Research Article
Investigation of the Complement-Dependent Effect of Heparin in a Liver Ischemia Reperfusion Model in Rats
Tulay D. Allahverdi*, Mahmut C. Yagmurdur, Hamdi Karakayali and Mehmet Haberal
Kafkas University Department of General Surgery, KARS, Turkey
*Address for Correspondence: Tulay Diken Allahverdi, Kafkas University Department of General Surgery, KARS, Turkey, Tel: +050- 6319651; E-mail: drtulaydiken@hotmail.com
Dates: Submitted: 08 September 2017; Approved: 30 September 2017; Published: 05 October 2017
Citation this article: Allahverdi TD, Yagmurdur MC, Karakayali H, Haberal M. Investigation of the Complement-Dependent Effect of Heparin in a Liver Ischemia Reperfusion Model in Rats. Int J Hepatol Gastroenterol. 2017;3(1): 050-055.
Copyright: © 2017 Allahverdi TD, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Liver; Heparin; Ischemia; Reperfusion
Abstract
The aim of the study was to investigate the damage created in tissue by using an in vivo isolated portal ischemia and reperfusion model in the rat liver and the effects of heparin administration on the complement system. A total of 25 male rats weighing 150-290 gr were used in the study. Following anesthesia with ketamine hydrochloride and xylazine hydrochloride, the incision area was shaved in all rats except the control group. The portal vein was isolated and clamped, and ischemia and reperfusion created. Two groups were sacrificed at the 24th hour and two at the 48th hour. Heparin was administered to one of the groups sacrificed at the 24th hour and not to the other group, and similarly one of the groups sacrificed at the 48th hour received heparin while the other did not. Biochemical and pathologic parameters were used to evaluate the damage using serum and liver tissue samples from the sacrificed rats. We used the liver GSH, MPO and C3 levels and the serum IL-6 level to evaluate the ischemia and reperfusion damage in the liver tissue. Heparin was shown to decrease the damage occurring after ischemia and reperfusion by decreasing complement activation and the MPO and IL-6 levels while increasing GSH levels as a result of the statistical analysis performed. Heparin was shown to prevent tissue damage after ischemia and reperfusion by decreasing complement activation and inflammation.
Introduction
Liver transplantation is the most important treatment option for both acute and terminal period liver disorders [1]. Improvements in surgical technique and the immunosuppressive treatments used after transplantation prolong survival but also lead to various complications [1]. These complications include hemorrhage, gall bladder leakage, bile fistula, bile duct stenosis, and vascular complications such as portal vein or hepatic artery thrombosis [1,2].
Minimizing the duration and effect of hepatic ischemia after the Pringle maneuver and keeping inflammatory events under control has become increasingly important to protect liver functions during surgery in recent years [3]. The Pringle maneuver leads to hepatic problems related to ischemia and reperfusion but the mechanism is not fully clear. Ischemia can develop due to arterial or venous occlusion. Vascular bed stasis is present in venous occlusion. The Pringle maneuver can be extended to 60-90 minutes at present but longer durations can lead to severe hepatic destruction and even shock and death [3]. Portal venous ischemia has also been reported to be fatal after 60 minutes in rats [4].
Polymorphonuclear Leukocyte (PMNL) activation, oxygen free radical formation, cytokine secretion, complement activation and eicosanoid production are the main results of ischemia and reperfusion damage. These mediators have been found at abnormally high levels in the circulation in patients with ischemia and reperfusion damage and their presence leads to the development of clinical signs and symptoms [5].
Heparin is an antithrombotic agent known to increase the survival rate in sepsis, shock, ischemia and reperfusion models [6,7]. Heparin has also been shown to prevent complement activation through the alternative route and decrease activation of the classic complement route with its C1 esterase inhibitor effect [8]. Heparin is known to play a role in decreasing inflammation in the ischemia and reperfusion model in this way [9]. It has been reported to decrease tissue damage by inhibiting complement activation after ischemia reperfusion damage [8,10].
The aim of this experimental study was to investigate whether heparin decreases tissue damage through complement inhibition in hepatic ischemia and reperfusion damage created via the portal vein.
