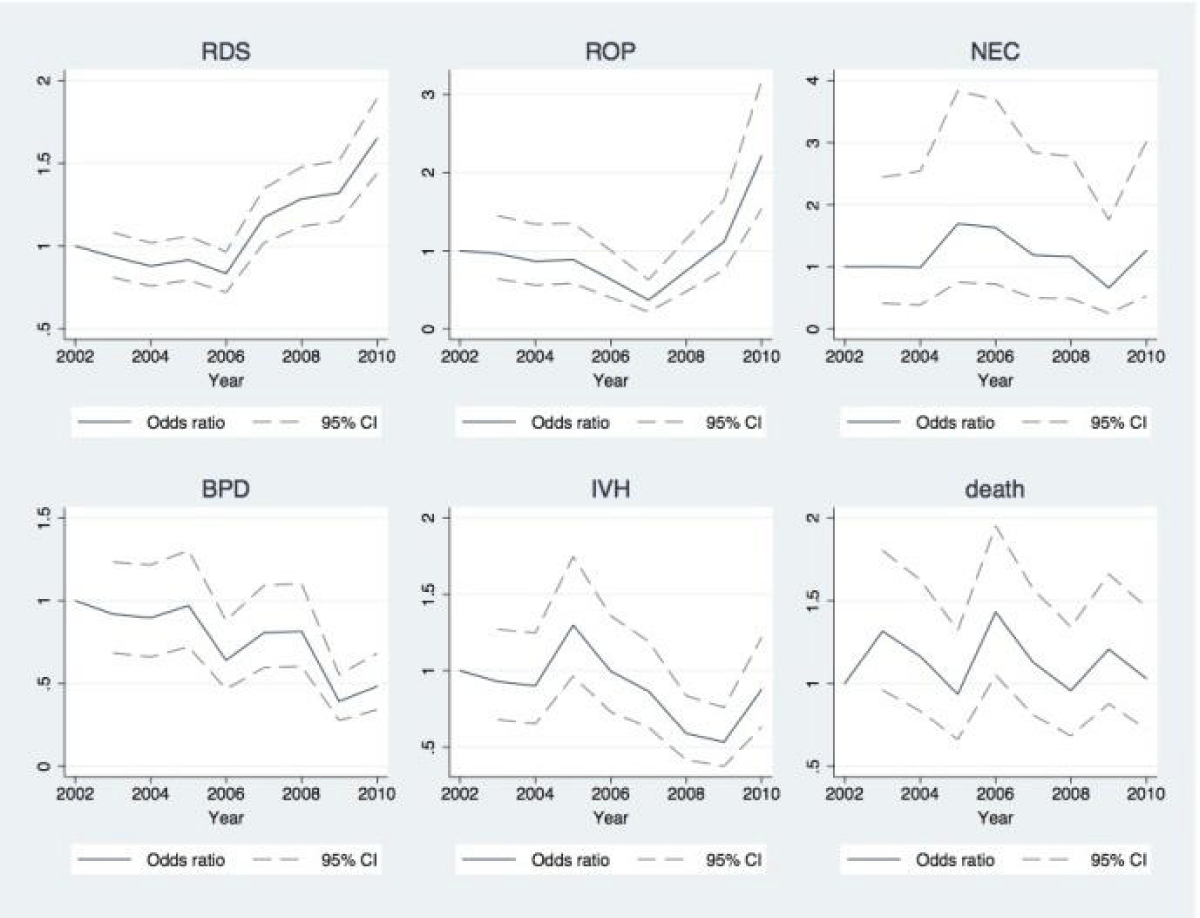
Table 3 shows the results of the multiple regressions. Figure 1 shows the change in OR for all outcome variables compared to the reference year 2002 in a multiple regression analysis with adjustments for birth weight, GA, SGA, male gender, multiple birth and PDA.
