Research Article
A Performance Comparison of Graft Copolymer Hydrogels Based on Functionalized-Tragacanth Gum/Polyacrylic Acid and Polyacrylamide as Antibacterial and Antifungal Drug Release Vehicles
Javid Monjezi1*, Rezvan Jamaledin1,2, Mousa Ghaemy3, Arash Moeini2 and Pooyan Makvandi3,4*
1Department of Chemistry, Islamic Azad University (I.A.U), Masjed-Soleiman Branch, Masjed-Soleiman, Iran
2Department of Chemichal engineering, University of Naples Federico II, Naples, Italy
3Department of Chemistry, University of Mazandaran, Babolsar, Iran
4Institute for Polymers, Composites and Biomaterials (IPCB), National Research Council (CNR), Naples, Italy
*Address for Correspondence: Pooyan Makvandi, Department of Chemistry, University of Mazandaran, Babolsar, Iran, and Institute for Polymers, Composites and Biomaterials (IPCB), National Research Council (CNR), Naples, Italy, Tel: +393-928-288-866; ORCID: orcid.org/0000-0003-2456-0961; E-mail: pooyan.makvandi@ipcb.cnr.it
Dates: Submitted: 10 May 2018; Approved: 18 May 2018; Published: 21 May 2018
Citation this article: Monjezi J, Jamaledin R, Ghaemy M, Moeini A, Makvandi P. A Performance Comparison of Graft Copolymer Hydrogels Based on Functionalized-Tragacanth Gum/Polyacrylic Acid and Polyacrylamide as Antibacterial and Antifungal Drug Release Vehicles. Am J Nanotechnol Nanomed. 2018; 1(1): 010-015.
Copyright: © 2018 Makvandi P, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Release profile; Antibacterial; Drug release; Antimicrobial; Antifungal
Abstract
The present article deals with design of antibacterial and antifungal pH-responsive hydrogels based on Quaternary Ammonium Functionalized-Tragacanth Gum (QTG) biopolymer as drug delivery systems. The antimicrobial effects of the graft-copolymer hydrogels QTG/ polyacrylic acid (QTG-AA) and QTG/polyacrylamide (QTG-AM) were investigated against five standard microorganisms including Candida albicans, Escherichia coli, Bacillus subtilis, Staphylococcus aureus, and Pseudomonas aeruginosa. The results of the in-vitro release of quercetin as a drug from the functionalized copolymer hydrogels exhibited dependence on the pH, immersion time, medium, and temperature. All copolymer hydrogels demonstrated antibacterial and antifungal properties and moreover, QTG-AM copolymers presented higher antimicrobial activity than QTG-AA copolymers.
Introduction
Drug carrier systems with controlled delivery can deliver drugs at predetermined rates prolonged release for predefined periods of time. Such systems have been invented to overcome the limitations of conventional drug formulations [1,2]. Hydrogels, especially natural based, have been utilized widely in the development of the smart delivery systems. Since gels exhibit many polymer characteristics without being dissolved in any solvent [3].
Tragacanth Gum (TG), as a natural biopolymer, has been used for producing of hydrogels and drug release vehicles [4,5]. TG is an exudate from a plant that founds in the western and northern of Iran. TG is a polysaccharide with molecular weight up to 850 kDa, thanks to its emulsifying ability, good thermal stability as well as high long shelf life; TG has been used as a natural thickener and emulsifier in cosmetics, food, drug, textile and adhesive industries. Moreover, this polysaccharide is durable over wide pH range and highly hydrophilic to absorb water. It is biocompatible and safe even for oral intake, medicinally imported carbohydrate, and possesses no cytotoxicity [6-8].
Antimicrobial hydrogels have been produced to combat infections. These materials can remain under physiological conditions while preserving microbicidal activity. These attributes make them as ideal materials for medical devices, implant/catheter coatings, skin infections and wound healing applications [9-13].
Quaternary ammonium compounds, which possess permanent positive charge, have been presented in declining bacterial growth in an extensive range of applications. The mechanism of bactericidal activity of these materials is via contact killing which consists of a long and lipophilic alkyl chain enters to the membranes of the bacterial cell which leads leakage, autolysis, and, finally, cell death of the bacteria [14-19].
In the present study, new antibacterial and antifungal copolymer hydrogels were prepared based on the synthesis of Quaternary Ammonium Functionalized-TG (QTG) as a natural biopolymer. The antimicrobial effects of the graft-copolymer hydrogels were evaluated against E. coli, P. aeruginosai (as Gram-negative) S. aureus, B. subtilis, (as Gram-positive) bacteria and a fungus C. albicans.
Materials and methods
Materials
The Tragacanth Gum (TG) with high quality was bought from a local pharmaceutical shop. Glycidyltrimethylammonium Chloride (GTMAC), Sodium Hydroxide (NaOH), N,N’-Methylenebisacrylamide (MBA), Ceric Ammonium Nitrate (CAN), Acrylamide (AM), Acrylic Acid (AA), and quercetin were purchased from Sigma-Aldrich. Except for AA, which was distilled before use, all the chemicals were of analytical grade and used without further purification.
Synthesis of Quaternary Ammonium TG (QTG)
Quaternary ammonium functionalized TG has been described elsewhere [1,2]. Briefly, in a two-necked flask equipped nitrogen gas inlet tube, TG (0.5 g) was dissolved in water (200 mL) and stirred for 24 h to achieve a homogenous mixture. Then, the pH was adjusted at 8 by addition NaOH. Then, 0.25 g GTMAC was added to mixture and temperature was raised to 65°C for 18 h. After neutralizing with nitric acid, the reaction mixture was poured into methanol to precipitate. Finally, the QTG product was filtered washed with acetone and then with ethanol and dried in vacuum oven at 40°C.
