Ekamol Tantisattamo*James L. Bailey
Ekamol Tantisattamo1*James L. Bailey2
1Multi-Organ Transplant Center, Division of Nephrology, Department of Internal Medicine, Oakland University William Beaumont School of Medicine, William Beaumont Hospital, Royal Oak, Michigan 48073 United States 2Renal Division, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia 30322 United States
*Address for Correspondence: Ekamol Tantisattamo, MD, FACP, FASN, FNKF, Multi-Organ Transplant Center, Division of Nephrology, Department of Internal Medicine, Oakland University William Beaumont School of Medicine, William Beaumont Hospital, 3535 West 13 Mile Road, Suite 644, Royal Oak, Michigan
Dates: Submitted: 20 May, 2016; Approved: 23 May, 2016; Published: 24 May, 2016
Citation this article: Tantisattamo E, Bailey JL. Is Nutritional Intervention a Management for Post-transplant Hypophosphatemia?. SRL Nutr Food Sci. 2016;1(1): 007-012
Copyright: © 2016 Tantisattamo E, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Chronic kidney disease-mineral bone disorders; Kidney Transplantation; Nutritional intervention, Post-transplant Hypophosphatemia
Post-transplant hypophosphatemia is a very common electrolyte disturbance following successful kidney transplantation. The incidence is up to 93% [1]. Similar to non-transplant patients, the pathogeneses of post-transplant hypophosphatemia includes shifts of intracellular phosphate, decreased phosphate intake, and excessive phosphate loss. Post-transplant factors may also contribute to hypophosphatemia. Shifts in intracellular phosphate are uncommon and post-transplant medications, including commonly used immunosuppressive medications, do not cause phosphate shifts. The majority of end-stage renal disease (ESRD) patients adhere to a dietary phosphate restriction and inadequate dietary phosphate intake may persistent and cause hypophosphatemia in the functioning renal allograft. Excessive phosphorus loss from urine plays a major role in causing post-transplant hypophosphatemia.
During the pretransplant period, the majority of chronic kidney disease (CKD) or ESRD patients have hyperphosphatemia secondary to decreased urinary phosphate excretion. To regulate this phosphate imbalance, expression of phosphaturic hormones such as parathyroid hormone (PTH) and fibroblast growth factor (FGF) 23 ensure an increase in renal phosphate excretion.
PTH and FGF 23 normalize with 3-6 months and 12 months respectively after successful kidney transplantation [2-4], and there is regression of renal phosphate wasting within 1 year post-transplantation [4]. However, some kidney transplant recipients may have persistently elevated levels of PTH and FGF 23 which cause decreased proximal phosphate reabsorption and renal phosphate wasting [5, 6]. Post-transplant hypophosphatemia is often secondary to a PTH-independent mechanism [7] which suggests that FGF 23 or other phosphaturic hormones play an important role in post-transplant renal phosphate wasting [4, 8-11]. Apart from the pretransplant factors causing urinary phosphate loss, post-transplant factors also worsen hypophosphatemia. Post-transplant renal tubular dysfunction, immunosuppressive medications such as calcineurin inhibitors [12], glucocorticoids [13, 14], vitamin D deficiency further enhance renal phosphate wasting. In addition, glucocorticoids [15, 16], and vitamin D deficiency increase intestinal phosphate losses.
Hypophosphatemia can cause mild to severe complications. In the early post-transplant period, hypophosphatemia can cause muscle weakness, rhabdomyolysis, hemolytic anemia, or even respiratory failure; however, these complications are rare. More prevalent are chronic kidney disease-mineral bone disorders (CMD-MBD), which are composed of the constellation of 1) Biochemical disturbances of calcium, phosphorus, PTH, and vitamin D 2) Soft tissue or vascular calcifications and 3) Renal bone diseases.
