Research Article
Computational Study of Carbon, Silicon and Boron Nitride Nanotubes as Drug Delivery Vehicles for Anti-Cancer Drug Temozolomide
Farshad Shahidi1* and Seyed Majid Hashemianzadeh2
1Department of Nano-Chemistry, Iran University of Science and Technology, Tehran, Iran
2Molecular Simulation Research Laboratory, Department of Chemistry, Iran University of Science and Technology, Tehran, Iran
*Address for Correspondence: Farshad Shahidi, Department of Nano-Chemistry, Iran University of Science and Technology, Tehran, Iran, Tel: +989-102-064-022; ORCID ID: orcid.org/0000-0002-1393-5687; Researcher ID: researcherid.com/rid/S-3234-2018; E-mail: shahidi_farshad@yahoo.com
Dates: Submitted: 10 September 2018; Approved: 28 September 2018; Published: 04 October 2018
Citation this article: Shahidi F, Hashemianzadeh SM. Computational Study of Carbon, Silicon and Boron Nitride Nanotubes as Drug Delivery Vehicles for Anti-Cancer Drug Temozolomide. Am J Nanotechnol Nanomed. 2018; 1(2): 048-054.
Copyright: © 2018 Shahidi F, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Nanotube; Drug delivery; Temozolomide; Encapsulation; Drug carrier
Abstract
Using various nanotubes for targeted drug delivery systems as well as interactions between drug and nanotubes with diverse properties such as gender, structure, and diameter are investigated based on Density Functional Theory (DFT) for the first time. A wide assortment of weak absorption on SWCNTs and SWBNNTs, it is demonstrated that Temozolomide (TMZ) molecule tend to be physical absorption into SWSiCNTs. The geometrical structures, energetics, and electronics properties of TMZ molecule and nanotubes obtained. There is a tolerable competing amount between decreasing of diameter and curvature effect that results indicate curvature has a much more salient effect. The results obtained by the Density of State (DOS) and Molecular Orbitals (MO) indicates adsorption of the drug into nanotubes is physical.
Graphical Abstract: Drug located into nanotube, and drug distance of the nanotube interior surface is equivalent
Introduction
Cancer is one of the great reasons for mortality overall in the whole world [1]. Generally, unshielded and direct using chemotherapy drugs for cancer treatment is one of the approaches collation with this disease that has pretty dangers for healthy cells [2]. In recent studies, many researchers have employed targeted Drug Delivery Systems (DDS) for obliterating cancer’s tissues, by taking nanostructures have been provided [3-6]. Nanotubes are the great materials that possess potential applications in the DDS domain [4,7-13]. Currently, the huge interior region, needles shape and the viable surface functionalization of the nanotubes, those have been converted to perfect Nano carriers for DDS [14-23]. Besides, most of the small drug molecules confined in CNTs have been investigated theoretically, including Doxorubicin (DOX), cisplatin, gemcitabine, ciprofloxacin, indomethacin, lamivudine, amantadine, and TMZ. Furthermore, for decreasing of the toxicity effect of the anti-cancer drugs developing DDS is necessary [24]. TMZ drug molecule has been continuously attracted attention some of the researchers because of its extended use as an oral alkylating agent in treatment dangerous brain tumors such as glioblastoma, astrocytoma, and melanoma which are serious types and offensive of the brain cancer. It is frequently used in the treatment of a type of tumor namely known glioma. Thus, this pharmaceutical molecule is in current first-line of chemotherapy for behaving against brain cancer. Despite this positive effect of the TMZ on cancer’s tissues, practically procedure of patient’s treatment is quite poor. On the other hand, this process totally rejects many factors such as to cross over the Blood-Brain Barrier (BBB), short half-life in circulation, low drug arrival and extreme drug permeating in the tumor cells [24-27]. TMZ has pretty inclined to join other combinations, especially, with the pharmaceutical feature materials. With this in mind, that has capable of constituting non-covalent bonds and building supra-molecular structures. Nevertheless, for the purposeful drug delivery, encapsulation drug into nanotubes are one of the most efficacious methods. Additionally, nanotubes have got analogous structures although they possess various properties including biocompatibility, solubility, functionalization, and cellular uptake. So, this diversity of the properties has been attributed to symmetry and type cross-link bonds on the nanotube walls, that on the base it, nanotubes had to divide into three diverse types consisting of an armchair, zigzag and chiral. Symmetry is one of the key factors in an interaction between drug and nanotubes. Moreover, a considerable number of studies have been done on the both of the experimental and theoretical about to use nanotubes as Nano-carriers of anti-cancer drugs [8,28-40].