Synthesis of QTG-AA and QTG-AM hydrogels
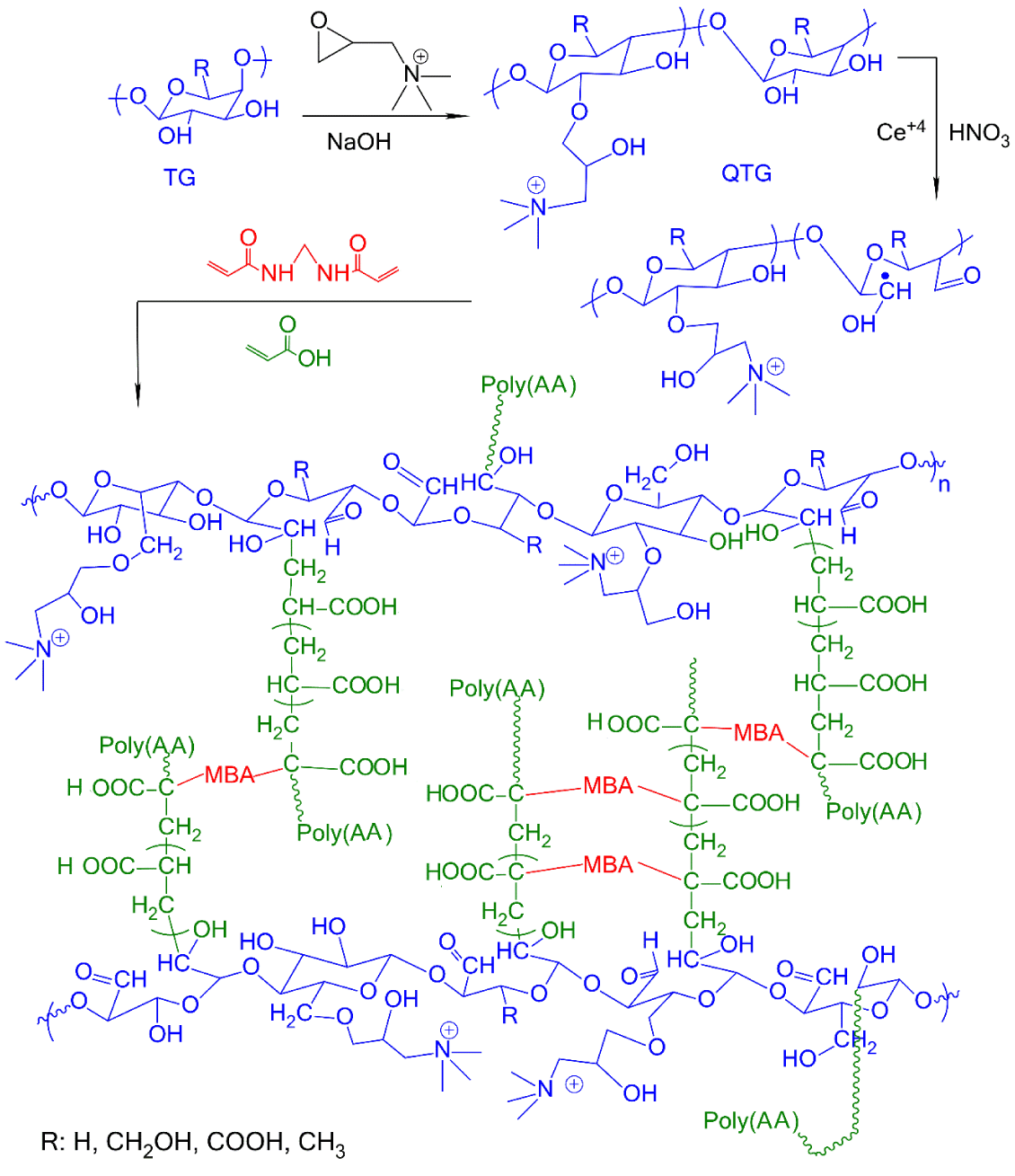
In a 250 mL two-necked flask equipped a nitrogen gas inlet tube, a certain weight of QTG, was dispersed in bi-distilled water with stirring to achieve a homogenous mixture. The initiator solution (10 % w/w ceric ammonium nitrate in HNO3) was added to the mixture and stirring was continued at 50˚C for about 1 h. Then, a certain amount of AA or AM monomer and MBA cross-linking agent (10 % based on the weight of AA or AM) were dissolved in water and added to the reaction mixture and stirred for 6 h. A continuous supply of nitrogen was maintained throughout the reaction period. Finally, a few droplets of a 10% aqueous NaOH solution were added to neutralize the mixture. The obtained hydrogel was dried in a vacuum oven at 40˚C till constant weight was reached. The reaction steps for graft copolymer hydrogel preparation of QTG/PolyAA are shown in figure 1.