Materials and Methods
The experimental study protocol was realized in accordance with the guidelines of the “National Institutes of Health Guide for the Care and Use of Laboratory Animals” after ethical and scientific approval from the Başkent University Medical Faculty Experimental Research Committee. The rats used in the experiment were obtained from the Experiment Animals Production Center affiliated with the Başkent University Research Center. This study was conducted at the Başkent University Experimental Research Center. A total of 25 (Wistar Albino) male rats weighing 150-290 gr were used in the study. They were transferred from the production center to the study center one week before the study started and prepared for the experiment by being kept at a stable environment (at 22°C) for 12 hours under daylight conditions and 12 hours under night conditions each day and being given standard rat food. The rats to be used in the experiment were left fasting and allowed to drink only water for 12 hours beforehand. Anesthesia was ensured by intraperitoneal administration of 50 mg/kg Ketamine Hydrochloride (Ketalar® Eczacibaşi Warner-Lambert İlaç Sanayi, Levent, Istanbul) and 7 mg/kg Xylazine Hydrochloride (Rompun® Bayer Şişli, Istanbul) under aseptic conditions . The subjects were divided into five main groups.
Group 1 (control group = 5)
The group where the parameters would be observed without administration of heparin or formation of ischemia reperfusion damage.
Group 2 (n = 5)
The group that was sacrificed 24 hours after the formation of ischemia reperfusion damage without the administration of the therapeutic drug.
Group 3 (n = 5)
The group that was sacrificed 24 hours after ischemia reperfusion damage was created and administration of heparin (Nevparin® Mustafa Nevzat Ilaç Sanayii, Gayrettepe, Istanbul).
Group 4 (n = 5)
The group that was sacrificed 48 hours after the formation of ischemia reperfusion damage without the administration of the therapeutic drug.
Group 5 (n = 5)
The group that was sacrificed 48 hours after ischemia reperfusion damage and administration of heparin.
The rats were weighed before the procedure. The incision site was shaved after anesthesia. Skin antisepsis was provided with povidone iodine and the arms and legs were fixed on the operating device. The device was held at a 30 degree incline to prevent the aspiration risk and the subject draped under sterile conditions with the incision site exposed. A midline incision was preferred. Following the laparotomy, the small intestines were taken out of the abdomen and the portal peduncle observed. Subsequently, the portal vein was isolated and dissected and then separated from the hepatic artery and biliary tract.
Ischemia creation
Ischemia was created with a microvascular clamp placed on the portal vein so as to avoid pressure on the hepatic artery (Figure 1). Ischemia duration was 60 minutes. The microclamp was then removed and reperfusion was provided. Each rat was kept under a heat lamp during this period in order to prevent heat loss. The incision was closed in 2 layers using 3.0 silk at the end of the surgery. The rats were not fed orally until the effects of anesthesia passed.
 Figure 1: Ischemia was created with a microvascular clamp placed on the portal vein so as to avoid pressure on the hepatic artery.
Figure 1: Ischemia was created with a microvascular clamp placed on the portal vein so as to avoid pressure on the hepatic artery.
Heparin administration
A 1200 IU/kg dose of heparin was administered from the tail vein right before ischemia was created.
Blood samples were taken from the inferior vena cava for biochemical investigation. The tissue samples taken from the liver were placed into sterile containers of known weight. The serum IL-6 levels and liver tissue GSH levels were measured as biochemical parameters at the Başkent University Hospital’s Biochemical Laboratory and recorded on the information form of each rat. Tissue samples taken from the left lobe of the liver were kept at -86°C until biochemical analyses were performed. The inferior vena cava blood samples were immediately centrifuged at 1500 g for 15 minutes to separate the serum for cytokine analyses and kept at -86°C until the analysis. All biochemical analyses were performed as dual studies. Serum TNF-α and IL-6 levels were measured with the ELISA method by using commercial kits (Biosource International Inc, California, USA; TNF-α: KRC3011 and IL-6: KRC0061) while cytokine analysis was with the “solid phase sandwich ELISA” method.