Yixuan et al. [41] looked into theoretically to adsorbing process of DOX as an anti-cancer drug on the surface, and into single-walled carbon nanotubes. Progressively, they were comprehension that drug encapsulated was better and stronger than the adsorption on a sidewall of the nanotubes, owing to the fact that there was in the process of drug encapsulation, hydrogen bonding, and π-π stacking were together, despite the adsorption on a surface of the nanotubes there was only have a little π-π stacking interaction. Thereby the process of drug encapsulation and drug release of nanotubes would occur slowly, and also little drug amounts would be missed before arriving at tumor’ tissues, that’s quite vital in DDS. Generally, the Interaction between TMZ and SWCNT open-ended have been investigated in various environments and temperatures by molecular mechanics and quantum mechanics methods. As a result, have been indicated that the most stable environment for the interaction between TMZ and SWCNT open-ended was water solvent in 310 K [42]. In fact, there were some made natural capsules bio-molecules for example proteins, peptides, and an anti-cancer drug molecule has been successfully done [43,44]. For this purpose, roosta et al had investigated the encapsulation process of the anti-cancer drug Gemcitabine (GMC) in Single-Walled Boron Nitride Nanotubes (SWBNNTs) open-ended, and Single-Walled Carbon Nanotubes (SWCNTs) open-ended by Molecular Dynamics (MD) simulations. Indeed, the study results of the encapsulation have shown that GMC inclined to come through in BNNT and located in its center [31,43]. Therefore, a suitable understanding of the behavior of drug molecules inside nanotubes in the encapsulation process is vital for the extension of drug delivery carriers [18].
In this study, the process of encapsulation of TMZ as an anti-cancer drug was investigated by quantum mechanics methods. As the first step in this direction, a wide variety of nanotubes was used, to predict the most favorable structure toward carrier of the drug to tumor cells including SWCNTs, SWBNNTs, and Single-Walled Silicon Carbide (SWSiCNTs) open-ended for the first. Equally important, effects of type (gender), structure and diameter of nanotubes in this research has been investigated. In conclusion, the totally optimized geometry of structures, including drug, nanotubes, and complexes ( like as drug@nanotubes) have been done, and binding energies of complexes have been calculated as well as electronic properties of structures such as Density of States (DOS) have been determined.
Computational Method
Preparation of initial models
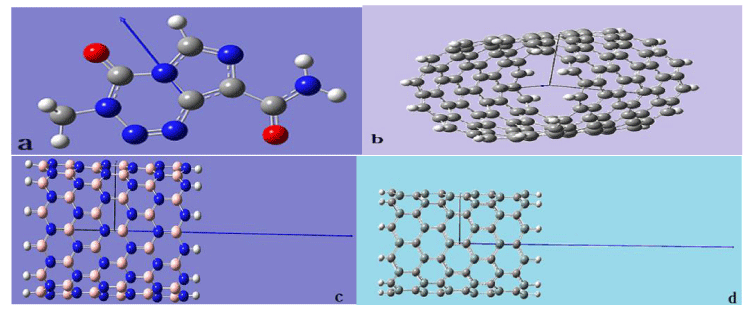
In this research, the zigzag and armchair open-ended single-walled nanotubes with a diameter ranging from 12 to 16 angstrom and length ranging from 17 to 24 angstrom have been considered, wherein, the fully optimized geometry structure of drug and nanotubes and information details them presented in figure 1 and table 1 [45]. For instance, nanotubes structure have been designed by NANOTUBE MODELER package [32,46]. The structure of drug has been constructed by GAUSS VIEW 5.0 package. The diameter of the drug is 9.171 angstrom. Optimization of drug and nanotubes geometry structure has been done by GAMESS-US/UK package [47]. All nanotubes are open-ended and for unfeeling dangling bond effect, and a decrease of time calculation, a mouth of nanotubes were enriched with H atoms [46,48].
 Figure 1: Fully optimized structures of a) CNT (10, 10) b) BNNT (10, 10) C) SiCNT (8, 8) d) CNT (10, 0) e) BNNT (10, 10) f) SiCNT (14, 0) g) Drug.
Figure 1: Fully optimized structures of a) CNT (10, 10) b) BNNT (10, 10) C) SiCNT (8, 8) d) CNT (10, 0) e) BNNT (10, 10) f) SiCNT (14, 0) g) Drug.