Characterization
FT-IR spectra were recorded in KBr pellets using a Bruker Tensor 27 spectrometer (Bruker, Germany). FT-IR spectra of all materials were collected in the 4000 cm-1 to 400 cm-1 region. 1H-NMR spectra were recorded on a 400 MHz (Bruker Avance DRX, Karlsruhe, Germany) instrument in Deuterium Oxide (D2O) by using tetramethylsilane as the internal standard. The integral values of 1H NMR spectroscopy were used to evaluate the Degree of Quaternization (DQ) of TG with Quaternary ammonium compound (QTG). DQ% was determined by the ratio of the integral area of methyl protons of quaternary ammonium compound (N,N,N-trimethyl) at δ 3.13 ppm (INMe3) to the integral area of the signal attributed to the methyl protons of the α-L-fucose unit at δ 1.10 ppm (IMe), as shown in, Eq. (1):
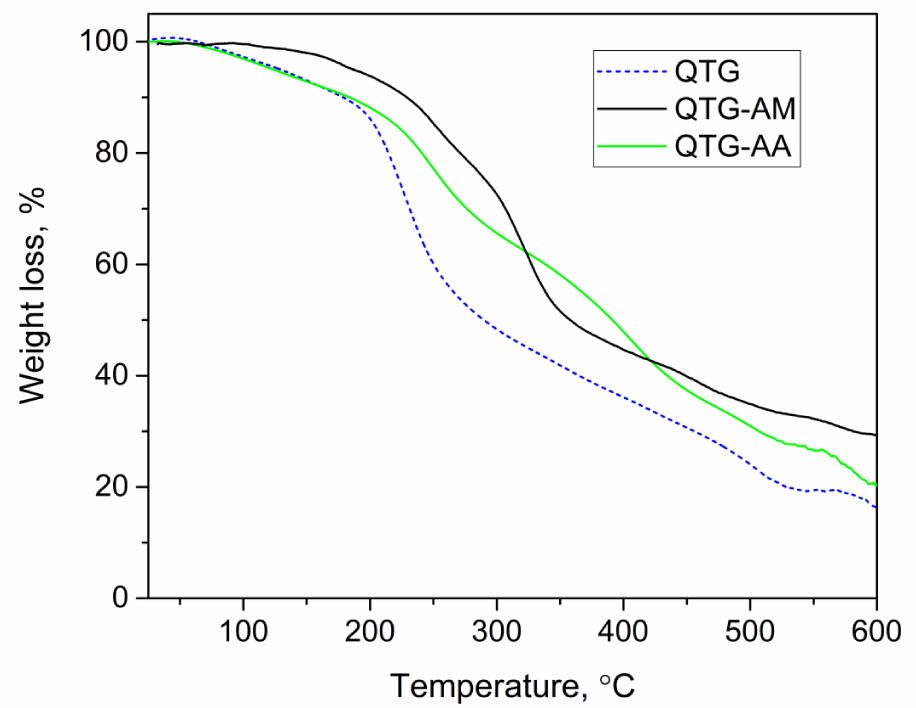
Thermal stability of QTG-AM and QTG-AN was examined using a thermogravimetric analysis (TGA Q50 V6.3 Build) under air atmosphere (flow of 20 ml min-1) with the heating rate 10˚C/min from ambient temperature to 600˚C.
Gel fraction
Freshly prepared QTG-AA and QTG-AM hydrogels were dried in an oven at 50˚C to a constant Weight (W0). The dried gels were extracted in a Soxhlet apparatus with hot distilled water for 24 h to remove the sol fraction. Then, the gels were dried to a constant Weight (Wg). The gel fraction percent was calculated using the flowing equation:
In vitro drug load
The quercetin solutions (1% w/v in PBS) were prepared at pH 7. A certain amount of dried samples of QTG-AA and QTG-AM was immersed in separate beakers each containing 25 mL quercetin solution and incubated at 37˚C for 2 days. The samples were then taken out of the solutions and the remaining solution was diluted to 50 mL. Then, the amount of quercetin left in the solution was measured by UV-vis spectrophotometer (PG+90 Instrument, United Kingdom) at λmax= 256 nm. The calibration curve standard at 256 nm was obtained by using diluted solutions of quercetin solution (1%, w/v). Entrapment Efficiency (EE) of quercetin in the hydrogels was calculated by using equation (3) [20]:
Where, Wi and Wf are the total amounts of quercetin in solution before and after loading, respectively.]
In vitro drug release
To study the release behavior of quercetin from the samples, the quercetin-loaded hydrogels were placed in individual beakers each containing 100 mL distilled water, 0.9% NaCl solution, and PBS at pHs 2.2, 7.4 and 9.2. The beakers were incubated at 37±0.1˚C under constant shaking at 100 rpm. From each beaker at predetermined time intervals, 4 mL of the release medium was taken out and replaced with 4 mL of fresh PBS to keep constant overall volume. The concentration of quercetin in the medium was determined by means of UV-vis spectrophotometer at 256 nm. All of the release experiments were performed in triplicate, and the averaged results were obtained.
Empirical analysis of in vitro release data
Data obtained from the release studies were fitted to various kinetic equations to find out the kinetics and mechanism of drug release from the samples. The drug released data were fitted to zero-order, first-order, Higuchi, Hixson–Crowell and Korsemeyer–Peppas models. Zero-order equation:
Where, Q is the amount of drug release at the time (t), k0 is the apparent dissolution rate constant or zero-order release constant and Q0 is the initial concentration of the drug in the solution resulting from a burst effect.
First-order equation:
Where, k1 is the first-order release constant, and in this case, the drug released at each time is proportional to the residual drug inside the dosage form.
Higuchi equation:
Where, Q is the amount of drug release at time (t) and kH is the Higuchi release constant. This is the most widely model to describe the drug release from pharmaceutical matrices.
Hixson–Crowell equation:
Where, Q0 is the initial amount of drug in the matrix tablet, Qt is the amount of drug remaining in the pharmaceutical dosage form at time (t), and ks is a constant incorporating the surface/volume ratio [4,21].
Korsmeyer–Peppas equation:
Where, Qt and Q∞ are the amounts of drug released at time (t) and at equilibrium, respectively. kk is the release rate constant which considers the structural and geometric characteristics of the tablet, and n is the diffusional exponent or release exponent, indicative of the drug release mechanism. The value of n = 0.5 indicates Fickian Diffusion (Higuchi Matrix), 0.5 < n < 1.0 indicates anomalous (non-Fickian) diffusion, n = 1.0 indicates case II transport (zero-order release) and n > 1.0 indicates super case II transport [22,23].