The OR of RDS was 65% higher in 2010 compared to 2002 (OR: 1.65; 95% CI: 1.43-1.89) (Table 3). The OR of BPD was 52% lower in 2010 compared to 2002 (OR 0.48, 95% CI 0.34-0.68) and a declining trend was seen over the study period (Figure 1) The OR of ROP decreased from 2002-2006 (not significant) but increased significantly from 2008, resulting in an OR of ROP of 2.21 in 2010 compared to 2002 (95% CI 1.53-3.17). The increase in RDS and ROP (2008-2010) was seen with a fairly similar pattern in all birth weight categories, but more profound in the lower weight categories. The OR of IVH decreased from 2004-2009, and increased again in 2010 to a level not significantly different from 2002. There was no change in NEC over time. There was no change in OR for in-hospital mortality 2002-2010. However, high in-hospital mortality rates were seen especially the first days of life in the lower birth weight categories (Figure 2).
Respiratory support
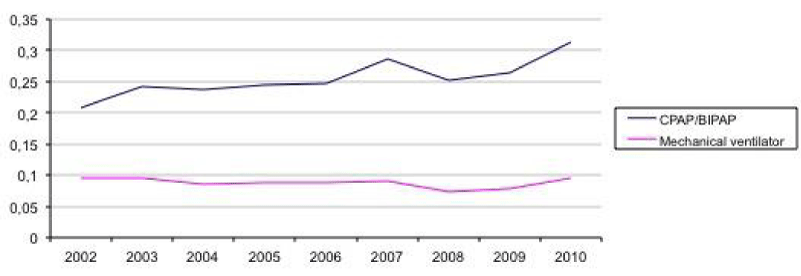
Due to missing data in seven hospitals in 2009, CPAP and mechanical ventilation was interpolated for these hospitals in 2009 as the average of 2008 and 2010 (Figure 3). Use of mechanical ventilation decreased and use of CPAP increased slightly 2002-2010.
Table 1
| Table 1: The study population 2002-2010. | ||||||||||
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Total | |
| RDS | 551 | 542 | 484 | 539 | 497 | 638 | 665 | 695 | 761 | 5,372 |
| BPD | 120 | 114 | 96 | 112 | 82 | 98 | 100 | 59 | 59 | 840 |
| ROP | 52 | 51 | 39 | 45 | 32 | 20 | 38 | 60 | 102 | 439 |
| IVH | 91 | 88 | 77 | 113 | 92 | 81 | 57 | 55 | 73 | 727 |
| NEC | 10 | 10 | 8 | 15 | 15 | 11 | 11 | 7 | 11 | 98 |
| Death | 72 | 87 | 69 | 60 | 95 | 71 | 66 | 83 | 58 | 661 |
| CPAP | 777 | 886 | 899 | 952 | 950 | 1,085 | 978 | 746* | 1,132 | 8,405 |
| Mechanical ventilator | 356 | 349 | 327 | 345 | 336 | 347 | 284 | 251* | 343 | 2,938 |
| N (preterm infants included in our study) | 3,731 | 3,649 | 3,778 | 3,874 | 3,847 | 3,802 | 3,865 | 3,712 | 3,624 | 33,882 |
| Term infants (not included in our study) | 42,323 | 43,202 | 43,580 | 43,228 | 44,562 | 44,626 | 46,095 | 47153 | 47,039 | 401,808 |
| *CPAP and MV were not reported to The Norwegian patient registry from seven hospitals in 2009, i.e. the decline in 2009 is a data artifact. When interpolating data for missing hospitals as average of 2008 and 2010, CPAP in 2009 was 994 and MV was 298 Respiratory Distress Syndrome (RDS), Continuous Positive Airway Pressure (CPAP), Retinopathy of Prematurity (ROP), Bronchopulmonary Dysplasia (BPD), Intraventricular Hemorrhage (IVH), Necrotizing Enterocolitis (NEC), Mechanical Ventilator (MV) | ||||||||||
Discussion
In this total patient registry study we found that the risk of RDS increased 2002-2010. The low average incidence of RDS in the extreme immaturity group (54%) might partly be explained by a mortality rate of 70% in the lower birth weight categories especially the first days of life. These infants may not have received all relevant diagnoses if they died early. Under diagnosing due to hospital financing in Norway where the Neonatal Intensive Care Units (NICUs) are being reimbursed for treating infants with a birth weight <1000g, irrespective of other diagnoses may be another possible explanation.