Table 1: Pristine nanotubes considered. |
||
| Species | Diameter (A°) | Length (A°)a |
| Pristine CC (10, 10) | 14.2 | 19.07 |
| Pristine CC (17, 0) | 13.8 | 17.8 |
| Pristine BN (10, 10) | 14.3 | 19.7 |
| Pristine BN (17, 0) | 13.6 | 18.1 |
| Pristine SiC (8, 8) | 14.3 | 22.6 |
| Pristine SiC (14, 0) | 14.07 | 22.2 |
aA*: Angstrom. |
||
Quantum mechanics
All calculations optimized geometry structures, and also complexes, binding energies have been done by density functional theory (DFT-Semi-core Pseudopods) method. Owing to the fact that the core electrons are dropped, the calculation is less computationally expensive, but because of these core potentials including some degree of relativistic effects, they can be very useful approximations for heavier elements. In the present work, Ab initio calculations have been performed on the Generalized-Gradient Approximation (GGA) with the Perdew-Wang 91 (PW91) functional, as well as all of the calculations performed on Double Numerical Polarization (DNP) as a basis set [41].
Results and Discussion
Under those circumstances, results being demonstrated a process of encapsulation of drug inside nanotubes, in the first stage, in order to understand the nanotube/drug interactions, the Binding Energy (Eb) of the drug onto nanotube is defined as shown in a table 2. All nanotubes are proportionate to the drug diameter. The diameter of the drug is 9.171 angstrom. In this regard, the negative Eb values indicate that the stability of complex nanotubes/drug is energetically favorable. Computation of binding energies described by the ensuing equation:
Table 2: Binding energy (Eb) of TMZ into SWNTs considered. |
|
| Species | Eads (kcal/mol) |
| CC (10, 10)@Drug | -5.3 |
| CC (17, 0)@Drug | -5.9 |
| BN (10, 10)@Drug | -7.7 |
| BN (17, 0)@Drug | -7.5 |
| SiC (8, 8)@Drug | -9.1 |
| SiC (14, 0)@Drug | -11.1 |
Eb= ENT-Drug – (ENT + EDrug)
Where, ENT-Drug, ENT, Drug are respectively total energies of Drug@nanotube complex, the isolated nanotubes, and isolated TMZ. According to the definition, the polarity of the drug molecule indicates that the process of drug encapsulation into polarity nanotubes are energetically favorable in figure 2. These results have been indicated structure and type (gender) of nanotubes play a vital role in the stability of drug into tubes (Figure 3).
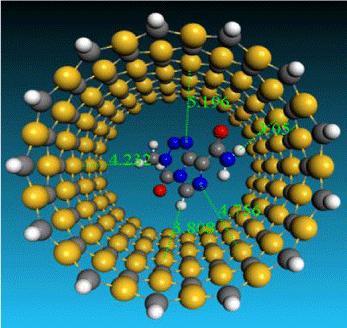 Figure 2: A final optimized geometries, drug located into the tube, and the equilibrium distances drug of interior’ surface of the tube.
Figure 2: A final optimized geometries, drug located into the tube, and the equilibrium distances drug of interior’ surface of the tube.
In order to understand the effect of diameter in the process of drug encapsulation into nanotubes, different diameters shown in the table 3 was considered.
| Table 3: Types of nanotubes considered. | |||||
| Species | Diameter (A°) | Length (A°) | Species@Drug | binding energy (kcal/mol) | Adsorption energy (kcal/mol) |
| Pristine CC (9, 9) | 12.923 | 19.074 | CC (9, 9)@Drug | -6.82 | -4.41 |
| Pristine CC (10, 10) | 14.227 | 19.076 | CC (10, 10)@Drug | -5.32 | -3.35 |
| Pristine CC (11, 11) | 15.528 | 19.074 | CC (11, 11)@Drug | -4.76 | -2.18 |
| Pristine CC (16, 0) | 12.599 | 17.821 | CC (16, 0)@Drug | -6.56 | -4.31 |
| Pristine CC (17, 0) | 13.818 | 17.818 | CC (17, 0)@Drug | -5.99 | -4.05 |
| Pristine CC (18, 0) | 14.184 | 17.819 | CC (18, 0)@Drug | -3.95 | -2.01 |
| Pristine BN (9, 9) | 13.207 | 19.686 | BN (9, 9)@Drug | -9.27 | -7.47 |
| Pristine BN (10, 10) | 14.363 | 19.389 | BN (10, 10)@Drug | -7.76 | -6.41 |
| Pristine BN (11, 11) | 15.518 | 19.268 | BN (11, 11)@Drug | -7.47 | -6.00 |
