Short Communication
High Sensitive Troponin T has an Incremental Value in Estimation of Cardiovascular Risk in Chronic Kidney Disease
Anna V. Olah1*, Timea Szalko2, Eszter Szantho1, Eva Varga3, Janos Matyus44 and Jozsef Varga5
1Department of Laboratory Medicine, Faculty of Medicine, University of Debrecen, Debrecen, Hungary
2Fejer County Hospital, Szekesfehervar, Hungary
33rd Department of Internal Medicine, Semmelweis University, Budapest, Hungary
4Department of Medicine, Faculty of Medicine, University of Debrecen, Debrecen, Hungary
5Department of Medical Imaging, Faculty of Medicine, University of Debrecen, Debrecen, Hungary
*Address for Correspondence: Anna V. Olah, Department of Laboratory Medicine, Faculty of Medicine, University of Debrecen, Nagyerdei krt 98, H-4032 Debrecen, Hungary
Dates: Submitted: 12 December 2016; Approved: 29 December 2016; Published: 27 January 2017
Citation this article: Olah AV, Szalko T, Szantho E, Varga E, Matyus J, et al. High Sensitive Troponin T has an Incremental Value in Estimation of Cardiovascular Risk in Chronic Kidney Disease. Int J Nephrol Ther. 2017;3(1): 001-006
Copyright: © 2017 Olah AV, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Cardiovascular mortality; Glomerular filtration rate; Proteinuria; Renal score; Troponin
Abstract
Relative risk of cardiovascular morbidity is increased in Chronic Kidney Disease (CKD). According to current KDIGO guideline cardiovascular risk can be estimated from Glomerular Filtration Rate (GFR) and proteinuria.
Aims: First aim was to evaluate renal score, is it enough to estimate cardiovascular risk in CKD. Then we checked whether high sensitive Troponin T (hsTnT) has an incremental value to predict cardiovascular risk in CKD.
Methods: Clinical cardiovascular score was established according to the Framingham study: age, BMI, blood pressure, lipids, patient history (diabetes mellitus, myocardial infarction, stroke). Renal severity scores (1-4) were determined from GFR and urinary albumin/creatinine or protein/creatinine. Biochemical parameters were determined by Cobas 6000.
Results: Results in Study A (n:20) Mean of GFR decreased (66 ± 12 vs. 47 ± 16 ml/min/1.73 m2) and renal score impaired (2.5 ± 1.1 vs. 3 ± 0.9) during four years. Clinical cardiovascular score was proportional to the renal score: clinical score was 21.8 ± 9.8 at renal score <3.5 and 29.2 ± 6.7 at renal score >3.5 (p:0.09). Proteinuria has prognostic value in lipid lowering therapy: atherogenic non-HDL was reducible from 3.62 ± 0.75 to 2.92 ± 0.63 mmol/ L without proteinuria. In Study B (n:21) mean of clinical cardiovascular score was 21.5 ± 2.1 at moderate renal damage and 27.4 ± 9.2 at severe renal damage. Mean of hsTnT was also lower (14.8 ± 11.3) at renal scores 1-2 compared to hsTnT (32.8 ± 20.9 ng/ L) at renal scores 3-4. Individual hsTnT correlated with clinical scores (R = 0.655, P < 0.001).
Conclusions: Impairment of renal score indicates higher cardiovascular risk. Permanent moderate elevation of hsTnT refers to increased cardiovascular risk, which has incremental value in the treatment of CKD.
Abbreviations
ACR: Urinary Albumin/Creatinine Ratio; CKD: Chronic Kidney Disease; GFR-EPI: estimated Glomerular Filtration Rate published by CKD-Epidemiology Collaboration; hsTnT: High Sensitive Troponin T; HbA1c: Hemoglobin A1c; non-HDL-C: difference between total cholesterol and HDL-cholesterol; Scr: Serum Creatinine; TPCR: Urinary Total Protein/Creatinine
Introduction
Cardiovascular mortality risk is increased in all stages of chronic kidney disease [1-4]. Although cardiac troponin is the first choice in the diagnosis of acute myocardial infarction, proper interpretation of slightly elevated troponin is challenging, because high sensitive troponin T tests applied since ~2009 detect even the smallest cardiac damage. Recent clinical studies proved that high sensitive troponin level depends on GFR, and it increases with the impairment of renal function. According to the current KDIGO 2013 guideline [5], automated reporting of estimated Glomerular Filtration Rate (GFR) in laboratories established the classification of Chronic Kidney Disease (CKD). However, recognition of chronic kidney disease in early stage is often based on albuminuria or proteinuria [6-15]. According to KDIGO 2013 guideline for the assessment of cardiovascular risk in chronic kidney disease GFR- and proteinuria-based renal score is recommended.