Liver tissue homogenates were prepared by using a glass homogenizer inside 0.15 M KCl (10%, w/v) for reduced glutathione (GSH) analyses in tissue samples. Tissue MDA concentration was used as the lipid peroxidation marker according to the method identified by Beuge and Aust [11]. Tissue GSH concentrations were evaluated using the tissue sulfhydryl group determination method of Ellman [12]. Protein analysis in tissue homogenates was according to the method of Lowry, et al. [13].
Pathology evaluation
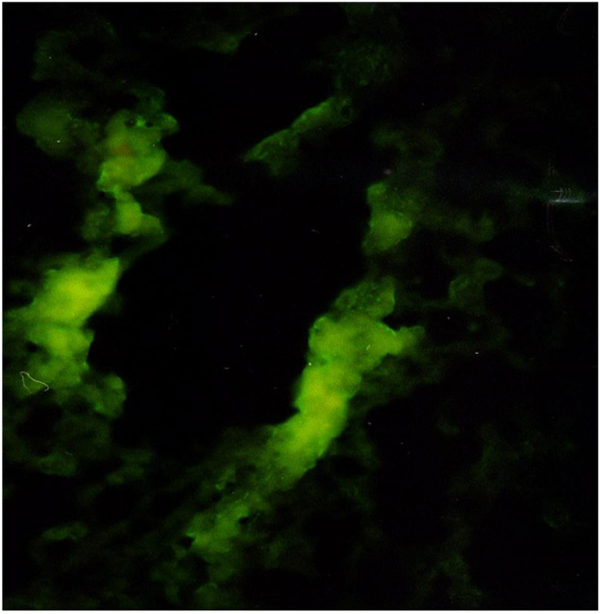
The liver tissue sample was covered with sterile gauze previously soaked with 0.9% Saline and sent to Başkent University Hospital’s Pathology Department inside a Petri Box for C3 evaluation in the fresh sample with the IF method. C3 (FITC-Conjugated Rabbit Anti-Human C3a Complement Code No. F 0201 Lot. 075 BIOKEM) presence was then investigated in liver tissue with the Immunofluorescent (IF) method. The study groups were monitored for 24 and 48 hours and the complement-dependent effects of heparin in the liver were investigated. The C3 levels in fresh liver tissue samples were determined with the IF method. The effects of heparin on the C3 level in the liver were evaluated.
C3 level determination
Samples taken from the fresh liver tissue were frozen in frozen section gel (Tissue freezing Medium/ JUNG) at -60°C and 6 µ sections placed on poly-lysine coated slides at -20°C. The sections were kept in acetone for 10 minutes. Once the acetone was vaporized, they were incubated with C3 Antibody (C3-b Complement/FITC-Rabbit Anti-Human/BİOKEM) at room temperature in an environment without light for 3 hours. The sections were then washed with distilled water and dried. They were covered with fluorescent covering gel (Fluorescent Mounting Medium/BIOKEM) and evaluated under the fluorescent microscope. The investigation was conducted by a single pathologist from the Başkent University Hospital and the samples graded semi quantitatively between +1 and +3 according to IF staining severity.
MPO Study
The 3 μ thick sections from paraffin blocks were placed on poly-L-lysine-coated slides and then kept in the oven at 56°C for 14 hours before being placed in xylene and alcohol for 30 minutes each. The slides were then washed under tap water and transferred into Citrate Buffer Solution (pH = 6.0). They were boiled at moderate temperature for 20 minutes and kept at room temperature for 20 minutes. The section were then incubated at room temperature for 2 hours with a 1:300 dilation of MPO (Myeloperoxidase DAKO polyclonal rabbit A 0398) and undiluted ready-to-use primary antibody INOS (INOS Rabbit polyclonal antibody RB-9242-R7) using the avidin-biotin complex method. Finally, the reaction was made visible using chromogen 3.3-diamino-benzidine-tetrahydrochloride.