| Pristine BN (16, 0) | 12.941 | 18.150 | BN (16, 0)@Drug | -9.00 | -7.17 |
| Pristine BN (17, 0) | 13.652 | 18.150 | BN (17, 0)@Drug | -7.54 | -5.74 |
| Pristine BN (18, 0) | 14.556 | 18.151 | BN (18, 0)@Drug | -7.65 | -5.94 |
| Pristine SiC (7, 7) | 12.662 | 24.185 | SiC (7, 7)@Drug | -10.91 | -9.39 |
| Pristine SiC (8, 8) | 14.349 | 22.635 | SiC (8, 8)@Drug | -9.12 | -7.91 |
| Pristine SiC (9, 9) | 16.109 | 24.184 | SiC (9, 9)@Drug | -8.64 | -7.60 |
| Pristine SiC (13, 0) | 12.916 | 21.094 | SiC (13, 0)@Drug | -11.54 | -10.27 |
| Pristine SiC (14, 0) | 14.072 | 22.191 | SiC (14, 0)@Drug | -11.18 | -9.98 |
| Pristine SiC (15, 0) | 14.098 | 21.098 | SiC (15, 0)@Drug | -9.56 | -8.40 |
The results indicate that a decrease in the diameter is responsible for the strongest of interactions. Also, effects Basis Set Superposition Error (BSSE) on the interaction energy between drug and nanotube can be obtained by the following equation:
Eads= ENT-Drug - (ENT + EDrug) + Ebsse
Primary calculation results show that among all selected nanotubes, an interaction between TMZ and SiCNT (7, 7), SiCNT (13, 0) is more favorable and reliable and promising than other tubes (Figure 4). In addition, investigation of the nanotubes diameters, before and after the encapsulation process, indicate that diameter of CNT (n, m) increasing after to encapsulate whereas for the CNT (n, 0) diameter decreased. Furthermore, the diameters of both of type (armchair, zigzag) BNNTs, SiCNTs are increased after the encapsulation process [8,35]. In the other words, whether the decrease in diameter is responsible for more interaction between the drug and the interior surface of the nanotube or effect of curvature. Obtained results have indicated according to the calculations performed in the DFT environment, the effect of curvature is more [48].
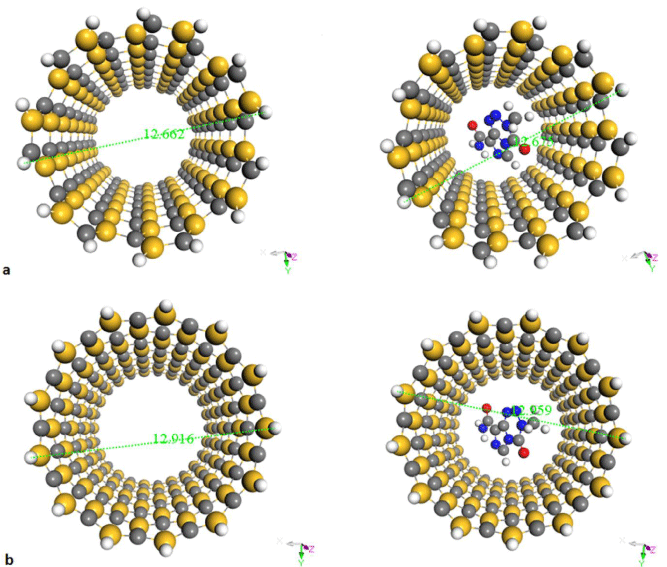 Figure 4: Calculated diameter of a) pristine SiC (7, 7) and complexes with drug b) pristine SiC (13, 0) and complexes with drug.
Figure 4: Calculated diameter of a) pristine SiC (7, 7) and complexes with drug b) pristine SiC (13, 0) and complexes with drug.
To the deepen understanding of values of the adsorption energy between TMZ and SWNTs and nature of bonds, we evaluated electronic properties based on the Density of States (DOSs) for the all of the complexes, isolated nanotubes, and isolated drug. As shown in figure 5. An insignificant change in the DOS spectra indicates that there isn’t a noticeable difference between the pristine nanotube and nanotube@drug complexes. Correspondingly, this data shows the presence of physical interaction between them. The above results were confirmed with more analyses. First, based on the change in the electronic structure of all complexes, could have been received that insignificant values of electrons are transferred in the process of encapsulation drug molecule between the drug molecule and nanotubes. Second, with respect to, results of Mulliken Population Analysis (MPA) exhibit that complexes charge transfer is negligible. Comparatively, in the CNTs, a charge transferred from the interior surface of the nanotube to drug molecule, but in the BNNTs, SiCNTs, the charge transferred from drug molecule to interior surface of the nanotube. As noted, the best interaction among complexes was related to SiCNT (7, 7)@Drug and SiCNT (13, 0)@Drug. A negligible change in the DOS spectra could be found for both desirable complexes, before and after the encapsulation process. In the other words, the low difference in the DOS plot before and after encapsulation process could be shown nature of bonds and also illustrated that adsorption was physical.