It is outside of the scope of this study to speculate on reasons for an observed increase in RDS over the study period. Changes in registration practices might have influenced this development. However, observing a distinct trend over more than one year reduces the risk of this being a coincidence or a result of changing registration practices.
Despite the increase in RDS, we found a decrease in BPD, a trend likely to be associated with less use of mechanical ventilation. In a Swedish study, the incidence of moderate-to-severe BPD in preterm infants with a mean birth weigh around 2 kg was 1.6-6.0% [13]. These numbers are somewhat higher than those found in the NPR where 0.98 % of infants with a birth weight 1500g-2000g and 7.43% of infants with a birth weight 1000-1499g were diagnosed with BPD. The incidence of ROP in the Swedish study of 2.7-6.6% was also higher that what we found in preterm infants of similar weight [13] [Table 2].
OR for ROP increased from 2007-2010 in our study. Some studies conducted the past 15 years indicate a decrease in the incidence of ROP [14], whereas others found no change [15-18]. A more cautious use of supplementary oxygen reduces the incidence of severe ROP [19]. However, major changes in oxygen targeting in preterm infants did not take place during the study years.
IVH decreased in our study except for 2004-2005 and 2009-2010. According to Volpe [20], the incidence of any grade IVH is 10-30%. In our data, the incidence was on average 2.15%, varying from 1.31% for moderately preterm infants to 17.06% for extremely preterm infants (GA <28 weeks) [Table 2].
We found a low and stable incidence of NEC, on average 0.29%, and 5.39% if birth weight 500-749g [Table 2].
Following the introduction of prenatal steroids and surfactant treatment, as well as multicenter randomized controlled trials such as the COIN trial [21,22], CPAP has replaced mechanical ventilator treatment in many preterm infants [23]. Data from the Norwegian NPR confirmed this trend when interpolating data per hospital.
Table 2
| Table 2: Fraction of preterm patients with RDS, BPD, IVH, ROP, NEC, in-hospital mortality, mechanical ventilator and CPAP treatment, by birth weight*. | |||||||||
| Preterm<28 weeks | |||||||||
| RDS | BPD | ROP | IVH | NEC | Mortality | CPAP | MV | Observations (N) | |
| <500 g or less | 56.76 % | 29.73 % | 10.81 % | 12.16 % | 4.05 % | 56.76 % | 47.30 % | 63.51 % | 74 |
| 500-749 g | 67.17 % | 33.40 % | 13.32 % | 20.83 % | 6.00 % | 34.33 % | 57.22 % | 65.48 % | 533 |
| 750-999 g | 64.53 % | 28.88 % | 13.35 % | 21.02 % | 2.93 % | 14.08 % | 78.79 % | 65.08 % | 547 |
| 1000-1499 g | 59.38 % | 19.79 % | 8.33 % | 18.40 % | 1.74 % | 9.03 % | 77.43 % | 54.51 % | 288 |