Statistical Analysis
The SPSS 9.0 software program was used for the statistical analyses of study results. Mean and standard deviations, which are central distribution criteria in statistical analysis, were calculated and intragroup differences of the nominal values determined using Fisher’s exact chi-square test for non-parametric data. A p value <0.05 was considered significant. The Kruskal-Wallis and Mann-Whitney U tests were used to analyze values obtained with measurement. For the groups where the difference was found to be significant with the Kruskal-Wallis test, we used the Mann-Whitney test to analyze the significance between two groups and p values <0.015 were accepted as significant following Bonferroni correction.
Results
Biochemical findings
The results obtained from the study were biochemically categorized (Table 1).
| Table1: IL-6, GSH, MPO mean and standard error values are presented in table 1. | ||||
| IL-6 (pg/ ml) | GSH (nmol/mg-protein) | MPO | ||
| Necrosis | Involvement | |||
| Control | 72.94 ± 55.56 | 23.58 ± 6.44 | 4.00 ± 8.94 | 5.00 ± 0.00 |
| Heparin (-) 24th Hour | 120.44 ± 141.98 | 31.22 ± 7.42 | 10.00 ± 22.36 | 21.25 ± 4.78 |
| Heparin (+) 24th Hour | 3.26 ± 7.28 | 38.42 ± 8.08 | 0.00 ± 0.00 | 9.00 ± 2.23 |
| Heparin (-) 48th Hour | 46.14 ± 41.13 | 25.74 ± 8.69 | 16.00 ± 23.02 | 22.00 ± 2.73 |
| Heparin (+) 48th Hour | 21.34 ± 47.71 | 30.44 ± 2.08 | 0.00 ± 0.00 | 9.00 ± 2.23 |
A statistically significant difference was found between all groups in terms of IL-6 (p = 0.001). Statistically significant differences were found between the groups sacrificed at 24 hours with and without heparin administration in pairwise comparison between the groups (p = 0.004). No statistically significant difference was found between the groups sacrificed at 48 hours with and without heparin administration (p > 0.017).
A statistically significant difference was found between all groups in terms of GSH (p = 0.001).Statistically significant differences were found between the groups sacrificed at 24 and 48 hours with and without heparin administration in pairwise comparisons (p = 0.002).
A statistically significant different was found between all groups in terms of MPO (p = 0.001).
Statistically significant differences were found between the groups sacrificed at 24 and 48 hours with and without heparin administration in pairwise comparisons (p = 0.001)
A statistically significant different was found between all groups in terms of MPO (p = 0.001)
Statistically significant differences were found between the groups sacrificed at 24 and 48 hours with and without heparin administration in pairwise comparisons (p = 0.002)
Pathology findings
C3 levels in liver tissue with the IF method: C3 levels in livertissue as determined with the IF method are presented (Table 2). C3 staining in the group without heparin administration (group 2 and 4) was stronger than in the groups administered heparin (group 3 and 5), (Figure 2).
| Table 2: Liver tissue C3 levels with the immunofluorescent method. | ||||
| Order No | Groups | Weight (gr) | Procedure Performed | Samples Taken |
| Pathology | ||||
| Liver Tissue C3 Levels with the Immunofluorescence Method. | ||||
| 1 | Group 1 | 180 | Control group | Negative |
| 2 | 160 | Negative | ||
| 3 | 160 | Negative | ||
| 4 | 150 | Negative | ||
| 5 | 150 | Negative | ||
| 6 | Group 2 | 190 | Ischemia and reperfusion damage at the 24th hour sacrifice without heparin administration | Negative |
| 7 | 210 | Diffuse +++ at vessel wall | ||
| 8 | 205 | Diffuse +++ at vessel wall | ||
| 9 | 210 | Focal + at vessel wall | ||
| 10 | 200 | Negative | ||
| 11 | Group 3 | 280 | Ischemia and reperfusion damage at the 24th hour sacrifice with heparin administration | Negative |
| 12 | 240 | Focal + at vessel wall | ||
| 13 | 195 | Negative | ||
| 14 | 220 | Negative | ||
| 15 | 250 | Focal + at vessel wall | ||
| 16 | Group 4 | 205 | Ischemia and reperfusion damage at the 48th hour sacrifice without heparin administration | Focal ++ at vessel wall |
| 17 | 190 | Negative | ||
| 18 | 190 | Focal ++ at vessel wall | ||
| 19 | 180 | Focal + at vessel wall | ||
| 20 | 285 | Negative | ||
| 21 | Group 5 | 290 | Ischemia and reperfusion damage at the 48th hour sacrifice with heparin administration | Focal + at vessel wall |
| 22 | 200 | Negative | ||
| 23 | 250 | Negative | ||
| 24 | 205 | Negative | ||
| 25 | 180 | Focal + at vessel wall | ||
A statistically significant difference was found between the groups in terms of C3 staining (p = 0.001).