In non-dialysis dependent CKD, hyperphosphatemia is associated with increased mortality [17]. Hyperphosphatemia is also associated with increased mortality in dialysis patients especially in African Americans [18]. Similar to non-transplant patients, hyperphosphatemia in post-transplant recipients has been associated with worsening patient survival as well as poorer renal allograft outcomes. On the other hand, post-transplant hypophosphatemia was associated with better graft function and survival.
Huber et al [19] conducted an observational study demonstrating that of 776 kidney transplant recipients with more than 6-month follow-up, 279 (36.1%) had hypophosphatemia (< 0.8 mmol/l) at 6 months. Hypophosphatemic patients had higher serum calcium levels, a lower Ca x phosphate product, and higher calcitriol levels. Overall patient survival, graft function and death-censored graft survival worsened in hyperphosphatemic patients. The authors concluded that hypophosphatemia is an indicator of excellent kidney function, but it does not have direct effects of on long-term outcomes.
Although post-transplant hypophosphatemia resolves in the majority of the patients, long-term complications of untreated hypophosphatemia especially CKD-MBD can occur. Therefore, hypophosphatemia should be treated early in kidney transplant recipients.
In ESRD patients, hyperphosphatemia is controlled by dialysis, phosphate blinders, and/or dietary phosphate restriction. Apart from dialysis, treatment for post-transplant hypophosphatemia can range from nutritional intervention to pharmacological therapy.
Ambuhl et al [1] randomized 28 kidney transplant recipients with stable functioning renal allograft (= 30 mL/min) and mild hypophosphatemia (0.3-0.75 mmol/L) into 2 groups: neutral sodium phosphate (Na2HPO4) versus sodium chloride (NaCl) therapy. After 12 weeks, both groups had normal and similar mean serum phosphate concentrations, but the number of patients with hypophosphatemia was higher in the NaCl group compared with the Na2HPO4 group (93% versus 67%). In addition, neutral phosphate did not affect serum calcium or PTH and significantly improved metabolic acidosis by increased urinary titratable acidity. The authors concluded that oral neutral phosphate corrects post-transplant hypophosphatemia, improves renal acid excretion, and had no effect on mineral metabolism.
Another prospective study by Caravaca et al [20] followed 32 kidney transplant recipients with well-functioning allografts with a mean time post-transplant of 41±18 months. After a 1-month washout period for an oral phosphate supplement, the subjects received neutral phosphate 1.5 g orally for 15 days with the usual diet. Serum phosphorus, PTH, and urinary phosphate excretion were significantly increased, while serum calcium, 1, 25-dihydroxycholecalciferol (1, 25-(OH)2 vitamin D), and urinary calcium excretion were significantly decreased. The authors concluded that phosphate supplement could worsen hyperparathyroidism in the late post-transplantation period.
In addition to phosphorus supplementations, calcimimetic agents and vitamin D are also utilized in kidney transplant recipients. Cinacalcet, a calcimimetic agent, indirectly activates the calcium sensing receptor (CaSR) by increasing calcium sensitivity of the receptor. The different alterations and the therapeutic implications for biochemical parameters of mineral and bone metabolism are outlined in Table 1.
In chronic dialysis patients, cinacalcet decreases serum calcium, phosphorus, and PTH [21]. The effect on serum phosphorus is due to decreased phosphorus release from bone, increased phosphorus bone uptake, and decreased intestinal phosphate absorption [22]. Serum calcium levels are also decreased with cinacalcet. There is decrease bone turnover; serum alkaline phosphatase is lower [23]; intestinal calcium absorption is reduced, and urinary calcium excretion is enhanced [24, 25]. The biochemical effects of cinacalcet on serum phosphorus and calcium are opposite to those of vitamin D [26], but are similar to the consequences of a medical parathyroidectomy [27, 28] and hungry bone syndrome [29]. As a calcimimetic, the action of cinacalcet on parathyroid glands causes CaSR-dependent PTH secretion and lower serum PTH values [21, 30].