Antimicrobial assay
The in-vitro antibacterial and antifungal activities of the samples were assayed using direct contact test with agar diffusion. For the preparation of tablets, about 100 mg of ground samples transferred to cylinder mold with a mobile shaft. Then under the force of 8 tons (Specac, USA) for 10 min, the tablets approximately with a thickness of 1 mm and 10 mm in diameter were fabricated. Then, the test compounds as tablets were placed on the surface of inoculated agar plates [15,24]. The antimicrobial activity of the samples was investigated against one fungal and four bacterial species. Test microorganisms consisted of C. albicans PTCC 5027, E. coli PTCC 1330, B. subtilis PTCC 1023, P. aeruginosai PTCC 1074, and S. aureus ATCC 35923. All tested gram-negative and gram-positive bacteria were preserved as well as used for direct contact agar diffusion test in Muller-Hinton broth (Merck) except C. albicans that were cultured in SABOURAUD Dextrose broth (Merck). Before using the cultures, they were standardized with a final cell density of approximately 108 CFU mL-1. The agar plates were inoculated from the standardized cultures of the test organisms by means of a sterile cotton swab and, then, it was spreaded out as uniformly as possible throughout the entire media. The tablet was placed on the upper layer of the seeded agar plate. The SABOURAUD Dextrose agar plate and Muller-Hinton agar plate incubated at 37°C for 24-48 hours. The antimicrobial activities of the hydrogels were compared with known antibiotic nystatin (100 Unit/disc), and gentamicin (10 µg/disc). Positive control plates were streaked with test microorganisms, but no tablet was used. Antimicrobial activity was evaluated by measuring the inhibition zone diameter (mm) on the surface of plates. Finally, the results were reported as mean ± SD after three repeats.
Results and Discussion
Synthesis and characterization
Figure 2A shows FT-IR spectra of pristine TG, QTG and the graft copolymer hydrogels. The pristine TG showed absorption bands at 1745 and 1645 cm-1 attributed to stretching vibrations of C = O groups of ester and carboxylic acid, respectively, at 1100-1200 cm-1 related to vibration of C–O bond, and at 3445 and 2925 cm-1 due to O-H and C-H stretching, respectively [25]. Due to the broad fingerprint of absorption bands of TG in the range of 1800 to 600 cm-1, some of the functional groups may have overlapped with each other. Therefore, it is hard to identify new functional groups’ vibrations for QTG. However, QTG showed new absorption bands in the region of 1000 to 600 cm−1 (at 921, 717, and 686 cm-1) which are due to stretching vibrations of the NR4+ complexes [15]. The absorption bands of C = O, N-H, and C-N overlapped with other absorption bands. The 1H NMR technique was used to characterize QTG and to determine the degree of substitution (DQ%). In 1H NMR spectrum of QTG, Figure 2B, the characteristic signal at δ 1.10 ppm corresponds to the methyl protons of α-L-fucose units, and the signal at 3.25 ppm is assigned to the vibrations of three pairs of protons of [N+(CH3)3] [26,27]. The solvent peak (D2O) is assigned at δ 4.80 ppm. DQ% was determined by 1H NMR and was about 41%.
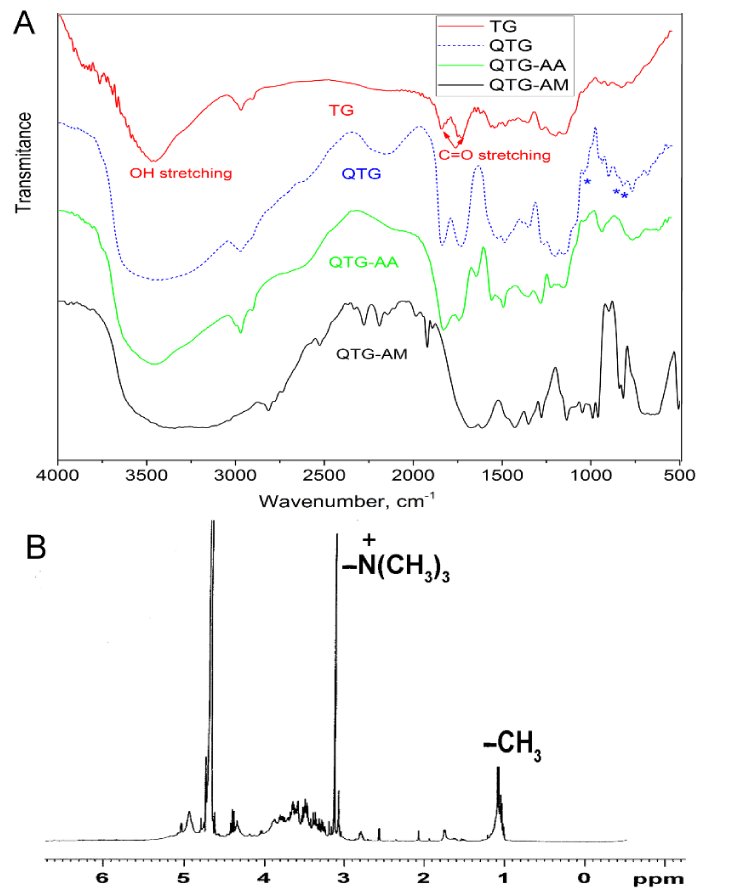 Figure 2: A. FT-IR spectra of native TG, QTG, QTG-AA and QTG-AM hydrogels. B. 1H NMR spectrum for QTG.
Figure 2: A. FT-IR spectra of native TG, QTG, QTG-AA and QTG-AM hydrogels. B. 1H NMR spectrum for QTG.
Figure 3 shows TGA curves of modified TG and the corresponding hydrogels from 30 to 600°C. For QTG, in the initial stage, there is dehydration and loss of volatile molecules which leads to the decomposition of modified TG, while in the lateral stage there is decomposition due to polymerization reaction [28]. In the TGA curves, the initial decomposition temperature and the char yield remained at 600°C revealed that QTG-AM and QTG-AA hydrogels were thermally more stable than modified tragacanth gum.