Discussion
Interventions on the liver lead to changes that are consistent with the ischemia and reperfusion model. While cell damage occurs during the ischemic period, this damage is further increased during the reperfusion period that follows. The damaging factors include oxygen free radicals, leukocyte migration and activation, sinusoidal endothelial cell damage, irregular microcirculation, and activation of the coagulation system and complement system [14,15].
Ischemia and reperfusion damage is an important pathological process leading to hepatic damage following shock and hepatic surgery. The hepatic damage caused by ischemia and reperfusion damage is believed to be through the pro-inflammatory cytokines and other inflammatory mediators secreted from the activated leukocytes. Therapeutic inhibition of leukocyte activation is therefore useful in preventing this hepatic damage. Inhibition of TNF-α production could be effective in preventing such damage. TNF-α plays an important role in the new microcirculatory distribution due to microthrombus formation. This microthrombus formation further increases TNF-α production in the hepatic damage caused by ischemia and reperfusion damage, leading to a vicious cycle as regards microcirculatory distribution. Heparin has also been shown to prevent neutrophil activation through increased hepatic PGI2 and to decrease the hepatic damage caused by ischemia and reperfusion. Heparin decreases this hepatic damage not only by inhibiting TNF-α production but also by increasing endothelial PGI2 production [16,17]. The fact that we did not find a difference between the groups for serum TNF-α levels could be due to the short half-life. More accurate results could be obtained by investigating tissue TNF-α levels.
Significant differences were found between the groups in the statistical analysis performed for serum IL-6 levels (p = 0.001). Statistically significant differences were found between Group 2 and Group 3 in pairwise comparisons (p = 0.004). No statistically significant differences were found in pairwise comparisons conducted between the other groups (p > 0.017). The maximum increase in serum IL-6 level was in Group 2. The maximum decrease in serum IL-6 level was in Group 3. When Group 4 and 5 were compared, no statistically significant difference was found. This result is explained with the rapid increase in serum cytokines after ischemia and reperfusion, decreasing to basal values at the 48th hour sacrifice due to the very short half-life. Although no statistically significant difference was found, IL-6 levels in the group sacrificed at the 48th hour were lower than in the control group. The IL-6 blood level reached is directly proportional to the amount of tissue damage and is therefore a valuable tissue damage indicator. The high IL-6 level found in Group 2 in our study indicates a high degree of tissue destruction in this group. The decrease in Group 3 could indicate decreased tissue destruction with the effect of heparin. The blood level of IL-6 increases during ischemia [14].
MDA in liver tissue reflects the presence of lipid peroxidation due to the free oxygen radicals in tissues following ischemia reperfusion and thus cellular damage [18]. The lack of a statistical difference between our groups for MDA results suggests that the ischemia that was created did not affect all liver tissue due to the presence of hepatic artery flow. However, one must remember that MDA is not specific.