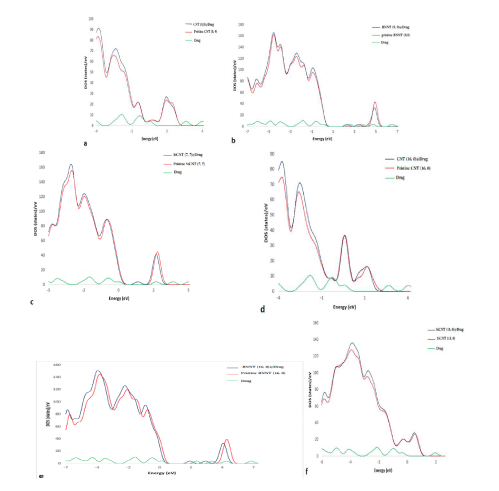 Figure 5: Calculated density of state for the complexes, pristine nanotubes and pure drug a) CNT (9, 9) b) BNNT (9, 9) c) SiCNT (7, 7) d) CNT (16, 0) e) BNNT (16, 0) f) SiCNT (13, 0).
Figure 5: Calculated density of state for the complexes, pristine nanotubes and pure drug a) CNT (9, 9) b) BNNT (9, 9) c) SiCNT (7, 7) d) CNT (16, 0) e) BNNT (16, 0) f) SiCNT (13, 0).
The final point which deserves some words here is that molecular orbital results analysis provides an impressive method for studying intra-and intermolecular bonding and interaction among bonds and provides a suitable basis for investigating charge transfer. Therefore, the electronic properties of pristine components and nanotube/drug systems are considered. The electron population analysis reveals that considerable charge transfer occurs during the adsorption and encapsulation processes. The Energy of the Highest Orbital Molecular Occupied (HOMO) and Lowest Orbital Molecular Unoccupied (LUMO) was calculated (Table 4). Fermi energy calculation for this systems indicates charge transfer happening between drug and tubes is negligible. As have been shown, the increase in diameter in the pristine armchair nanotubes, the gap energy amount is increased. In the light of, gap energy of armchair nanotubes complexes is increased while the number of gap energies for the zigzag pristine nanotubes and complexes at first increased and afterward decreased which displayed that chirality plays a salient role in the nanotube reactivity [35].
| Table 4: The calculated energies of HOMO (eV), LUMO (eV) and gap energy of Complexes, pristine nanotubes and drug. | ||||
| Species | Fermi energy (eV) | EHOMO (eV) | ELUMO (eV) | Gap energy (eV) |
| Pristine CC (9, 9) | -0.148255 | -3.955 | -3.737 | 0.218 |
| Pristine CC (10, 10) | -0.14866 | -3.987 | -3.731 | 0.256 |
| Pristine CC (11, 11) | -0.148823 | -3.971 | -3.713 | 0.258 |
| Pristine CC (16, 0) | -0.145842 | -3.896 | -3.744 | 0.152 |
| Pristine CC (17, 0) | -0.14598 | -3.914 | -3.892 | 0.022 |
| Pristine CC (18, 0) | -0.146021 | -3.979 | -3.9 | 0.079 |
| Pristine BN (9, 9) | -0.212046 | -5.527 | -0.941 | 4.586 |
| Pristine BN (10, 10) | -0.211638 | -5.588 | -1 | 4.588 |
| Pristine BN (11, 11) | -0.211 | -5.531 | -1.037 | 4.494 |
| Pristine BN (16, 0) | -0.210681 | -5.438 | -1.267 | 4.171 |
| Pristine BN (17, 0) | -0.21048 | -5.446 | -1.26 | 4.186 |
| Pristine BN (18, 0) | -0.210603 | -5.741 | -1.355 | 4.386 |
| Pristine SiC (7, 7 | -0.183764 | -4.816 | -2.495 | 2.321 |
| Pristine SiC (8, 8) | -0.183316 | -4.824 | -2.5 | 2.324 |
| Pristine SiC (9, 9) | -0.182771 | -4.984 | -2.652 | 2.332 |
| Pristine SiC (13, 0) | -0.146874 | -3.814 | -3.572 | 0.242 |
| Pristine SiC (14, 0) | -0.146439 | -3.797 | -3.62 | 0.177 |
| Pristine SiC (15, 0) | -0.146003 | -3.811 | -3.623 | 0.188 |
| CC (9, 9)@Drug | -0.147789 | -4.028 | -3.673 | 0.355 |
| CC (10, 10)@Drug | -0.148219 | -4.036 | -3.655 | 0.381 |
| CC (11, 11)@Drug | -0.148782 | -4.047 | -3.648 | 0.399 |
| CC (16, 0)@Drug | -0.145505 | -3.9 | -3.848 | 0.052 |
| CC (17, 0)@Drug | -0.145729 | -4.005 | -3.952 | 0.053 |
| CC (18, 0)@Drug | -0.145715 | -3.972 | -3.893 | 0.079 |
| BN (9, 9)@Drug | -0.210703 | -5.72 | -3.527 | 2.193 |
| BN (10, 10)@Drug | -0.210583 | -5.719 | -3.371 | 2.348 |
| BN (11, 11)@Drug | -0.210252 | -5.72 | -3.373 | 2.347 |
| BN (16, 0)@Drug | -0.2091 | -5.676 | -3.562 | 2.116 |
| BN (17, 0)@Drug | -0.20954 | -5.698 | -3.476 | 2.222 |
| BN (18, 0)@Drug | -0.209094 | -5.695 | -3.366 | 2.329 |
| SiC (7, 7)@Drug | -0.183405 | -4.992 | -3.376 | 1.231 |