Cinacalcet may decrease FGF 23 in non-dialysis CKD and kidney transplant patients [31, 32], and decreases urinary phosphate excretion. This leads to an increase in serum phosphorus level [25, 31, 33-35]. As a result of its action on CaSR, cinacalcet decreases PTH secretion. In turn, there is decreased PTH action on proximal renal tubular epithelial cells which results in decreased formation of 1, 25-(OH)2 vitamin D [36, 37].
Hypocalcemia in the pretransplant period generally normalizes after successful kidney transplantation as secondary hyperparathyroidism (SHPT) resolves. In the setting of post-transplant hypophosphatemia, 1, 25-(OH)2 vitamin D activity should be high and even normal vitamin D levels are inappropriately low. Vitamin D therapy could be used to increase serum levels of calcium and phosphorus. Vitamin D directly stimulates intestinal calcium and phosphate absorption while suppressing PTH excretion. As a result, urinary phosphate excretion is decreased. However, some patients may develop persistent SHPT which results in post-transplant hypercalcemia, This generally occurs in the first 10 days of transplantation but in some instances it may occur more than six months later [38, 39]. In addition, resolved uremia and improved nutritional status following successful kidney transplantation normalize hypoalbuminemia and can cause a mild post-transplant hypercalcemia. This limits the use of vitamin D therapy.
Pharmacological intervention for hypophosphatemia can potentially cause unfavorable side effects (Table 2). As mentioned earlier, phosphate supplementation can worsen hyperparathyroidism in the late post-transplantation period. The administration of cinacalcet can lead to persistent post-transplant hypocalcemia. The PTH level is depressed with cinacalcet while vitamin D supplementation can further depress PTH. However, over suppression of PTH should be avoided in these patients as it can result in low bone turnover or adynamic bone disease. This can be diagnosed by bone biopsy and bone histomorphometry, but it is not commonly performed in clinical practice.
Since there are numerous potential adverse effects of pharmacological intervention, nutritional intervention for post-transplant hypophosphatemia should always be an integral part of bone management.
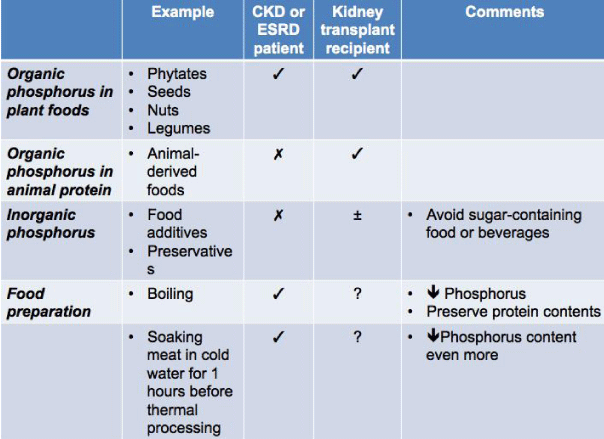
There are 3 sources of dietary phosphorus: organic phosphorus in plant foods, organic phosphorus in animal protein, and inorganic phosphorus. Organic phosphorus in plant foods e.g. phytates, seeds, nuts, legumes, etc. have the lowest phosphorus bioavailability (20-40%) due to a lack of the enzyme phytase. The phosphorus bioavailability in animal-derived foods is up to 40-60% because this source of phosphorus is easily hydrolyzed and absorbed. Inorganic phosphorus has 100% bioavailability [40, 41] and is commonly found in food additives and preservatives.
The major source of dietary phosphorus is derived from protein and there is a strong association between dietary protein and phosphorus intake [40]. However, given the varied bioavailability of phosphorus from different sources and inorganic additives in processed foods [42], the estimated phosphorus content may not be accurate [43]. For CKD patients, it is recommended that they consume food that are low inorganic phosphorus or have foods with a low phosphorus-to-protein ratio. Adequate protein content foods [40] can be derived from nondairy products and animal-derived foods e.g. egg white [41]. In addition, food preparation such as boiling can decrease the overall phosphorus content of food but still preserve the overall protein content [44]. Even soaking meat in cold water for 1 hour before thermal processing can further lower the phosphorus content of the food [45].