Gel fraction and in vitro quercetin load
The gel content was obtained after 24 h hot extraction with distilled water. The gel fraction of QTG-AA and QTG-AM was in the range of 60-62 wt%.
The Entrapped Efficiency (EE%) of quercetin in the hydrogels was determined by UV-vis spectrophotometer and the results for the QTG-AA and QTG-AM were very close to 65% and 61%, respectively. This similarity in the EE values is probably due to their polarity and interaction with quercetin. Quercetin release was investigated from drug loaded QTG-AA and QTG-AM hydrogels in PBS at three different pH values (2.2, 7.4, and 9.2).
In vitro quercetin release
The release measurements were carried out during 24 h and the obtained results are shown in figure 4. As can be seen, more release has been observed in pH 9.2 buffer for QTG-AA hydrogel as compared to the release in pHs 2.2 and 7.4. This can be due to higher swelling of QTG-AA copolymer hydrogel at pH 9.2, as shown in figure 4A. However, for QTG-AM copolymer (Figure 4B) drug release in pH 2.2 buffer is more than the release in other buffers. This can be due to protonation of amine group in AM units, which causes repulsion force in the network. The drug diffusion from the drug-loaded polymers is directly related to the polymer swelling [29]. The network structure and pH sensitivity make these QTG based hydrogels candidate to be used as carriers for controlled drug release.
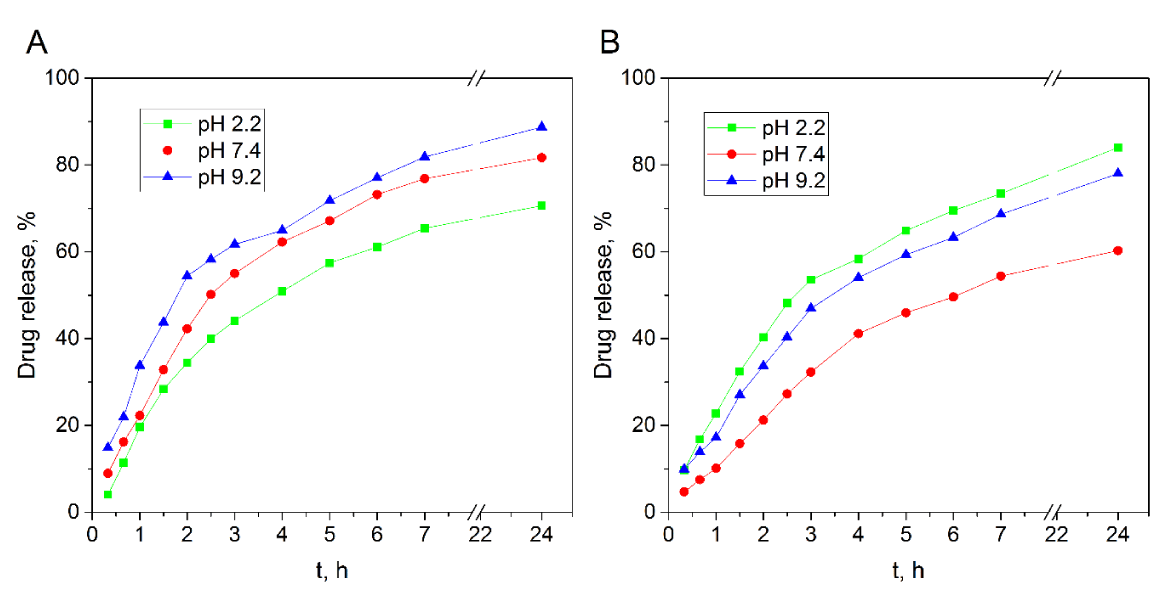 Figure 4: Effect of time on in-vitro drug release profiles of QTG-AA
A. QTG-AM. B. Hydrogels in PBS at different pHs.
Figure 4: Effect of time on in-vitro drug release profiles of QTG-AA
A. QTG-AM. B. Hydrogels in PBS at different pHs.
Release kinetic study
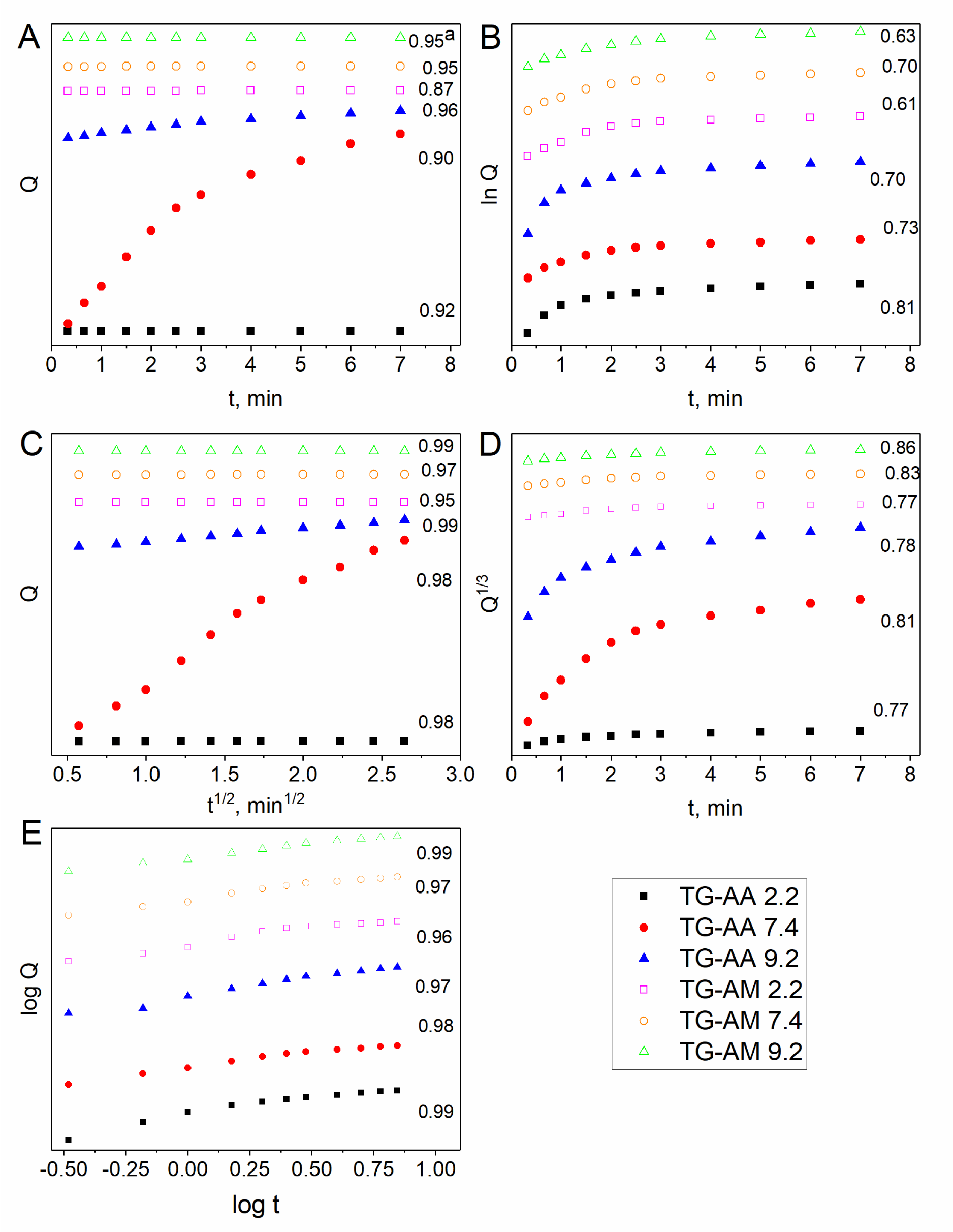
The release data of quercetin from the drug-loaded hydrogels were plotted according to the equations of relevant models and they are presented in Figure 5. The release of drug from the loaded hydrogels depends on the swelling of the polymer matrix and solubility of the drug. Drug release from hydrophilic matrices has been shown to be a complex interaction between swelling, diffusion and erosion mechanisms [30]. In the present study, the higher swelling has been observed at pH 9.2 as compared to the swelling at pHs 2.2 and 7.4. Mathematical modeling of drug release can be very useful to speed up product development and to comprehend better the mechanisms controlling drug release from advanced delivery systems. According to the values of diffusion exponent ‘n’, 0.5 < n < 1.0, in table 1, swelling of the hydrogels occurred through non-Fickian diffusion