| 1500-1999 g | 38.46 % | 7.69 % | 0.00 % | 0.00 % | 0.00 % | 7.69 % | 46.15 % | 30.77 % | 13 |
| 2000-2499 g | 1.56 % | 0.00 % | 0.00 % | 1.56 % | 0.00 % | 0.00 % | 3.13 % | 1.56 % | 64 |
| >2500 g | 13.75 % | 6.32 % | 4.09 % | 5.95 % | 0.00 % | 12.27 % | 15.99 % | 12.64 % | 269 |
| Total | 54.08 % | 24.22 % | 10.46 % | 17.06 % | 3.13 % | 20.25 % | 58.45 % | 53.02 % | 1,788 |
| Preterm 28-37 weeks of GA | |||||||||
| RDS | BPD | ROP | IVH | NEC | Mortality | CPAP | MV | Observations (N) | |
| <500 g or less | 34.78 % | 17.39 % | 17.39 % | 4.35 % | 0.00 % | 39.13 % | 39.13 % | 52.17 % | 23 |
| 500-749 g | 57.79 % | 30.52 % | 22.73 % | 11.04 % | 3.25 % | 15.58 % | 70.78 % | 51.95 % | 154 |
| 750-999 g | 57.76 % | 15.10 % | 11.02 % | 7.14 % | 2.04 % | 3.47 % | 80.00 % | 40.82 % | 490 |
| 1000-1499 g | 44.97 % | 6.10 % | 4.94 % | 6.40 % | 0.56 % | 2.81 % | 61.88 % | 22.63 % | 2,673 |
| 1500-1999 g | 21.01 % | 0.96 % | 0.34 % | 1.81 % | 0.09 % | 1.01 % | 35.84 % | 7.57 % | 5,522 |
| 2000-2499 g | 10.19 % | 0.29 % | 0.00 % | 0.65 % | 0.03 % | 0.57 % | 19.04 % | 3.56 % | 9,018 |
| >2500 g | 5.23 % | 0.28 % | 0.06 % | 0.27 % | 0.03 % | 0.47 % | 10.55 % | 2.49 % | 14,214 |
| Total | 13.73 % | 1.27 % | 0.79 % | 1.31 % | 0.13 % | 0.93 % | 22.93 % | 6.20 % | 32,094 |
| All preterm infants | |||||||||
| Total | RDS | BPD | ROP | IVH | NEC | Mortality | CPAP | MV | Observations (N) |
| <500 g or less | 51.55 % | 26.80 % | 12.37 % | 10.31 % | 3.09 % | 52.58 % | 45.36 % | 60.82 % | 97 |
| 500-749 g | 65.07 % | 32.75 % | 15.43 % | 18.63 % | 5.39 % | 30.13 % | 60.26 % | 62.45 % | 687 |
| 750-999 g | 61.33 % | 22.37 % | 12.25 % | 14.46 % | 2.51 % | 9.06 % | 79.36 % | 53.62 % | 1,037 |
| 1000-1499 g | 46.37 % | 7.43 % | 5.27 % | 7.57 % | 0.68 % | 3.41 % | 63.39 % | 25.73 % | 2,961 |
| 1500-1999 g | 21.05 % | 0.98 % | 0.34 % | 1.81 % | 0.09 % | 1.03 % | 35.86 % | 7.62 % | 5,535 |
| 2000-2499 g | 10.13 % | 0.29 % | 0.00 % | 0.66 % | 0.03 % | 0.56 % | 18.93 % | 3.55 % | 9,082 |
| >2500 g | 5.39 % | 0.39 % | 0.13 % | 0.38 % | 0.03 % | 0.69 % | 10.65 % | 2.68 % | 14,483 |
| Total | 15.86 % | 2.48 % | 1.30 % | 2.15 % | 0.29 % | 1.95 % | 24.81 % | 8.67 % | 33,882 |
| >*The change in fractions in each weight category follows fairly the same pattern as described in Figure 1, Respiratory Distress Syndrome (RDS), Continuous Positive Airway Pressure (CPAP), Retinopathy of Prematurity (ROP), Bronchopulmonary Dysplasia (BPD), Intraventricular Hemorrhage (IVH), Necrotizing Enterocolitis (NEC), Mechanical Ventilator (MV) |
|||||||||
Table 3
Extraction of multiple logistic regression estimating time trends in odds ratio (OR) of RDS, BPD, ROP, IVH. NEC and in hospital mortality, coefficients of time trend in Figure 1.