For kidney transplant recipients with hypophosphatemia, there is no strong evidence supporting any particular high phosphorus-containing diet. High quality animal-derived protein, dairy products, or dark-color beverages are commonly prescribed for hypophosphatemic patients. However, in our opinion, food selection should be individualized in order to avoid the unintentional side effects previously listed for these high phosphorus-containing diets. For instance, a soft drink rich in phosphorus is frequently prescribed in newly transplanted kidney patients with hypophosphatemia because they need not only high phosphorus intake, but they also require adequate hydration in order to avoid dehydration. However, these beverages also have a high sugar content, which should be avoided in kidney patients especially those with concomitant diabetes mellitus.
Sources of dietary phosphorus and methods of food preparations recommended for CKD and ESRD patients as well as those suggested for kidney transplant recipients are summarized in Table 3.
Successful therapy for hyperphosphatemia in advanced CKD and ESRD requires understanding and adhering with a renal specific diet, phosphate binders, and dialysis [46]. Unlike patients with advanced CKD or on dialysis, kidney transplant recipients with well-functioning allografts do not need to be restricted by a "renal diet" or a fluid restriction. They are advised to eat a "normal diet" and drink plenty of fluids. If these patients are unfamiliar with high phosphorus containing food, they are prone to develop hypophosphatemia. This may worsen or prolong their hypophosphatemia. Therefore, education which provides the rational for these changes, will increase understanding and enhance adherence to an appropriate diet in post-transplant patients [47].
Although a phosphate restriction is not applicable to a patient with good renal allograft function, a diet with a high phosphorus-to-protein ration and rich in inorganic phosphorus-containing preservatives should be avoided in kidney transplant recipients with failing allografts.
Conclusion
Post-transplant hypophosphatemia is a very common electrolyte disturbance especially in the early post-transplant period. Although it rarely leads to serious complications, the potential for long-term consequences such as metabolic bone disorders is of concern. Pharmacological therapy may worsen these disorders with serious alterations in other electrolytes and hormones. Nutritional intervention should always be an initial strategy to correct hypophosphatemia and avoid adverse effects of pharmacological intervention. However, with limited evidence, further studies are required to help guide us as to what constitutes optimal therapy.
Table 1
The effects of cinacalcet on biochemical parameters of mineral and bone metabolism.
1, 25-(OH)2 vitamin D, 1, 25-dihydroxy vitamin D FGF 23, fibroblast growth factor 23 NA, not applicable.
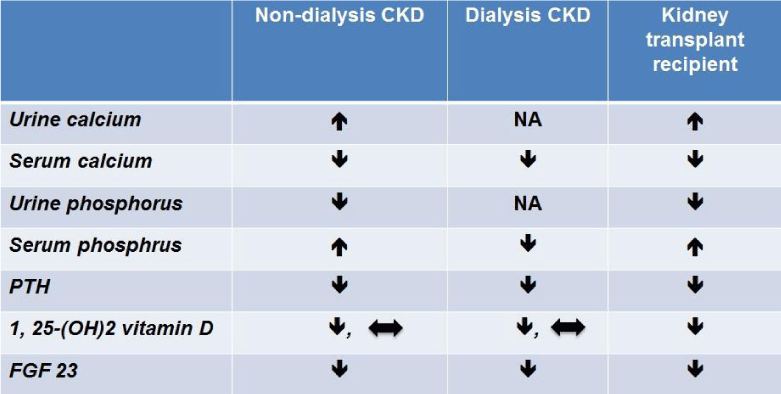
Table 2
Pharmacological interventions for post-transplant hyperphosphatemia and their adverse effects PTH, parathyroid hormone.
