Research Article
Protective Effect of Mallow Leaves Extract against Loperamide-induced Oxidative Stress in Rat Jejunum
Mohamed Amine Jabri*, Najla Hajji, Dalanda Wannes, Lamjed Marzouki and Hichem Sebai
Laboratory of Physiology Functional and Valorization of Bio-Resources - Higher Institute of Biotechnology of Beja, University of Jendouba, Avenue Habib Bourguiba - B.P. 382-9000 Beja, Tunisia
*Address for Correspondence: Mohamed-Amine Jabri, Laboratory of Physiology Functional and Valorization of Bio-Resources - Higher Institute of Biotechnology of Beja, University of Jendouba, Avenue Habib Bourguiba - B.P. 382-9000 Beja, Tunisia, Tel: +216-93-484-751; Fax: +216-78-459-098; E-mail: jabri.amino@gmail.com
Dates: Submitted: 16 July 2017; Approved: 25 July 2017; Published: 28 July 2017
Citation this article: Jabri MA, Hajji N, Wannes D, Marzouki L, Sebai H. Protective Effect of Mallow Leaves Extract against Loperamide-induced Oxidative Stress in Rat Jejunum. Int J Hepatol Gastroenterol. 2017;3(1): 022-027.
Copyright: © 2017 Jabri MA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Loperamide; Malva sylvestris; Yohimbine; Lipid peroxidation; Jejunum; Oxidative balance; Thiol group; Free iron
Abstract
Loperamide (LOP) is an antidiarrheal agent that works by slowing gastrointestinal transit and reducing intestinal secretions. The aim of the study is to evaluate the effect of loperamide consumption for five days on the intestinal oxidative balance, as well as the putative protective effect of mallow leaves extract. Animals were divided into one normal control group and five experimental groups. LOP, LOP + the different doses of the extract (100, 200, and 400 mg/ kg, b.w.), and LOP+ yohimbine (2 mg/ kg, b.w. p.i.), used as reference drug. Loperamide (3 mg/ kg, b.w. p.o) was administered twice a day, for 5 days. Treatment with mallow extract or yohimbine protected against the lipid peroxidation, antioxidant enzymes activity depletion, the fall in the thiol group and reduced glutathione level as well as jejunal free iron and H2O2 overload induced by loperamide intoxication. Thereby, Malva sylvestris aqueous extract (MSAE) attenuates the pathogenicity of loperamide.
Introduction
The digestive tract comprises several organs. When we eat, we chew the food in our mouth, and then they pass into the esophagus and then into the stomach where they are crushed, mixed, processed by various substances: enzymes and acids [1]. In the small intestine the essential phase of life takes place: intestinal absorption. The small intestine is an important organ, both by its size and surface area, as well as by its contribution to the endogenous synthesis of glucose [1,2] Foods (proteins, carbohydrates, trace elements, vitamins ...) will be transformed into energy. As regards nutrients, the absorption takes place mainly at the small intestine [1,3]. At the end, the ileum enters the colon and at this level, we have about 800 mL to 1L of fecal fluid. The role of the colon is therefore to absorb water and some minerals [4]. Loperamide is a synthetic opiate derivative lacking central effects that acts as antidiarrheal by decreasing hydration of digestive contents and slowing down transit. Loperamide also exhibits calcium blocking and calmodulin inhibitory effects [5-7].
Oxidative stress is excessive production of reactive oxygen species and the body’s ability to neutralize and repair oxidative damage [8]. Free radicals are molecules with one or more unpaired electrons are very unstable and react quickly with other components, trying to capture the electron necessary for stability [8,9]. The excessive production of free radicals causes direct lesions of biological molecules such as oxidation of DNA, proteins, lipids and carbohydrates, but also secondary lesions due to the cytotoxic and mutagenic nature of the released metabolites, especially during the oxidation of lipids [10].
Malva sylvestris L. (Malvaceae family) is a hairy plant, from 30 to 60 cm in height, with a stem often spread out and crenulated leaves of a shape similar to those of ivy, the flowers are pink-purple with darker veins on the petals. Mallow is biennial, but may be perennial by underground buds [11,12]. The mallow flowering occurs between May-June and September [13]. Numerous studies on the use of medicinal plants have demonstrated the importance of M. sylvestris in the traditional world medicine as a medicated feed. The mallow was used as a mild laxative, a tonic liver cleanser against heartburn [14-16].
Accordingly, the objective of this study is evaluation of the protective effect of mallow leaves (Malva sylvestris L.) extract against oxidative stress induced by loperamide intoxication and the mechanism involved in such protection.
Materials and Methods
Chemicals
Butylated hydroxytoluene (BHT), bovine catalase, Epinephrine, trichloroacetic acid, 2-Thio-barbituric acid (TBA) and yohimbine were from Sigma chemicals Co (Germany). All other chemicals used were of analytical grade.