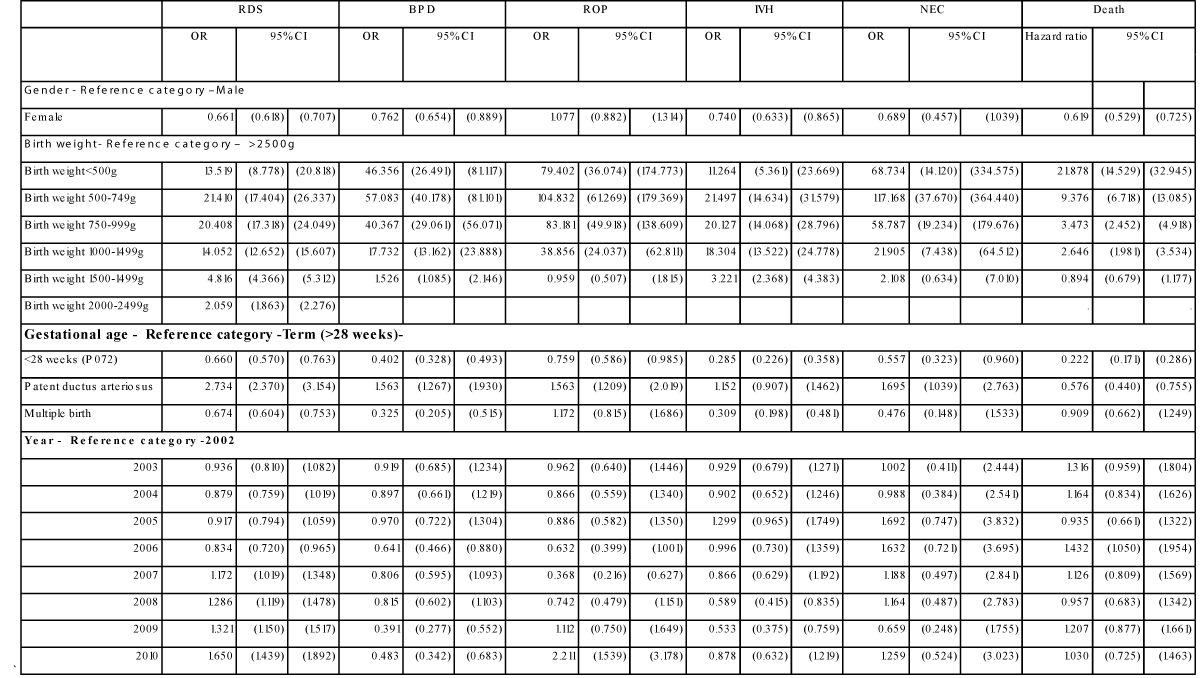
Figure 1
Estimated time trend in Respiratory Distress Syndrome (RDS), Retinopathy of Prematurity (ROP), Bronchopulmonary Dysplasia (BPD), Intraventricular Hemorrhage (IVH), Necrotizing Enterocolitis (NEC) and hazard ratio of in-hospital mortality 2002-2010. Adjusted for low birth weight, prematurity, patent ductus arteriosus, gender and multiple birth. Odds ratio using 2002 as reference year, coefficients from the rest of the multiple regression analysis presented in Table 3.
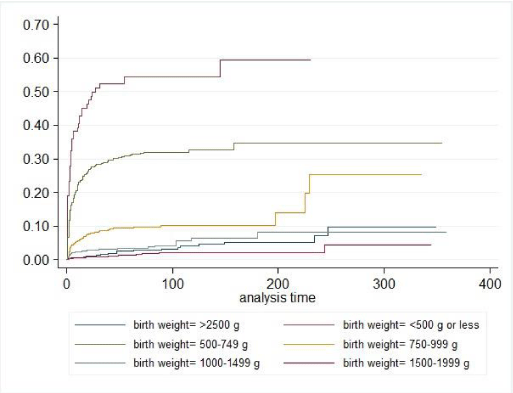
Figure 3
Fraction of patients who received Continuous Positive Airway Pressure (CPAP) or mechanical ventilation treatment once or more, 2002-2010 (2009 interpolated in seven hospitals as average of 2008 and 2010).