The first aim of this study was to evaluate whether renal score is enough to estimate cardiovascular risk in CKD for a long run. Therefore, in Study A we retrospectively evaluated renal scores, GFR, proteinuria and the effect of proteinuria on the efficacy of lipid lowering therapy during a four-year period.
The second aim was to prove whether high sensitive troponin T (hsTnT) has an incremental value to predict cardiovascular risk. For this we evaluated hsTnT at different stages of CKD and correlation with clinical cardiovascular score (Study B). As the permanently elevated hsTnT is common in CKD, correct evaluation of cardiac state is a crucial task at emergency departments, nephrology or cardiac centers.
Subjects and Methods
Study A
First in a retrospective study we reviewed the clinical and laboratory data of 400 patients, who were followed regularly at the Nephrology Outpatient Centre, University of Debrecen in a four-year period (2009-2012) without acute cardiac events. To calculate Framingham clinical cardiovascular scores and renal scores, we could enroll only 20 patients who had a complete medical history (Table 1) and all laboratory results (serum creatinine, GFR, urinary protein (or albumin), creatinine from the first morning urine, serum cholesterol, LDL-C, HDL-C, triglyceride and HbA1c in diabetes mellitus) each year (2009-2012). At the beginning of Study A median age of the 20 patients (15 men, 5 women) was 65 years (38-88 years). The clinical score indicating the risk of cardiovascular mortality was estimated from the parameters of the Framingham study [16,17]. The patient's history and traditional cardiovascular risk markers were taken into account as it is detailed in Table 1 (age, BMI, blood pressure, cardiac events, myocardial infarction, stroke, blood lipids, hyperglycemia, diabetes mellitus, HbA1c). Renal score was estimated from the glomerular filtration rate (GFR-EPI) and urinary protein/creatinine or albumin/creatinine ratio (Table 2). The patients were sorted into four groups of severity based on renal scores (1: low, 2: moderate, 3: high, 4: extremely high cardiovascular risk). The advantage of GFR-EPI formula is the reliability up to 90 ml/min/1.73 m2; it was calculated as Levey et al published for adults in 2009 [14]:
Female if Scr <62 μmol/ L: eGFR=144X(Scr/61.6)-0,329X(0.993)age
Female if Scr >62 μmol/ L: eGFR=144X(Scr/61.6)-1,209X(0.993)age
Male if Scr <80 μmol/ L: eGFR=141X(Scr/79.2)-0,411X(0.993)age
Male if Scr >80 μmol/ L: eGFR=141X(Scr/79.2)-1,209X(0.993)age
Fasting blood samples were collected in Becton-Dickinson vacuum tubes with a gel separator. After centrifugation (1800 g, 10 min) clinical biochemical tests were performed on a Cobas-6000 analyzer (Roche Diagnostics Ltd, Mannheim). Serum cholesterol and triglyceride were determined by enzymatic assay, HDL-cholesterol and LDL-cholesterol were measured by homogenous enzymatic assay. For monitoring the efficacy of lipid lowering therapy, non-HDL-C the sum of atherogenic lipoproteins was calculated from the difference of total cholesterol and HDL-cholesterol. We considered non-HDL-C<3.3 mmol/L as the target value in chronic kidney disease. Urinary protein, albumin and creatinine were determined from the first morning urine. Urinary albumin was analyzed by immunoturbidimetry, urinary total protein was measured by turbidimetric assay. To compensate diurnal variation, first morning urinary albumin/creatinine and protein/creatinine were taken into consideration. Urinary and serum creatinine was determined by kinetic Jaffe method.
In Study A during a four-year period (2009-2012) 50% of the patients was treated with atorvastatin (10-40 mg/ day), 25% received simvastatin (10-40 mg/ day), and 25% were treated with ezetimibe (20-40 mg/ day).