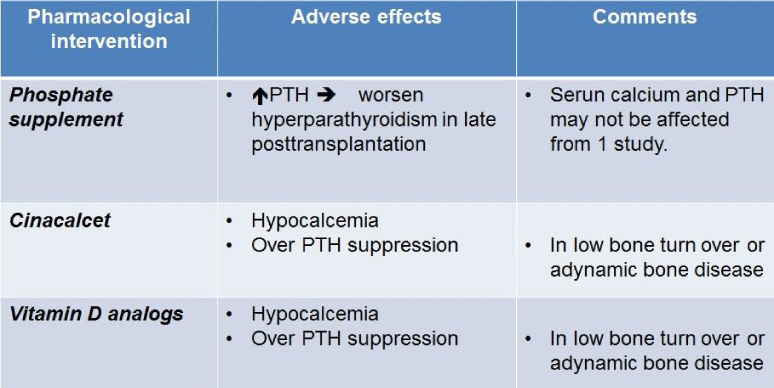
Table 3
Three different sources of dietary phosphorus and methods for food preparation reducing its phosphorus content for CKD and ESRD patients and kidney transplant recipients.
? = Recommended
? = Not recommended
? = Unclear
References
- Ambuhl PM, Meier D, Wolf B, Dydak U, Boesiger P, Binswanger U. Metabolic aspects of phosphate replacement therapy for hypophosphatemia after renal transplantation: impact on muscular phosphate content, mineral metabolism, and acid/base homeostasis. Am J Kidney Dis. 1999;34:875-83.
- Torres A, Lorenzo V, Salido E. Calcium metabolism and skeletal problems after transplantation. J Am Soc Nephrol. 2002;13:551-8.
- Sirilak S, Chatsrisak K, Ingsathit A, Kantachuvesiri S, Sumethkul V, Stitchantrakul W, et al. Renal phosphate loss in long-term kidney transplantation. Clin J Am Soc Nephrol. 2012;7:323-31.
- Evenepoel P, Meijers BK, de Jonge H, Naesens M, Bammens B, Claes K, et al. Recovery of hyperphosphatoninism and renal phosphorus wasting one year after successful renal transplantation. Clin J Am Soc Nephrol. 2008;3:1829-36.
- Lotscher M, Scarpetta Y, Levi M, Halaihel N, Wang H, Zajicek HK, et al. Rapid downregulation of rat renal Na/P(i) cotransporter in response to parathyroid hormone involves microtubule rearrangement. J Clin Invest. 1999;104:483-94.
- Kalantar-Zadeh K, Molnar MZ, Kovesdy CP, Mucsi I, Bunnapradist S. Management of mineral and bone disorder after kidney transplantation. Curr Opin Nephrol Hypertens. 2012;21:389-403.
- Bhan I, Shah A, Holmes J, Isakova T, Gutierrez O, Burnett SM, et al. Post-transplant hypophosphatemia: Tertiary 'Hyper-Phosphatoninism'? Kidney Int. 2006;70:1486-94.
- Evenepoel P, Naesens M, Claes K, Kuypers D, Vanrenterghem Y. Tertiary 'hyperphosphatoninism' accentuates hypophosphatemia and suppresses calcitriol levels in renal transplant recipients. Am J Transplant. 2007;7:1193-200.
- Trombetti A, Richert L, Hadaya K, Graf JD, Herrmann FR, Ferrari SL, et al. Early post-transplantation hypophosphatemia is associated with elevated FGF-23 levels. Eur J Endocrinol. 2011;164:839-47.
- Levi M. Post-transplant hypophosphatemia. Kidney Int. 2001;59:2377-87.
- Green J, Debby H, Lederer E, Levi M, Zajicek HK, Bick T. Evidence for a PTH-independent humoral mechanism in post-transplant hypophosphatemia and phosphaturia. Kidney Int. 2001;60:1182-96.