| SiC (8, 8)@Drug | -0.183035 | -4.979 | -3.586 | 1.393 |
| SiC (9, 9)@Drug | -0.182582 | -4.968 | -3.496 | 1.472 |
| SiC (13, 0)@Drug | -0.147434 | -4.127 | -3.917 | 0.21 |
| SiC (14, 0)@Drug | -0.146863 | -4.105 | -3.949 | 0.156 |
| SiC (15, 0)@Drug | -0.146241 | -4.1 | -3.917 | 0.183 |
| Drug | -0.215385 | -5.861 | -3.441 | 2.42 |
Conclusion
In summary, according to the materials we have investigated the geometrical structures, energetics, and electronic properties as well as interactions between the drug molecule and nanotubes. In this work, we have reported a theoretical study of encapsulation of TMZ drug molecule into both armchair and zigzag different nanotubes. A wide variety of nanotubes with various properties like gender, structure, and diameter considered. The results obtained show that the drug molecule inclined to locate into SWSiCNT by physical adsorption. In case, the polarity of the drug molecule indicates that the process of drug encapsulation into polarity nanotubes are energetically favorable. Curvature effect plays a vital role in the encapsulated process. Especially, when the nanotube diameter goes decrease. It should be noted results obtained from DOS plot indicate the drug adsorption into nanotube was physical. It also negligible changes in amounts of energy between HOMO and LUMO indicates the drug molecule bonds with nanotubes are quite weak which it is a critical issue in releasing the drug molecule in vitro process. In case, some theoretical findings such as binding energies, charge transfer, a density of states plots, and total density introduced the nanotubes, typically SiCNT an efficacious nano-carrier for delivery of TMZ drug at nano-medical predominant. In that case, it’s hoped that the research will broaden our understanding of encapsulation behavior of drug molecules into nanotubes that have a vital role in developing drug delivery vehicles at the nano domain.
References
- Gmeiner WH, Ghosh S. Nanotechnology for cancer treatment. Nanotechnol Rev.2014; 3: 111-122. https://goo.gl/1wLnRU
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376: 687-697. https://goo.gl/VS5USG
- Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008; 14: 1310-1316. https://goo.gl/NTLxg2
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007; 2: 751-760. https://goo.gl/Y9edwC
- Hughes GA. Nanostructure-mediated drug delivery. Nanomedicine. 2005; 1: 22-30. https://goo.gl/M9oEJm
- Brannon PL, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004; 56: 1649-1659. https://goo.gl/5t1TtD
- Bhadra U, Manika Pal Bhadra, Jagannadh Bulusu, Yadav JS. Organic Nanotubes: promising vehicles for drug delivery. 2014. https://goo.gl/f7qtLU
- Rezvani M, Ganji DM, Faghihnasiri M. Encapsulation of lamivudine into single walled carbon nanotubes: A vdW-DF study. Physica E: Low-dimensional Systems and Nanostructures. 2013; 52: 27-33. https://goo.gl/iZQdHD
- Ji SR, Liu C, Zhang B, Yang F, Xu J, Long J, et al. Carbon nanotubes in cancer diagnosis and therapy. Biochim Biophys Acta. 2010; 1806: 29-35. https://goo.gl/pp6vuB
- Liu Z, Tabakman S, Welsher K, Dai H. Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano res. 2009; 2: 85-120. https://goo.gl/HxnGFK
- Hilder TA, Hill JM. Modeling the loading and unloading of drugs into nanotubes. Small. 2009; 5: 300-308. https://goo.gl/tRiszt
- Chopra NG, Luyken RJ, Cherrey K, Crespi VH, Cohen ML, Louie SG. Boron nitride nanotubes. Science. 1995; 269: 966-967. https://goo.gl/UTXrUV
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991; 354: 56-58. https://goo.gl/1mNs7H