Study B
We enrolled 21 CKD patients (19 men, 2 women); their median age was 67 years (32-89 years). They were monitored regularly at the Nephrology Outpatient Centre and had no previous acute cardiac event. We enrolled patients who had complete medical history and laboratory results to calculate clinical cardiovascular score and renal score. Their hsTnT was determined from a fasting serum sample by chemiluminescent immunoassay on Cobas 6000 analyzer.
Ethics approval
The work is conforming to the guiding principle of the Declaration of Helsinki; the patients gave informed consent for the study approved by the Institutional Committee on Human Research, Debrecen (RKEBI/IKEBI 3764-2012).
Table 1
| Table 1: Clinical score as a basic marker of cardiovascular risk was calculated from parameters of the Framingham study [16,17]. | ||||||
| Age (years) | <40 | 40-60 | 60-70 | >70 | ||
| score | 1 | 2 | 3 | 4 | ||
| Hypertension | ||||||
| duration (years) | <5 | 5-10 | >10 | |||
| <140 Hgmm | 1 | 2 | 3 | |||
| 140-160 Hgmm | 4 | 5 | 6 | |||
| >160 Hgmm | 7 | 8 | 9 | |||
| Diabetes mellitus | ||||||
| duration (years) | <5 | 5-10 | >10 | |||
| HbA1c= 6.5-7.5 | 2 | 4 | 6 | |||
| HbA1c >7.5 | 4 | 6 | 8 | |||
| Cardiac event | ||||||
| Acute myocardial infarction, stroke | 10 | |||||
| Cholesterol | ||||||
| level (mmol/L) | <4.5 | 4.5-5 | >5 | |||
| score | 0 | 3 | 6 | |||
| Triglyceride (mmol/L) | <1.7 | 1.7-2.5 | ||||
| score | 0 | 3 | ||||
| LDL-C (mmol/L) | <2.5 | 2.5-3 | >3 | |||
| score | 0 | 3 | 6 | |||
| HDL-C (mmol/L) | >1 male, >1.3 female | <1 male, <1.3 female | ||||
| score | 0 | 3 | ||||
| Body mass index | 23-25 | 25-30 | >30 | |||
| score | 2 | 4 | 6 | |||
| Cardiac symptoms 2 scores/symptom |
chest pain oedema palpitation fatigue asphyxia arrhythmia cardiac decompensation |
|||||
| Abbreviations: HbA1c: Hemoglobin A1c; LDL-C: Low Density Lipoprotein; HDL-C: High Density Lipoprotein | ||||||
Table 2
| Table 2: Renal score based on GFR and proteinuria shows relative risk of cardiovascular mortality in chronic kidney disease [6,7]. | |||||
| Relative risk of cardiovascular mortality in CKD on the base of GFR and proteinuria |
Stages of albuminuria or proteinuria (mg/ mmol) | ||||
| A1: normal ACR <3 |
A2: moderate ACR: 3-30, or TPCR: 15-50 |
A3: severe ACR >30, or TPCR: 51-350 |
A3n: nephrotic TPCR >350 |
||
| GFR stages (ml/min/ 1.73 m2) | G1: normal, or elevated >90 | Low | Moderate | High | Extremely High |
| G2: mild decrease 60-89 | Low | Moderate | High | Extremely High | |
| G3a: mild-moderate decrease 45-59 | Moderate | High | Extremely High | Extremely High | |
| G3b: moderate-severe decrease 30-44 | High | Extremely High | Extremely High | Extremely High | |
| G4: severe decrease 15-29 | Extremely High | Extremely High | Extremely High | Extremely High | |
| G5: end-stage renal failure <15 | Extremely High | Extremely High | Extremely High | Extremely High | |
| Low risk: 1 (relative risk: 1-1.49), moderate risk: 2 (relative risk: 1.5-2.29), high risk: 3 (relative risk: 2.3-3.69), extremely high risk: 4 (relative risk >3.7) ACR: urinary Albumin/Creatinine (mg/mmol), GFR: Glomerular Filtration Rate, TPCR: urinary Total Protein/Creatinine (mg/ mmol). |
|||||
Statistical Analysis
Statistical calculations were done using the SPSS package version 22 (IBM, Somers, NY). In the retrospective Study A we analyzed the changes of the renal score, GFR, proteinuria and non-HDL with time by Friedman's test. The average yearly changes of GFR and non-HDL demonstrated normal distribution, so one-sample t-test could be applied to test whether the changes were significant. The change rates of the groups with and without proteinuria were compared using two-sample t-test. In Study B we compared troponin T levels and clinical scores between the groups with mild vs. severe renal damage by Welch test, since the variances of the groups were significantly different by Levene's test. We tested whether the troponin T level and the renal score were independent predictors of the clinical cardiovascular score using stepwise forward linear regression analysis.