- Demeule M, Beliveau R. Cyclosporin inhibits phosphate transport and stimulates alkaline phosphatase activity in renal BBMV. Am J Physiol. 1991;260:F518-24.
- Levi M, Shayman JA, Abe A, Gross SK, McCluer RH, Biber J, et al. Dexamethasone modulates rat renal brush border membrane phosphate transporter mRNA and protein abundance and glycosphingolipid composition. J Clin Invest. 1995;96:207-16.
- Loffing J, Lotscher M, Kaissling B, Biber J, Murer H, Seikaly M, et al. Renal Na/H exchanger NHE-3 and Na-PO4 cotransporter NaPi-2 protein expression in glucocorticoid excess and deficient states. J Am Soc Nephrol. 1998;9:1560-7.
- Rosental R, Babarykin D, Fomina O, Smelters G, Valiniece M, Baumann V. Hypophosphatemia after successful transplantation of the kidney. Clinico-experimental study. Z Urol Nephrol. 1982;75:393-9.
- Borowitz SM, Granrud GS. Glucocorticoids inhibit intestinal phosphate absorption in developing rabbits. J Nutr. 1992;122:1273-9.
- Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Outcomes associated with serum phosphorus level in males with non-dialysis dependent chronic kidney disease. Clin Nephrol. 2010;73:268-75.
- Scialla JJ, Parekh RS, Eustace JA, Astor BC, Plantinga L, Jaar BG, et al. Race, Mineral Homeostasis and Mortality in Patients with End-Stage Renal Disease on Dialysis. Am J Nephrol. 2015;42:25-34.
- Huber L, Naik M, Budde K. Frequency and long-term outcomes of post-transplant hypophosphatemia after kidney transplantation. Transpl Int. 2013;26:e94-6.
- Caravaca F, Fernandez MA, Ruiz-Calero R, Cubero J, Aparicio A, Jimenez F, et al. Effects of oral phosphorus supplementation on mineral metabolism of renal transplant recipients. Nephrol Dial Transplant. 1998;13:2605-11.
- Cinacalcet: AMG 073, Calcimimetics--Amgen/NPS Pharmaceuticals, KRN 1493, NPS 1493. Drugs R D. 2003;4:349-51.
- Fukagawa M, Yumita S, Akizawa T, Uchida E, Tsukamoto Y, Iwasaki M, et al. Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrol Dial Transplant. 2008;23:328-35.
- Belozeroff V, Goodman WG, Ren L, Kalantar-Zadeh K. Cinacalcet lowers serum alkaline phosphatase in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:673-9.
- Esposito L, Rostaing L, Gennero I, Mehrenberger M, Durand D, Kamar N. Hypercalciuria induced by a high dose of cinacalcet in a renal-transplant recipient. Clin Nephrol. 2007;68:245-8.
- Borchhardt KA, Heinzl H, Mayerwoger E, Horl WH, Haas M, Sunder-Plassmann G. Cinacalcet increases calcium excretion in hypercalcemic hyperparathyroidism after kidney transplantation. Transplantation. 2008;86:919-24.
- Coyne DW, Grieff M, Ahya SN, Giles K, Norwood K, Slatopolsky E. Differential effects of acute administration of 19-Nor-1,25-dihydroxy-vitamin D2 and 1,25-dihydroxy-vitamin D3 on serum calcium and phosphorus in hemodialysis patients. Am J Kidney Dis. 2002;40:1283-8.
- Wang HY, Yu CC, Huang CC. Successful treatment of severe calciphylaxis in a hemodialysis patient using low-calcium dialysate and medical parathyroidectomy: case report and literature review. Ren Fail. 2004;26:77-82.
- Narayan R, Perkins RM, Berbano EP, Yuan CM, Neff RT, Sawyers ES, et al. Parathyroidectomy versus cinacalcet hydrochloride-based medical therapy in the management of hyperparathyroidism in ESRD: a cost utility analysis. Am J Kidney Dis. 2007;49:801-13.