- Maicon PL, Claudio de O, Augusto FO, Luciana G, Helio AD. Structural, electronic, and mechanical properties of single-walled halloysite nanotube models. The Journal of Physical Chemistry C. 2010; 114: 11358-11363. https://goo.gl/7x6QeM
- Ciofani G. Potential applications of boron nitride nanotubes as drug delivery systems. Expert Opin Drug Deliv.2010; 7: 889-893. https://goo.gl/vq395b
- Zhang Z, Guo W, Dai Y. Stability and electronic properties of small boron nitride nanotubes. Journal of Applied Physics. 2009; 105: 084312. https://goo.gl/AguP5m
- Melanko JB, Pearce ME, Salem AK. Nanotubes, nanorods, nanofibers, and fullerenes for nanoscale drug delivery, in Nanotechnology in Drug Delivery. Springer. 2009; 105-127. https://goo.gl/FYVkMH
- Hilder TA, Hill JM. Encapsulation of the anticancer drug cisplatin into nanotubes. in Nanoscience and Nanotechnology. International Conference. IEEE. 2008. https://goo.gl/9EwBRb
- Mpourmpakis G, Froudakis GE.Why boron nitride nanotubes are preferable to carbon nanotubes for hydrogen storage?: an ab initio theoretical study. Catalysis today. 2007; 120: p. 341-345. https://goo.gl/Z7SFs9
- Taguchi T, Igava N, Yamamoto H, Jitsukawa S. Synthesis of silicon carbide nanotubes. Journal of the American Ceramic Society. 2005; 88: 459-461. https://goo.gl/iDykij
- Cao G. Synthesis, properties and applications. World Scientific. 2004.
- Sun XH, Li CP, Wong WK, Wong NB, Lee CS, Lee ST, et al. Formation of silicon carbide nanotubes and nanowires via reaction of silicon (from disproportionation of silicon monoxide) with carbon nanotubes. J Am Chem Soc. 2002; 124: 14464-14471. https://goo.gl/Z9MiSR
- Peng S, Cho K. Chemical control of nanotube electronics. Nanotechnology. 2000; 11: 57. https://goo.gl/WKkdJV
- Martincic M, Tobias G. Filled carbon nanotubes in biomedical imaging and drug delivery. Expert Opin Drug Deliv. 2015; 12: 563-581. https://goo.gl/mhqZPG
- Kasende OE, Matondo A, Muya JT, Scheiner S. Interaction between temozolomide and HCl: preferred binding sites. Computational and Theoretical Chemistry. 2016; 1075: 82-86. https://goo.gl/7Rz7QA
- Saravanan G, Ravikumar M, Jadhav MJ, Suryanarayana MV, Someswararao N, Acharyulu PVR. A stability-indicating LC assay and degradation behavior of temozolomide drug substances. Chromatographia. 2007; 66: 291-294. https://goo.gl/y6FYc4
- Mejri A, Vardanega D, Tangour B, Gharbi T, Picaud F. Encapsulation into carbon nanotubes and release of anticancer cisplatin drug molecule. J Phys Chem B. 2015; 119: 604-611. https://goo.gl/tyDLxE
- Liu Z, Robinson JT, Tabakman SM, Yang K, Dai H. Carbon materials for drug delivery & cancer therapy. Materials today. 2011; 14: 316-323. https://goo.gl/fLvxtW
- Zhang S, Yang K, Liu Z. Carbon nanotubes for in vivo cancer nanotechnology. Science China Chemistry. 2010; 53: 2217-2225. https://goo.gl/9RR7j4
- Liu Z, Yang K, Lee ST. Single-walled carbon nanotubes in biomedical imaging. Journal of Materials Chemistry. 2011; 21: 586-598. https://goo.gl/yR7rka
- Roosta S, Nikkhah SJ, Sabzali M, Hashemianzadeh SM. Molecular dynamics simulation study of boron-nitride nanotubes as a drug carrier: from encapsulation to releasing. RSC Advances. 2016; 6: 9344-9351. https://goo.gl/WsjhPV
- Li Z, Tozer T, Alisaraie L. Molecular dynamics studies for optimization of noncovalent loading of vinblastine on single-walled carbon nanotube. The Journal of Physical Chemistry C. 2016; 120: 4061-4070. https://goo.gl/wU38Fy