Confounders
Birth weight and GA have been used as predictors of long-term disability [24-26]. In our study, birth weight and GA as well as SGA and male gender were predictors of RDS in a multiple regressions analysis. Interestingly, multiple birth was associated with a lower incidence of RDS compared to singletons of the same weight, GA etc. Contrary to common beliefs and population data [11], gender did not influence any of the main outcomes, except RDS when we adjusted for SGA, weight and GA.
Limitations
We did not adjust for prenatal steroids and surfactant treatment. However, these factors can be assumed to have been constant throughout the study period. We did not differentiate between different stages of ROP, moderate and severe BPD; or grading of IVH. The retrospective nature of the study, and the fact that the results rely on routine reporting practices pose challenges to interpretation of the results. Changes in reporting practices associated with the introduction of the prospective Norwegian registry of neonatal quality of care cannot completely explain changes in OR after 2008, since there is no consistent trend in OR for all the different outcomes 2008-2010. However, as can be seen from Table 2, a number of infants with GA <28 weeks has been registered with a birth weight >2500g. This may be due to missing data resulting in a 'default' registration of 'normal birth weight' or alternatively, the infant's weight at the time of transfer to a local level 2 NICU has been registered as birth weight. Finally, the temporary ID numbers assigned to newborn infants in Norway make registry data less reliable the first year of life. Some of the infants in our study have been included multiple times when admitted to several hospitals with the same diagnoses, and incidence of the study outcomes except mortality is likely to be overestimated. Despite this, except for IVH we found fewer complications in the extremely preterm group than in the prospective Swedish EXPRESS study [7]. Some of the differences in outcome data in the NPR and the EXPRESS study should be attributed to the retrospective nature of our study.
Despite these limitations, we claim that the NPR can be used to describe temporal changes in the outcomes of interest. The results do not indicate that the variables described in this paper were influenced systematically by the introduction of the Norwegian registry of neonatal quality of care in 2008, since some variables increased, some decreased, whereas other variables remained stable after 2008.
Strengths of our study include the population-based design and the fact that our data reflect preterm morbidity the last decade. Many epidemiological studies of extremely preterm infants are from the early 2000s [2]. Also, few studies have studied temporal changes the way we did in the present study.
In conclusion, the risk of RDS increased whereas BPD risk decreased. ROP decreased from 2002-2006 and IVH from 2005-2009 followed by an increase. There was neither a change in NEC, nor in in-hospital mortality. Use of mechanical ventilation decreased and use of CPAP increased slightly. There is a need for cautious interpretation of data from the national patient registry. However, the data can be useful for estimating time trends to be further explored.
References
1. Minin~o AMM S.L, Xu J, Kochanek K.D. National Vital Statics Reports: Deaths: Final Data for 2008. Hyattsville, MD: National Center for Health Statistics, 2011 December 2011. Report No.: Contract No.: 10.
2. Markestad T, Kaaresen PI, Ronnestad A, Reigstad H, Lossius K, Medbo S, et al. Early death, morbidity, and need of treatment among extremely premature infants. Pediatrics. 2005; 115: 1289-1298.
3. Fanaroff AA, Hack M, Walsh MC. The NICHD neonatal research network: changes in practice and outcomes during the first 15 years. Semin Perinatol. 2003; 27: 281-287.
4. Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005; 115: 997-1003.
5. Emsley HC, Wardle SP, Sims DG, Chiswick ML, D'Souza SW. Increased survival and deteriorating developmental outcome in 23 to 25 week old gestation infants, 1990-4 compared with 1984-9. Arch Dis Child Fetal Neonatal Ed. 1998; 78: F99-F104.
6. Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005; 90: F134-F140.
7. Fellman V, Hellstrom-Westas L, Norman M, Westgren M, Kallen K, Lagercrantz H, et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. 2009; 301: 2225-2233.