Results
In the retrospective Study A during a four-year period (2009-2012) we observed significant impairment of the GFR (P < 10-6) and renal score (P < 10-4; Friedman test for all patients). While the individual GFR values decreased 0-15 ml/min/1.73 m2 in a year, urinary protein/creatinine fluctuated. During the four-year period, considerable impairment of renal function was observed in three patients: proteinuria increased and GFR decreased to the value of <30 ml/min/1.73 m2. The mean of GFR decreased from 66 ± 12 to 47 ± 16 ml/min/1.73 m2 in four years. In most cases the decrease of GFR was followed by progressing proteinuria. The clinical cardiovascular score was proportional to the renal score: mean of clinical score was 21.8 ± 9.8 at renal score <3.5 and 29.2 ± 6.7 at renal score >3.5 (p:0.09). Our results denote that impairment of renal score based on GFR and proteinuria indicates higher cardiovascular risk.
Efficacy of lipid lowering therapy also depended on proteinuria: the atherogenic non-HDL-cholesterol (non-HDL-C) decreased significantly in the 11 patients without proteinuria (P < 0.05, two-sample t-test), but it was not reducible in the 9 patients with proteinuria (Figure 1). Mean value of non-HDL-C decreased from 3.62 ± 0.75 to 2.92 ± 0.63 mmol/ L without proteinuria, and remained 3.5 ± 1.2 mmol/L with significant proteinuria in spite of lipid lowering therapy (target value of non-HDL-C<3.3 mmol/ L).
In Study B (n:21) we investigated whether high sensitive Troponin T (hsTnT) has an incremental value to predict cardiovascular risk in CKD. Both cardiac hsTnT level (Welch test: P = 0.02) and clinical cardiovascular score (P = 0.017) were significantly higher at severe kidney damage (renal scores 3 or 4) than at mild kidney damage (renal scores 1 or 2), (Figure 2). Mean of hsTnT was higher (32.8 ± 20.9 ng/L) at severe renal damage than at mild renal damage (14.8 ± 11.3). The upper limit of reference range is hsTnT<14 ng/L (99 percentile value). Mean of clinical cardiovascular score was also higher (27.4 ± 9.2) at severe renal damage than at mild renal damage (21.5 ± 2.1), (Figure 2).
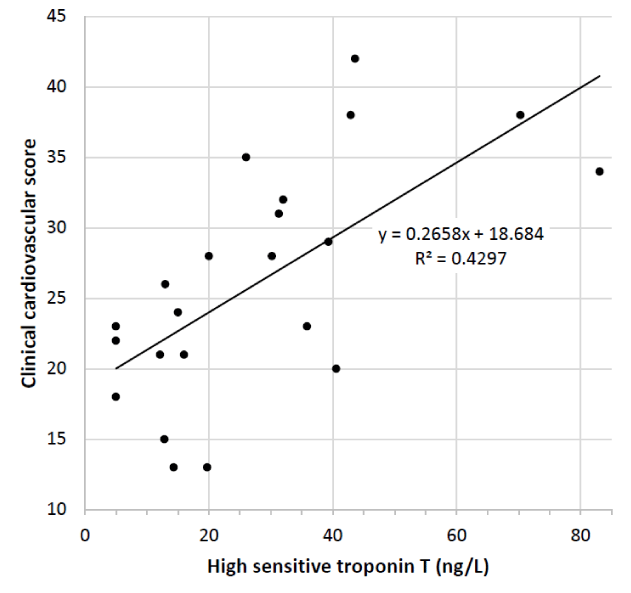
The correlation between clinical cardiovascular score and hsTnT (R = 0.655, p < 0.001) indicates that permanent moderate elevation of hsTnT has incremental value in predicting cardiovascular risk in CKD (Figure 3).