- Lazar ES, Stankus N. Cinacalcet-induced hungry bone syndrome. Semin Dial. 2007;20:83-5.
- Valle C, Rodriguez M, Santamaria R, Almaden Y, Rodriguez ME, Canadillas S, et al. Cinacalcet reduces the set point of the PTH-calcium curve. J Am Soc Nephrol. 2008;19:2430-6.
- Charytan C, Coburn JW, Chonchol M, Herman J, Lien YH, Liu W, et al. Cinacalcet hydrochloride is an effective treatment for secondary hyperparathyroidism in patients with CKD not receiving dialysis. Am J Kidney Dis. 2005;46:58-67.
- Serra AL, Wuhrmann C, Wuthrich RP. Phosphatemic effect of cinacalcet in kidney transplant recipients with persistent hyperparathyroidism. Am J Kidney Dis. 2008;52:1151-7.
- Coyne DW. Cinacalcet should not be used to treat secondary hyperparathyroidism in stage 3-4 chronic kidney disease. Nat Clin Pract Nephrol. 2008;4:364-5.
- Chonchol M, Locatelli F, Abboud HE, Charytan C, de Francisco AL, Jolly S, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of cinacalcet HCl in participants with CKD not receiving dialysis. Am J Kidney Dis. 2009;53:197-207.
- Serra AL, Braun SC, Starke A, Savoca R, Hersberger M, Russmann S, et al. Pharmacokinetics and pharmacodynamics of cinacalcet in patients with hyperparathyroidism after renal transplantation. Am J Transplant. 2008;8:803-10.
- Slatopolsky E, Brown A, Dusso A. Pathogenesis of secondary hyperparathyroidism. Kidney Int Suppl. 1999;73:S14-9.
- Ba J, Friedman PA. Calcium-sensing receptor regulation of renal mineral ion transport. Cell Calcium. 2004;35:229-37.
- David DS, Sakai S, Brennan BL, Riggio RA, Cheigh J, Stenzel KH, et al. Hypercalcemia after renal transplantation. Long-term follow-up data. N Engl J Med. 1973;289:398-401.
- Garvin PJ, Castaneda M, Linderer R, Dickhans M. Management of hypercalcemic hyperparathyroidism after renal transplantation. Arch Surg. 1985;120:578-83.
- Kalantar-Zadeh K, Gutekunst L, Mehrotra R, Kovesdy CP, Bross R, Shinaberger CS, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:519-30.
- Noori N, Sims JJ, Kopple JD, Shah A, Colman S, Shinaberger CS, et al. Organic and inorganic dietary phosphorus and its management in chronic kidney disease. Iran J Kidney Dis. 2010;4:89-100.
- Uribarri J. Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Semin Dial. 2007;20:295-301.
- Kalantar-Zadeh K. Patient education for phosphorus management in chronic kidney disease. Patient Prefer Adherence. 2013;7:379-90.
- Cupisti A, D'Alessandro C, Baldi R, Barsotti G. Dietary habits and counseling focused on phosphate intake in hemodialysis patients with hyperphosphatemia. J Ren Nutr. 2004;14:220-5.
- Vrdoljak I, Panjkota Krbavcic I, Bituh M, Vrdoljak T, Dujmic Z. Analysis of different thermal processing methods of foodstuffs to optimize protein, calcium, and phosphorus content for dialysis patients. J Ren Nutr. 2015;25:308-15.
- Sutton D. Phosphate control: who, how and when? A comment. J Ren Care. 2009;35 Suppl 1:84-5.
- Jamieson NJ, Hanson CS, Josephson MA, Gordon EJ, Craig JC, Halleck F, et al. Motivations, Challenges, and Attitudes to Self-management in Kidney Transplant Recipients: A Systematic Review of Qualitative Studies. Am J Kidney Dis. 2015.
Authors submit all Proposals and manuscripts via Electronic Form!




