8. Lund JL, Froslev T, Deleuran T, Erichsen R, Nilsson T, Pedersen AN, et al. Validity of the Danish National Registry of Patients for chemotherapy reporting among colorectal cancer patients is high. Clin Epidemiol. 2013;5:327-334.
9. Wildenschild C, Mehnert F, Thomsen RW, Iversen HK, Vestergaard K, Ingeman A, et al. Registration of acute stroke: validity in the Danish Stroke Registry and the Danish National Registry of Patients. Clin Epidemiol. 2013; 6: 27-36.
10. Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand. 2000; 79: 440-449.
11. Farstad T, Bratlid D, Medbo S, Markestad T, Norwegian Extreme Prematurity Study G. Bronchopulmonary dysplasia - prevalence, severity and predictive factors in a national cohort of extremely premature infants. Acta Paediatr. 2011; 100: 53-58.
12. Westby Wold SH, Sommerfelt K, Reigstad H, Ronnestad A, Medbo S, Farstad T, et al. Neonatal mortality and morbidity in extremely preterm small for gestational age infants: a population based study. Arch Dis Child Fetal Neonatal Ed. 2009; 94: F363-367.
13. Ortenstrand A, Westrup B, Brostrom EB, Sarman I, Akerstrom S, Brune T, et al. The Stockholm Neonatal Family Centered Care Study: effects on length of stay and infant morbidity. Pediatrics. 2010; 125: e278-8e285.
14. Zin A, Gole GA. Retinopathy of prematurity-incidence today. Clin Perinatol. 2013; 40: 185-200.
15. Allegaert K, de Coen K, Devlieger H. Threshold retinopathy at threshold of viability: the EpiBel study. Br J Ophthalmol. 2004; 88: 239-242.
16. Bullard SR, Donahue SP, Feman SS, Sinatra RB, Walsh WF. The decreasing incidence and severity of retinopathy of prematurity. J AAPOS. 1999; 3: 46-52.
17. Hussain N, Clive J, Bhandari V. Current incidence of retinopathy of prematurity, 1989-1997. Pediatrics. 1999; 104: e26.
18. Blair BM, O'Halloran H S, Pauly TH, Stevens JL. Decreased incidence of retinopathy of prematurity, 1995-1997. J AAPOS. 2001; 5: 118-122.
19. Askie LM, Henderson-Smart DJ, Ko H. Restricted versus liberal oxygen exposure for preventing morbidity and mortality in preterm or low birth weight infants. Cochrane Database Syst Rev. 2009; CD001077.
20. Volpe JJ. Neurology of the newborn. 4th ed. Philadelphia ; London: Saunders. 2001; xiii: 912 p. p.
21. Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008; 358: 700-708.
22. Roehr CC, Proquitte H, Hammer H, Wauer RR, Morley CJ, Schmalisch G. Positive effects of early continuous positive airway pressure on pulmonary function in extremely premature infants: results of a subgroup analysis of the COIN trial. Arch Dis Child Fetal Neonatal Ed. 2011; 96: F371-F373.
23. Sweet D, Bevilacqua G, Carnielli V, Greisen G, Plavka R, Saugstad OD, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome. J Perinat Med. 2007; 35: 175-186.
24. Friedlander Y, Paltiel O, Deutsch L, Knaanie A, Massalha S, Tiram E, et al. Birthweight and relationship with infant, child and adult mortality in the Jerusalem perinatal study. Paediatr Perinat Epidemiol. 2003; 17: 398-406.
25. Li CI, Daling JR, Emanuel I. Birthweight and risk of overall and cause-specific childhood mortality. Paediatr Perinat Epidemiol. 2003; 17: 164-170.
26. Samuelsen SO, Magnus P, Bakketeig LS. Birth weight and mortality in childhood in Norway. Am J Epidemiol. 1998; 148: 983-991.
Authors submit all Proposals and manuscripts via Electronic Form!