Figure 1
The efficacy of lipid lowering therapy depends on proteinuria: atherogenic non-HDL was successfully decreased in the group (n:11) without proteinuria (P < 0.05, two-sample t-test), while change in non-HDL-C was Not Significant (NS) in patients (n:9) with proteinuria during four years long lipid lowering therapy in Study-A. SEE: Standard Error of the Estimate
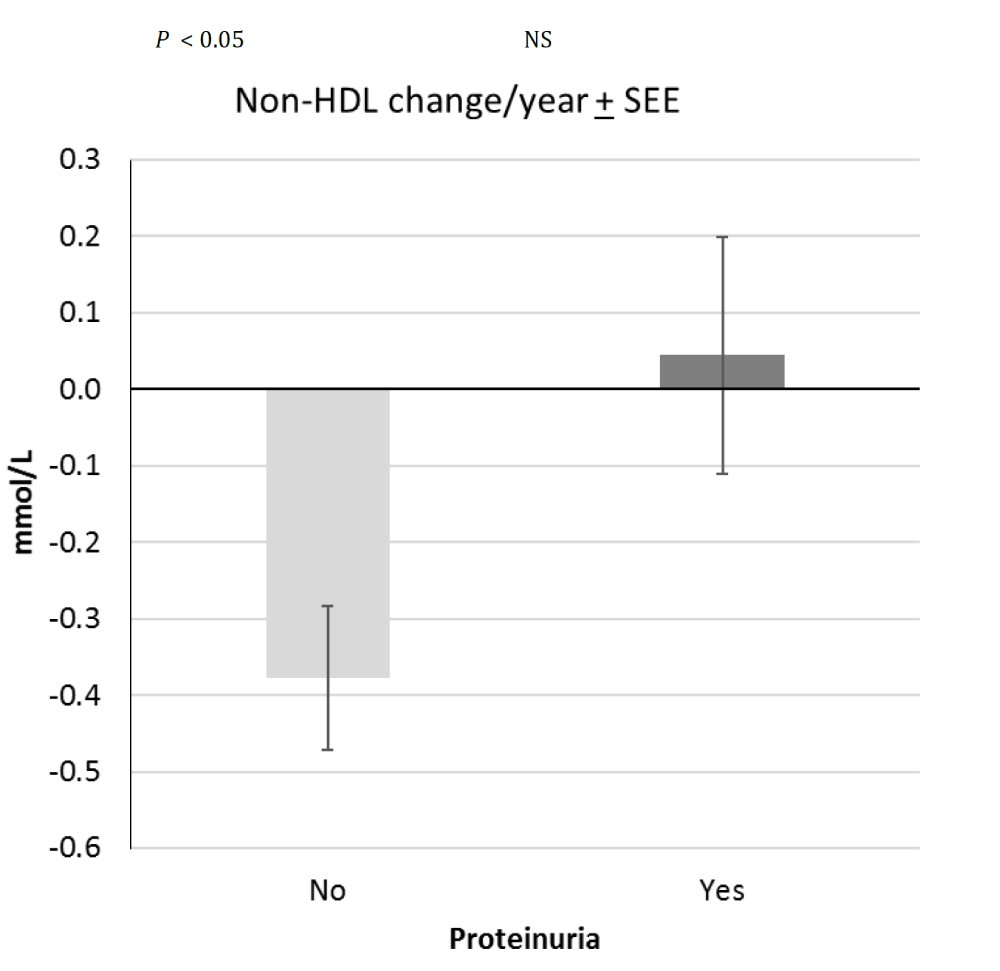
Figure 2
Both high sensitive troponin T (P = 0.020, Welch test) and clinical cardiovascular score (P = 0.017) were significantly higher at severe kidney damage (renal scores 3 or 4) than at mild kidney damage (renal scores 1 or 2). Study-B, n:21. Upper limit of the reference range is 14 ng/ L for hsTnT.

Figure 3
Clinical cardiovascular score (based on Framingham study) correlates with high sensitive troponin T level in chronic kidney disease (R = 0.655, P < 0.001). Study-B, n:21.