- Rajarajeswari M, Iyakutti K, Lakshmi I, Rajeswarapalanichamy R, Kawazoe Y. Functionalized single-walled carbon nanotube (5, 0) as a carrier for Isoniazid-A tuberculosis drug. International Journal of Computational Materials Science and Engineering. 2015; 4: 1550014. https://goo.gl/5QWrjA
- Panczyk T, Konczak L, Michalek JN, Pastorin G. Corking and uncorking carbon nanotubes by metal nanoparticles bearing ph-cleavable hydrazone linkers. Theoretical analysis based on molecular dynamics simulations. The Journal of Physical Chemistry C. 2015; 120: 639-649. https://goo.gl/r7TeZM
- Khorsand A, Jamehbozorgi S, Ghiasi R, Rezvani M. Structural, energetic and electrical properties of encapsulation of penicillamine drug into the CNTs based on vdW-DF perspective. Physica E: Low-dimensional Systems and Nanostructures. 2015; 72: 120-127. https://goo.gl/7YBvxt
- Khatti Z, Hashemianzadeh SM. Investigation of thermodynamic and structural properties of drug delivery system based on carbon nanotubes as a carboplatin drug carrier by molecular dynamics simulations. Journal of Inclusion Phenomena and Macrocyclic Chemistry. 2015; 83: 131-140. https://goo.gl/njdDGJ
- Taghavi F, Javadian S, Hashemianzadeh SM. Molecular dynamics simulation of single-walled silicon carbide nanotubes immersed in water. Journal of Molecular Graphics and Modelling. 2013; 44: 33-43. https://goo.gl/GzT7X3
- Li Y, Hou T. Computational simulation of drug delivery at molecular level. Curr Med Chem. 2010; 17: 4482-4491. https://goo.gl/PTJcG1
- Zhao JX, Ding YH. Theoretical studies of the interaction of an open-ended Boron Nitride Nanotube (BNNT) with gas molecules. The Journal of Physical Chemistry C. 2008; 112: 20206-20211.
- Hilder TA, Hill JM. Theoretical comparison of nanotube materials for drug delivery. IET Micro & Nano Letters. 2008; 3: 18-24. https://goo.gl/cxqWK1
- Wang Y, Xu Z. Interaction mechanism of doxorubicin and SWCNT: protonation and diameter effects on drug loading and releasing. RSC Adv. 2016; 6: 314-322. https://goo.gl/vjbUB4
- Mollaamin F, Najafi F, Khaleghian M, Hadad BK, Monajjemi M. Theoretical study of different solvents and temperatures effects on single-walled carbon nanotube and temozolomide drug: a QM/MM study. Fullerenes, Nanotubes and Carbon Nanostructures. 2011; 19: 653-667. https://goo.gl/u9DYpn
- Roosta S, Hashemianzadeh SM, Ketabi S. Encapsulation of cisplatin as an anti-cancer drug into boron-nitride and carbon nanotubes: Molecular simulation and free energy calculation. Mater Sci Eng C Mater Biol Appl. 2016; 67: 98-103. https://goo.gl/zcovLE
- Kang Y, Wang Q, Liu YC, Shen JW, Wu T. Diameter selectivity of protein encapsulation in carbon nanotubes. J Phys Chem B. 2010; 114: 2869-2875. https://goo.gl/yp4iA1
- Mananghaya M, Promentilla MA, Aviso K, Tan R. Theoretical investigation of the solubilization of COOH-functionalized single wall carbon nanotubes in water. Journal of Molecular Liquids. 2016; 215: 780-786. https://goo.gl/An4hg7
- Sedghamiz E, Jamalizadeh E, Ali Hosseini SM, Sedghamiz T, Zahedi E. Molecular dynamics simulation of boron nitride nanotube as a drug carrier. Arabian Journal for Science and Engineering. 2014; 39: 6737-6742. https://goo.gl/MDNkvr
- Schaftenaar G, Noordik JH. Molden: a pre-and post-processing program for molecular and electronic structures. Journal of computer-aided molecular design.2000; 14: 123-134. https://goo.gl/AZV7sW
- Zaboli M, Raissi H. The influence of nicotine on pioglitazone encapsulation into carbon nanotube: the investigation of molecular dynamic and density functional theory. J Biomol Struct Dyn. 2016; 35: 520-534. https://goo.gl/bCVxiQ
Authors submit all Proposals and manuscripts via Electronic Form!