The consumption of glutathione through conjugation during ischemia and reperfusion damage is one of the causes of cellular GSH level reduction. The decrease in the glutathione amount is compensated by biosynthesis in hepatocytes or exogenous GSH intake. The decrease in intracellular GSH increases GSH biosynthesis through an adaptive cellular response at an early stage [19]. A decrease in the high GSH level is considered to indicate mitochondrial damage and weakening of the intracellular defense system. Heparin sulfate proteoglycans bind to the cell surface and inhibit the damage created by free oxygen radicals. The GSH levels were highest in Group 3 in our study and higher here than in Group 2. This result shows that heparin decreases oxidative damage and increases the level of antioxidant enzymes. No significant difference was found when Group 4 and 5 were compared. The reason for the decrease in tissue GSH levels in Group 4 and 5 could be increased oxidative stress as a result of the decreased lipid peroxidation caused by active neutrophils in these groups and thus inadequate GSH enzyme system activation. This result indicates that heparin reduces oxidative stress in the first 24 hours and leads to the maintenance of high GSH levels. Elucidating the mechanism of this effect will require more advanced studies.
One of the most important indicators of leukocyte migration into tissue that leads to tissue damage is the tissue MPO level. Myeloperoxidase is released from the primary granules of leucocytes and catalyzes the reaction of hypochloric acid formation that is harmful to tissue with the chloride ions of hydrogen peroxide [20,21]. Toxic digestive enzymes such as neutrophil elastase, lactoferrin, β-glucuronidase, N-acetylglucosaminidase, and in particular MPO are released with neutrophil degranulation. This reaction causes tissue destruction and decreased immune resistance [20,21]. MPO activity is considered to be a sensitive quantitative indicator of neutrophil sequestration and has been found to be associated with acute liver damage in many studies based on the ischemia-reperfusion model [22]. We evaluated MPO activity of liver tissue with the immunohistochemical method based on data showing a relationship between MPO activity and number of neutrophils in the tissue [20]. Evaluation of the MPO activity of the liver tissue revealed a statistically significant difference between the groups (p = 0.003). Increased MPO activity was observed in the groups not administered heparin. Low MPO activity was found in most control group subjects. The groups with the most increase in tissue MPO activity were those where ischemia was created without heparin administration. Tissue necrosis was also observed in these groups. There was a decrease in the MPO and no tissue necrosis in the groups administered heparin. These results suggest that heparin can prevent ischemia-related damage in the liver by decreasing cytokines and that this action is independent of the complement system [23,24]. Our results were consistent with the literature. The MPO and IL-6 levels also reflect tissue damage. We observed heparin to decrease tissue damage as reflected in the reduced MPO and IL-6 levels in our study. Heparin could be producing an immunosuppressive effect through its anti-complement action. It has been shown to have a tissue-protective effect at doses of 400 to 2000 IU/kg in various ischemia and reperfusion models [15]. We found mortality to increase at heparin doses of 1200 IU/kg and above in the ischemia and reperfusion model in pilot studies while creating our experimental model and therefore determined our heparin dose as 1200 IU/kg. The reason for administering heparin as a single intravenous bolus is that heparin infusion requires immobilization of the rat causing additional stress. We considered that stress could affect many parameters of our study and therefore used a single intravenous bolus.
Heparin can decrease hepatic damage independently of its anticoagulant effect. Heparin and derivatives also have an effect on the complement system and the most important one is inhibition of the alternative route through C3b. Inhibition of complement and its derivatives by heparin has been demonstrated with residual C3 [25]. C3 inhibition then inhibits the production of C3a, C5a and MAC (C5b-9) that increase leukocyte chemotaxis. Heparin is a C1 inhibitor and prevents complement activation through the classic route [26]. C1 inhibitors have shown damage-decreasing effects in models where experimental ischemia and reperfusion damage were created in the liver, myocardium and brain [14,27]. The protective effect of C1 inhibitors in human myocardial ischemia and reperfusion damage has been demonstrated [27]. This protective effect suggests that this substance may play a role in ischemia and reperfusion damage in other organs. However, the exact mechanism of these effects of C1 inhibitors is not yet clear. Specific inhibition of MAC formation can also play a protective role against ischemia and reperfusion damage in certain tissues. Heparin has been shown to inhibit C3 convertase and C5b-9 [28,29]. We did not include C5b-9 in our study as the other studies were performed in humans and not in rats [28,29].