Discussion
Our retrospective study detected the impairment of renal score in a four-year period. The results support that clinical cardiovascular score is proportional to renal score and it indicates the diagnostic merit of the combined renal score for the estimation of Framingham-based cardiovascular risk-assessment. In chronic kidney disease, for the estimation of cardiovascular mortality risk both the glomerular filtration rate and albumin/proteinuria should be monitored. Permanent proteinuria alone implies an elevated cardiovascular risk, and it indicates the progression of vascular damage. In case of diabetes mellitus urinary albumin is recommended as a first, more sensitive test. Even the first grade of albuminuria (3-30 mg/ mmol urinary albumin/creatinine) indicates high risk of cardiovascular morbidity, if GFR is decreased to as low as 45-59 ml/min/1.73 m2 (Table 2). It is in accordance with the opinion of Astor, et al. [18], who established that 10 ml/min/1,73 m2 decrease in GFR at GFR<60 ml/min/1.73 m2 is associated with 1.29 and doubling of albuminuria with 1.06 relative risk of cardiovascular mortality.
Among the cardiovascular lipid markers non-HDL-C decreased in response to lipid lowering therapy only in those patients who did not have proteinuria. The results indicate that proteinuria may be a predictor of non-successful lipid-lowering therapy. These findings underline the importance of monitoring proteinuria and GFR not only in kidney failure, but in other cardiovascular diseases as well. One limitation of our study might be the different concentration of the first morning urine samples, which might cause a scattering in proteinuria, even if it was normalized to urinary creatinine. Another limitation was the low number of cases, which was due to the fact that to calculate Framingham clinical cardiovascular and renal scores, we could only enroll the patients who had a complete medical history and all laboratory results. However, the four-year follow-up proved the utility of renal score in the estimation of cardiovascular risk.
Our results confirm that slightly elevated cardiac troponin is common in CKD. Although a permanent slight elevation of cardiac troponin is considered generally as a consequence of decreased GFR [19-22], we found a moderate correlation between non-renal clinical cardiovascular score and hsTnT. According to our results permanently elevated troponin can be considered as a marker of increased cardiovascular risk. Our opinion agrees with recent publications in CKD [23-26]. If the renal score shows a rapid impairment, it probably indicates the progression of vascular or myocardial damage, which can be detected early by high sensitive troponin T. Elevation of troponin depends on GFR and at end-stage renal impairment it correlates with cardiac events and total mortality [26]. It agrees with our opinion that in CKD hsTnT is predominantly a marker of cardiovascular risk. Individual troponin T measured in balanced conditions may have importance at the emergency unit when a patient has atypical complaints and slightly elevated troponin. For the differentiation between acute and chronic cardiac damage, repeated troponin testing is proposed. The recent guidelines recommended comparison of 0/1-hour or 0/3-hour values of hsTnT with a test-specific cutoff, and it yields reliable evaluation [27,28]. The permanent slight elevation of hsTnT can be considered as increased cardiovascular risk. Elevated baseline troponin has an incremental prognostic value [29] beside GFR and albumin/proteinuria in the diagnosis and treatment of chronic kidney disease.
References
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007; 298: 2038-47.
- Parikh N, Hwang SJ, Larson MG, Levy D, Fox CS. Chronic kidney disease as a predictor of cardiovascular disease (from the Framingham Heart Study. Am J Cardiol. 2008; 102: 47-53.
- McCullough PA, Li S, Jurkovitz CT, Stevens L, Collins AJ, Chen SC, et al. Chronic kidney disease, prevalence of premature cardiovascular disease, and relationship to short-term mortality. Am Heart J. 2008; 156: 277-83.
- Apple FS, Jesse RL, Newby LK, Wu AH, Christenson RH, Cannon CP, et al. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: analytical issues for biochemical markers of acute coronary syndromes. Clin Chem. 2007; 53: 547-51.
- CKD Workshop Group KDIGO. Clinical Practice Guideline for Evaluation and Management of Chronic Kidney Disease. Kidney Inter Suppl. 2013; 3: 1-150.
- Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010; 375: 2073-81.