mechanism. In this mechanism, the rate of diffusion of water molecules into the hydrogels matrix and rate of polymer chains relaxation are comparable. On the other hand, the non-Fickian release mechanism implies that the release is controlled by diffusion or a combination of diffusion and macromolecular chain relaxation mechanisms. The results showed that the Higuchi and Korsmeyer–Peppas equation gave the best fit with the highest correlation coefficient (r2) for all the hydrogels.
| Table 1: r2 parameter of drug release obtained by fitting release data by different release kinetics models at different pHs. | |||||||
| samples | pH | Zero order | First order | Higuchi | Korsmeyer | Hixson-Crowell | |
| QTG-AA | 2.2 | 0.95 | 0.63 | 0.99 | n | 0.99 | 0.86 |
| 7.4 | 0.95 | 0.7 | 0.97 | 0.85 | 0.97 | 0.83 | |
| 9.2 | 0.87 | 0.6 | 0.95 | 0.71 | 0.96 | 0.77 | |
| QTG-AM | 2.2 | 0.96 | 0.7 | 0.99 | 0.92 | 0.97 | 0.78 |
| 7.4 | 0.9 | 0.73 | 0.98 | 0.77 | 0.98 | 0.81 | |
| 9.2 | 0.92 | 0.81 | 0.98 | 0.72 | 0.99 | 0.77 | |
Antimicrobial activity testing
Nowadays nanotechnology has expanded its applications in biomedical field including fighting and preventing of diseases using atomic scale functional materials. Figure 6 shows the results of antimicrobial activity of samples. The finding towards inhibition of microorganisms was correlated with the standard antibiotics such as chloramphenicol and nystatin. The TG samples showed no inhibition zone while all other samples containing quaternary ammonium showed inhibition zones. Researchers have shown that acrylic acid43 and acrylamide44 hydrogels do not have the antibacterial activity against gram-negative and gram-positive bacteria. All samples in this study, except TG, showed good activity against gram-negative and positive bacteria. In addition, antifungal activity revealed that the samples containing quaternary ammonium showed a good growth inhibitory effect against C. albicans. The antifungal activity of the tested samples was more effective than nystatin. Among the tested hydrogels, QTG-AM hydrogels exhibited the higher antibacterial activity than QQTG-AA hydrogels. This may be due to the presence of the cationic amine (NH3+) in QTG-AM hydrogels [31]. Therefore, the improved antibacterial efficacy of QTG-AM hydrogel in comparison to the antibacterial activity of QTG-AA might be due to presence cationic amine. The antibacterial mechanism of quaternary ammonium salt (cationic amine) is widely thought to be “contact killing”: a long, lipophilic alkyl chain penetrates bacterial cell membranes to produce leakage, autolysis, and cell death of bacteria [32].