Heparin has been found to show a protective effect by inhibiting complement in kidney tissue with the C3 IF staining technique in an experimental ischemia reperfusion model [15]. We aimed to show the relationship between heparin and complement with the IF method in the rat liver in this study. Complement staining in Group 2 and 4 (the groups that were not administered heparin) was more intense than in Group 3 and 5 (the groups administered heparin). Group 2 complement staining was more intense than in Group 3. Diffuse involvement of the vessel wall was seen in group 2 while there was no involvement or only focal involvement in Group 3. Complement staining was more intense in Group 4 than in Group 5. Complement staining was less intense in the groups administered heparin. Our results have demonstrated the anti-complement effect of heparin.
Portal vein thrombosis after liver transplantation is a rare complication. However, it needs to be diagnosed and treated early in symptomatic patients as it carries a risk of mesenteric ischemia. Retransplantation may be required if it occurs in the early period after liver transplantation. Heparin and coumadin are used in the treatment of portal vein thrombosis. We showed heparin to decrease the ischemia and reperfusion damage that occurs after portal vein clamping in this study. The results of the clinical use of heparin can be better evaluated in cases of portal vein thrombosis occurring after liver transplantation.
References
- Tokunaga Y, Tanaka K, Uemoto S, Tanaka A, Morimoto T, Yamaoka Y. Risk factors and complications in living-related liver transplantation. Transplant Proc. 1994; 26: 140-143. https://goo.gl/6zybyc
- Mohkam K, Darnis B, Rode A, Hetsch N, Balbo G, Bourgeot JP, et al. Rescue arterial revascularization using cryopreserved iliac artery allograft in liver transplant patients. Exp Clin Transplant. 2017; 15: 420-424. https://goo.gl/Gs4A7K
- Usami M, Furuchi K, Shiroiwa H, Saitoh Y. Effect of repeated portal triad cross clamping during partial hepatectomy on hepatic regeneration in normal and cirrhotic rats. J Surg Res. 1994; 57: 541-548. https://goo.gl/3P5MDi
- Boyce FF, Lampert R, McFetridge EM. Occlusion of the portal vein. J Lab Clin Med. 1935; 20: 935-943.
- Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001; 181: 160-166. https://goo.gl/LeJKsR
- Griffin MP, Gore DC, Zwischenberger JB, Lobe TE, Hall M, Traber DL, et al. Does heparin improve survival in experimental porcine gram-negative septic shock? Circ Shock. 1990; 31: 343-349. https://goo.gl/ikpDKP
- Schirmer WJ, Schirmer JM, Naff GB. Heparin’s effect on the history of sepsis in the rat. Circ Shock. 1987; 21: 363.
- Weiler JM, Edens RE, Linhardt RJ, Kapelanski DP. Heparin and modified heparin inhibit complement activation In-vivo. J Immunol. 1992; 148: 3210-3215. https://goo.gl/x3pG1R
- Fruchterman TM, Spain DA, Wilson MA, Harris PD, Garrison RN. Complement inhibition prevents gut ischemia and endothelial cell dysfunction after hemorrhage/resuscitation. Surgery. 1998; 124: 782-792. https://goo.gl/Q9B9QV
- Hein E1, Munthe Fog L, Thiara AS, Fiane AE, Mollnes TE, Garred P. Heparin-coated cardiopulmonary bypass circuits selectively deplete the pattern recognition molecule ficolin-2 of the lectin complement pathway In-vivo. Clin Exp Immunol. 2015; 179: 294-299. https://goo.gl/pP2sbW
- Beuge JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978; 52: 302-310. https://goo.gl/KP6Wtu
- Elman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82:70-77. https://goo.gl/6kT2eR
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951; 193: 265-275. https://goo.gl/8Kpqy1