- Olah AV, Fodor B, Matyus J. Current questions on proteinuria. J Clin Metab & Diab. 2012; 3: 26-32.
- Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type- 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008; 371: 1800-1809.
- Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002; 106: 1777-82.
- Zhang QL, Brenner H, Koenig W, Rothenbacher D. Prognostic value of chronic kidney disease in patients with coronary heart disease: role of estimating equations. Atherosclerosis. 2010; 211: 342-7.
- National Kidney Foundation Guidelines. November 2010: https://www.kidney.org/professionals/guidelines/guidelines
- Methven S, MacGregor MS, Traynor JP, O'Reilly DS, Deighan CJ. Assessing proteinuria in chronic kidney disease: protein-creatinine ratio versus albumin-creatinine ratio. Nephrol Dial Transplant. 2010; 25: 2991-6.
- Methven, MacGregor MS, Traynor JP, Hair M, O'Reilly DS, Deighan CJ. Comparison of urinary albumin and urinary total protein as predictors of patients outcomes in CKD. Am J Kidney Dis. 2011; 57: 21-28.
- Levey A, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150: 604-612.
- Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012; 379: 165-80.
- Castelli WP, Anderson K, Wilson PW, Levy D. Lipids and risk of coronary heart disease. The Framingham Study. Ann Epidemiol. 1992; 2: 23-8.
- Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998; 97: 1837-47.
- Astor BC, Hallan S, Miller ER, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause Mortality in the US population. Am J Epidemiol. 2008; 167: 1226-1234.
- Khan NA, Hemmelgarn BR, Tonelli MR, Thompson CR, Levin A. Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: A meta-analysis. Circulation. 2005; 112: 3088-96.
- Kristian T; Johannes M; Hugo K; Mario P; Per V; Paul C, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J. 2010; 31: 2197-2204.
- Pianta TJ, Horvath AR, Ellis VM, Leonetti R, Moffat C, Josland EA, Brown MA, et al. Cardiac high sensitivity troponin T measurement: A layer of complexity in managing haemodialysis patients. Nephrology. 2012; 17: 636-641.
- Szantho E, Szabo Z, Varga J, Paragh G, Olah AV. [Interpretation of high sensitive troponin assays: acute or chronic myocardial damage?] Orv Hetil. 2011; 152: 1528-1534.
- Goicoechea, M, Garca de Vinuesa, S, Gomez-Campdera F, Gutierrez MJ, Blanco P, Amann R, Luno J, et al. Clinical significance of cardiac troponin T levels in chronic kidney disease patients: predictive value for cardiovascular risk. Am J Kidney Dis. 2004; 43: 846-853.
- Marcinkowska E, Flisinski M, Manitius J. [Diagnostic and prognostic value of cardiac troponins in patients with chronic kidney disease]. Pol Merkur Lekarski. 2007; 137: 375-381.
- Wang F, Ye P, Luo L, Xu R, Bai Y, Wu H Association of glomerular filtration rate with high-sensitivity cardiac troponin T in a community-based population study in Beijing. PLoS One. 2012; 7: e38218.
- Jain N, Hedayati SS. How should clinicians interpret cardiac troponin values in patients with ESRD? Semin Dial. 2011; 24: 398-400.
- Muller C, Giannitsis E, Christ M, Ordonez-Llanos J, deFilippi C, McCord J, et al. Multicenter Evaluation of a 0-hour/1-hour algorithm in the diagnosis of myocardial Infarction with hs cardiac troponin-T. Ann Emerg Med. 2016; 68: 76-87.
- Fahim MA, Hayen AD, Horvath AR, Dimeski G, Coburn A, Tan KS, et al. Biological variation of high sensitive cardiac troponin T in stable dialysis patients: implications for clinical practice. Clin Chem Lab Med. 2015; 53: 715-722.
- Acharji S, Baber U, Mehran R, Fahy M, Kirtane AJ, Lansky AJ, et al. Prognostic significance of elevated baseline troponin in patients with acute coronary syndromes and chronic kidney disease treated with different antithrombotic regimens:a substudy from the ACUITY trial. Circulation Cardiovascular Interventions. 2012; 5: 157-165.
Authors submit all Proposals and manuscripts via Electronic Form!




