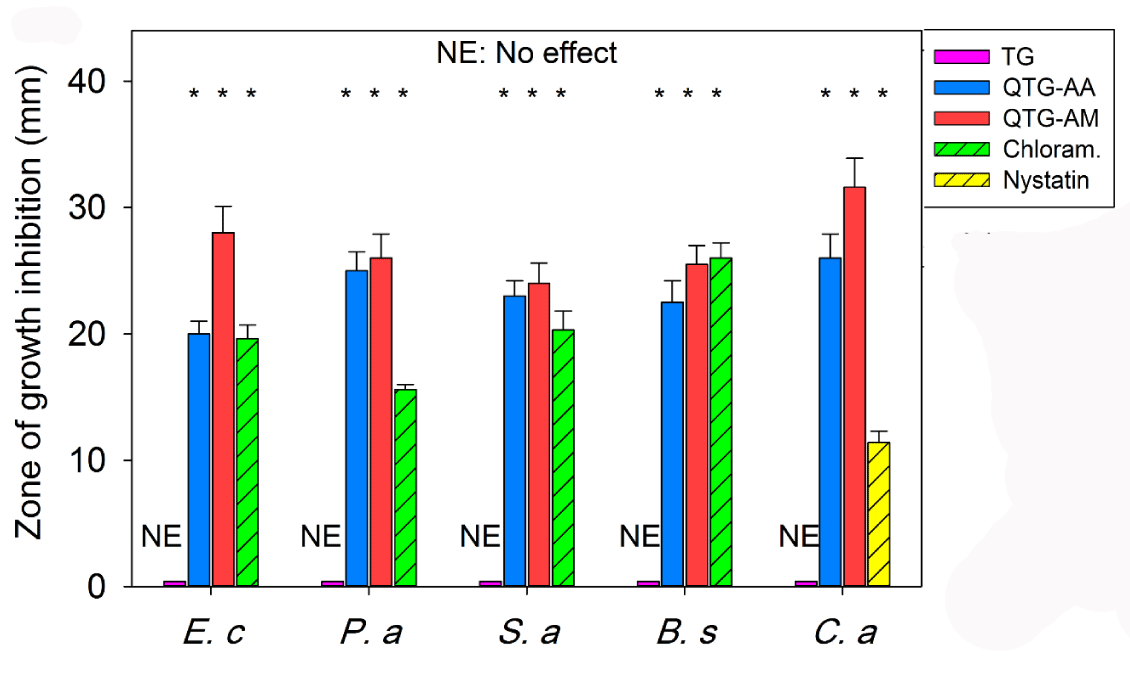 Figure 6: The antimicrobial activity of samples against E. coli, P. aeruginosai (Gram-negative), S. aureus, B. subtilis, (Gram-positive) bacteria and a fungus C. albicans. The samples were compared with known antibiotics: nystatin, and chloramphenicol. *P <0.05 as compared to TG. Each error bar represents 1 standard deviation and serves as the estimate of standard uncertainty.
Figure 6: The antimicrobial activity of samples against E. coli, P. aeruginosai (Gram-negative), S. aureus, B. subtilis, (Gram-positive) bacteria and a fungus C. albicans. The samples were compared with known antibiotics: nystatin, and chloramphenicol. *P <0.05 as compared to TG. Each error bar represents 1 standard deviation and serves as the estimate of standard uncertainty.
Conclusions
Two types of antimicrobial graft copolymer hydrogels (QTG-AA and QTG-AM) based on natural biopolymer TG were synthesized and characterized. The synthesized antimicrobial graft copolymer hydrogels released the loaded drug in a controlled manner with salt- and pH-responsiveness properties. In addition, both QTG-AA and QTG-AM hydrogels revealed desirable antibacterial and antifungal properties against the tested microorganisms.
References
- Monjezi J, Jamaledin R, Ghaemy M, Makvandi P. Antimicrobial modified-tragacanth gum/acrylic acid hydrogels for the controlled release of quercetin. Quarterly Journal of Applied Chemical Research. 2018.
- Monjezi J, Jamaledin R, Ghaemy M, Makvandi P. Novel quaternary ammonium modified-tragacanth gum-acrylamide drug carrier with antimicrobial activity. Journal of Physical & Theoretical Chemistry. 2018.
- Xu X, Jha AK, Harrington DA, Farach-Carson MC, Jia X. Hyaluronic acid-based hydrogels: from a natural polysaccharide to complex networks. Soft Matter. 2012; 8: 3280-3294. https://goo.gl/j8f9cM
- Singh B, Sharma V. Influence of polymer network parameters of tragacanth gum-based pH responsive hydrogels on drug delivery. Carbohydr Polym. 2014; 101: 928-940. https://goo.gl/acWoSv
- Hemmati K, Masoumi A, Ghaemy M. Tragacanth gum-based nanogel as a superparamagnetic molecularly imprinted polymer for quercetin recognition and controlled release. Carbohydr Polym. 2016; 136: 630-640. https://goo.gl/Qp3vDa
- Greenwald H, Luskin L, Davidson R. Handbook of Water Soluble Gums and Resins. 1980. https://goo.gl/gQWDTP
- Weiping W, Phillips G, Williams P. Tragacanth and karaya. Cambridge: Woodhead Publishing Ltd; 2000.