- Saurim R, Koike MK, Bonservizi WG, Felix GA, Silva SM, Taha MO, et al. Cardiac effect of ischemic preconditioning and heparin following intestinal ischemia and reperfusion in rats. Transplant Proc. 2014; 46: 1852-1856. https://goo.gl/VmEDXM
- Yagmurdur MC, Çolak T, Emiroglu R, Karabay R, Bilezikçi B, Türkoglu S, et al. Antiinflammatory action of heparin via the complement system in renal ischemia-reperfusion. Transplantation Proceedings. 2003; 35: 2566-2570. https://goo.gl/oEGsE7
- Okano K, Kokudo Y, Okajima K, Hossain MA, Ishimura K, Yachida S, et al. Protective effects of Antithrombin III supplementation on warm ischemia and reperfusion injury in rat liver. World J Surg. 1996; 20: 1069-1075. https://goo.gl/isPr41
- Opal SM, Kesler CM, Roemisch J, Knaub S. Antithrombin, heparin, heparan sulfate. Crit Care Med. 2002; 30: 325-331. https://goo.gl/G4TQP6
- Dimitrios G, Georgios P, Stavros I, Nicholas K, Evanthia K, Aikaterine P, et al. Intramuscular administration of very high dose of alfa Tocopheral protects liver from severs ischemia/reperfusion injury. World J Surg. 2002; 26: 872-877. https://goo.gl/u1yfxz
- Jaeschke H. Molecular mechanisms of hepatic ischemia reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003; 125: 917-36. https://goo.gl/qqfsqR
- Allan G, Bhattacherjee P, Brook CD, Read NG, Parke AJ. Myeloperoxidase activity as a quantitative marker of polymorphonuclear leukocyte accumulation into an experimental myocardial infarct-the effect of ibuprofen on infarct size and polymorphonuclear leukocyte accumulation. J Cardivasc Pharmacol. 1985; 7: 1154-1160. https://goo.gl/FLm5BU
- Gute DC, Ishida T, Yarimizu K, Korthuis RJ. Inflammatory responses to ischemia and reperfusion in skeletal muscle. Moll Cell Bio. 1998; 179: 169-187. https://goo.gl/bGZ6Rz
- Grace PA. Ischemia-reperfusion Injury. Br J Surg. 1994; 81: 637-647. https://goo.gl/m9duZb
- Gaines GC, Welborn MB, Moldawer LL. Attenuation of skeletal muscle ischemia/reperfusion injury by inhibition of tumor necrosis factor. J Vasc Surg. 1999; 29: 370-376. https://goo.gl/hC8jXb
- Kyriakides C, Austen WG Jr, Wang Y, Favuzza J, Moore FD Jr, Hechtman HB. Neutrophil mediated remote organ injury after lower torso ischemia and reperfusion is selectin and complement dependent. J Trauma. 2000; 48: 32-38. https://goo.gl/7mB6Eu
- Linhardt RJ, Loganathan D. Heparin, heparinoids and heparin oligosaccharides: Structure and biological activities, in biomimetic polymers. C. G. Gebelein, ed. Plenum Publishing Corp. New York; 1990. p.135. https://goo.gl/UvYVzd
- Schreiber RD, Morrison DC, Podack ER, Mullereberhard HJ. Bactericidal Activity Of The Alternative Complement Pathway Generated From 11 Isolated Plasma-Proteins. J Exp Med. 1979; 149: 870-882. https://goo.gl/ECkUkX
- Zwaan C, Kleine AH, Diris JH,Glatz JF, Wellens HJ, Strengers PF, Tissing M, Hack CE, van Dieijen-Visser MP, Hermens WT. Continuous 48-h C1 inhibitor treatment, following reperfusion therapy, in patients with acute myocardial infarction. Eur Heart J. 2002; 23: 1670-1677. https://goo.gl/VcHHma
- Bos IG, van Mierlo GJ, Bleeker WK, Rigter GM, te Velthuis H, Dickneite G, et al. The potentiation of human C1-inhibitor by dextran sulphate is transient in vivo: studies in a rat model. International Immunopharmacology 2001; 1: 1583-1595. https://goo.gl/MG6TQn
- Park JL, Tanhehco EJ, Kilgore KS, Gralinski MR, Lucchesi BR. Reviparin-sodium prevents complement-mediated myocardial injury in the isolated rabbit heart. J Cardiovasc Pharmacol. 1997; 30: 658-66. https://goo.gl/x2um11
Authors submit all Proposals and manuscripts via Electronic Form!