- Balbir SK, Rajiv J, Rachna S. Study of ionic charge dependent salt resistant swelling behavior and removal of colloidal particles using reduced gum rosin-poly (acrylamide)-based green flocculant. Iran Polym J. 2016; 25: 349-362. https://goo.gl/j3zm7U
- Li Y, Fukushima K, Coady DJ, Engler AC, Liu S, Huang Y, et al. Broad‐spectrum antimicrobial and biofilm‐disrupting hydrogels: stereocomplex‐driven supramolecular assemblies. Angew Chem Int Ed Engl. 2013; 52: 674-678. https://goo.gl/LjcUw9
- Zhou C, Li P, Qi X, Sharif AR, Poon YF, Cao Y, et al. A photopolymerized antimicrobial hydrogel coating derived from epsilon-poly-L-lysine. Biomaterials. 2011; 32: 2704-2712. https://goo.gl/TNZHXj
- Daphne AS, Darrin JP, Joel PS. Design of an injectable β‐hairpin peptide hydrogel that kills methicillin‐resistant Staphylococcus aureus. Adv Materials. 2009; 21: 4120-4123. https://goo.gl/D3DqiJ
- Juby KA, Dwivedi C, Kumar M, Kota S, Misra HS, Bajaj PN. Silver nanoparticle-loaded PVA/gum acacia hydrogel: synthesis, characterization and antibacterial study. Carbohydr Polym. 2012; 89: 906-913. https://goo.gl/dTVxg7
- Pooyan M, Nasseer N, Naser SS, Nader TQ. Effect of silver nanoparticle on the properties of poly(methyl methacrylate) nanocomposite network made by in situ photoiniferter-mediated photopolymerization. Bull. Mater. Sci. 2015; 38: 1625-1631. https://goo.gl/LwDyzm
- Sekhavat PZ, Makvandi P, Ghaemy M. Performance properties and antibacterial activity of crosslinked films of quaternary ammonium modified starch and poly (vinyl alcohol). Int J Biol Macromol. 2015; 80: 596-604. https://goo.gl/VvzCaE
- Pooyan M, Mousa G, Mojtaba M. Synthesis and characterization of photo-curable bis-quaternary ammonium dimethacrylate with antimicrobial activity for dental restoration materials. Eur Polym J. 2016; 74: 81-90. https://goo.gl/Tr6NKR
- Makvandi P, Ghaemy M, Ghadiri AA, Mohseni M. Photocurable, antimicrobial quaternary ammonium–modified nanosilica. J Dent Res. 2015; 94: 1401-1407. https://goo.gl/GPTvEq
- Pooyan M, Carola EC, Federica P, Anna LG, Francesco M, Rezvan J, et al. Antimicrobial modified hydroxyapatite composite dental bite by stereolithography. Polym Adv Technol. 2017; 29: 364-371. https://goo.gl/MYayAJ
- Makvandi P, Jamaledin R, Jabbari M, Nikfarjam N, Borzacchiello A. Antibacterial quaternary ammonium compounds in dental materials: a systematic review. Dent Mater. 2018; 34: 851-867. https://goo.gl/wrMw3J
- Qian Y, Yanan S, Xiaomei S, Chunye X, Yuezhen B. Preparation and properties of chitosan derivative/poly (vinyl alcohol) blend film crosslinked with glutaraldehyde. Carbohydr Polym. 2011; 84: 465-470. https://goo.gl/4nLt37
- Khadijeh H, Arameh M, Mousa G. pH responsive tragacanth gum and poly (methyl methacrylate-co-maleic anhydride)-g-poly (caprolactone) conetwork microgel for in vitro quercetin release. Polymer. 2015; 59: 49-56. https://goo.gl/Q1qo1P
- Anita GS, Lata SM, Tejraj MA. Novel pH-sensitive hydrogels prepared from the blends of poly (vinyl alcohol) with acrylic acid-graft-guar gum matrixes for isoniazid delivery. Ind Eng Chem Res. 2010; 49: 7323-7329. https://goo.gl/oAxSwb
- Richard WK, Robert G, Eric D, Pierre B, Nikolaos AP. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983; 15: 25-35. https://goo.gl/ePxTZF
- Costa P, Sousa LJM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001; 13: 123-133. https://goo.gl/giDTyS
- Method for antifungal disk diffusion susceptibility testing of yeasts. Approved Guidelines-second edition. Clinical & Laboratory Standards Institute. 2009; 29.
- Zahra M, Mohammad JZ, Kourosh K, Mohammad RN. Tragacanth gum-graft-polyacrylonitrile: synthesis, characterization and hydrolysis. Journal of Polymer Research. 2008; 15: 173-180. https://goo.gl/t91qhw
- Novac O, Lisa G, Profire L, Tuchilus C, Popa MI. Antibacterial quaternized gellan gum based particles for controlled release of ciprofloxacin with potential dermal applications. Mater Sci Eng C Mater Biol Appl. 2014; 35: 291-299. https://goo.gl/gZgzt5
- Pooyan M, Carola EC, Federica P, Anna LG, Francesco M, Rezvan J, et al. Antimicrobial modified hydroxyapatite composite dental bite by stereolithography. Polym Adv Technol. 2017; 29: 364-371. https://goo.gl/MYayAJ
- Saruchi, Balbir SK, Rajiv J, Kapur G, Vaneet K. Synthesis, characterization and evaluation of gum tragacanth and acrylic acid based hydrogel for sustained calcium chloride release–enhancement of water-holding capacity of soil. Journal of the Chinese Advanced Materials Society. 2014; 2: 40-52. https://goo.gl/yP8UgB
- Bertz A, Wohl-Bruhn S, Miethe S, Tiersch B, Koetz J, Hust M, et al. Encapsulation of proteins in hydrogel carrier systems for controlled drug delivery: influence of network structure and drug size on release rate. J Biotechnol. 2013; 163: 243-249. https://goo.gl/LdvLK9
- Reynolds TD, Gehrke SH, Hussain AS, Shenouda LS. Polymer erosion and drug release characterization of hydroxypropyl hethylcellulose matrices. J Pharm Sci. 1998; 87: 1115-1123. https://goo.gl/vJp7Pq
- Kim SS, Lee J. Antibacterial activity of polyacrylonitrile–chitosan electrospun nanofibers. Carbohydr Polym. 2014; 102: 231-237. https://goo.gl/oKBYYw
- Ahlstrom B, Thompson RA, Edebo L. The effect of hydrocarbon chain length, pH, and temperature on the binding and bactericidal effect of amphiphilic betaine esters on Salmonella tvphimurium. APMIS. 1999; 107: 318-324. https://goo.gl/UK371A
Authors submit all Proposals and manuscripts via Electronic Form!