Sampling and extract preparation
Mallow (Malva sylvestris L.) was collected during March, 2016 from Beja governorate (Tunisia). The Mallow leaves were dried in an incubator at 40°C during 72 hours, and then ground in an electric mixer. The mallow leaves powder was then dissolved in distilled water and incubated at room temperature for 24 h under magnetic stirring. Sample was centrifuged at 10 000 g for 10 min and the supernatant was lyophilized, aliquoted and stored at -80°C until use.
Animals
Adult male Wistar rats (200-220 g, 15 weeks old) were provided by Pasteur Institute of Tunis and used in accordance with the Tunis University ethics committee for the use and care of Laboratory animals and in accordance with the NIH recommendations [17]. They were provided with food and water ad libitum and maintained at a room temperature of 22-25°C.
Loperamide induced-oxidative stress in rats
Oxidative stress was induced in the rats by oral administration of 1 mL of loperamide solution (3 mg/ kg body weight in 0.9% NaCl solution for 5 days) at 09:00 and at 18:00h, while the control groups were received only the saline solution [4,18].
The animals were divided as follows:
Group I: normal control, treated with NaCl (0.9%, p.o.).
Group II: loperamide control treated with NaCl and intoxicated by loperamide on the last day of treatment.
Group III: treated with MSAE (100 mg/kg, b.w. p.o) during 5 days and intoxicated by loperamide on the last day of treatment.
Group IV: treated with MSAE (200 mg/kg, b.w. p.o) during 5 days and intoxicated by loperamide on the last day of treatment.
Group V: treated with MSAE (400 mg/kg, b.w. p.o) during 5 days and intoxicated by loperamide on the last day of treatment.
Group VI: treated with yohimbine (2 mg/kg, b.w. p.i.) during 5 days and intoxicated by loperamide on the last day of treatment.
At the end of experiments, the animals were sacrificed and the jejunum tissues were immediately removed cleaned and homogenized to measure the biochemical parameters
Biochemical estimations
The protein content was assayed by Hartree [19] which is a slight modification of the Lowry method. Malondialdehyde (MDA) levels were determined using the thiobarbituric acid method [20]. GSH (reduced glutathione) levels determination was carried out by Sedlak and Lindsay method [21] and sulfhydryl groups by Ellman’s method [22]. The method described by Flohe and Gunzler [23] was used to determine the activity of jejunal Glutathione peroxidase (GPx) and the method of Misra and Fridovich [24] to determine the Superoxide dismutase (SOD) activity. Catalase (CAT) activity was determined according to the method described by Aebi [25]. Jejunal tissues non haem iron was measured according to the ferrozine method as described by Leardi, et al. [26]. Finally, the hydrogen peroxide (H2O2) levels were determined according to the method described by of Dingeon, et al. [27].
Statistical analysis
All the data were expressed as mean ± Standard Error of the Mean (S.E.M.). Differences between the experimental groups were assessed by one-way ANOVA followed by Duncan’s test. Values were considered statistically significant when p < 0.05.
Results
Effects of MSAE and loperamide on lipid peroxidation
The inhibition of intestinal secretion by loperamide intoxication produced a significant increase in jejunal MDA content compared with the normal group. M. sylvestris aqueous extract (100, 200 and 400 mg/ kg, b.w. p.o) treatment for 5 days significantly decreased MDA content as compared with loperamide group. yohimbine (2 mg/ kg, b.w. p.i.), also significantly protect (P < 0.001) against jejunal MDA overload induced by loperamide (Figure 1).
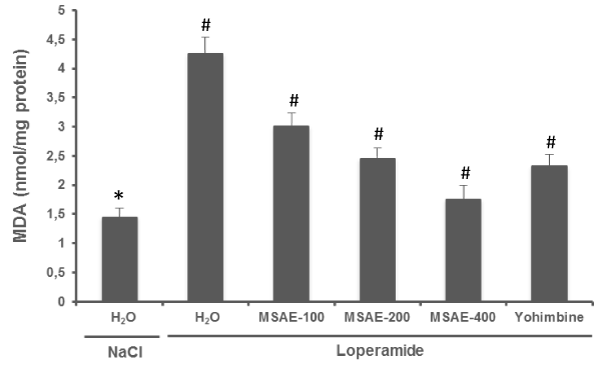 Figure 1: Effects of Malva sylvestris aqueous extract (MSAE) and yohimbine on jejunal MDA level during loperamide intoxication. Animals were treated with various doses of MSAE (100, 200 and 400 mg/kg, b.w., p.o.), reference molecule (yohimbine, 2 mg/ kg, b.w., i.p.) or vehicle (NaCl 0.9%) after loperamide (Lop, 3 mg/kg b.w., p.o.) intoxication.
*: p < 0.05 compared to control group
#: p < 0.05 compared to loperamide group.
Figure 1: Effects of Malva sylvestris aqueous extract (MSAE) and yohimbine on jejunal MDA level during loperamide intoxication. Animals were treated with various doses of MSAE (100, 200 and 400 mg/kg, b.w., p.o.), reference molecule (yohimbine, 2 mg/ kg, b.w., i.p.) or vehicle (NaCl 0.9%) after loperamide (Lop, 3 mg/kg b.w., p.o.) intoxication.
*: p < 0.05 compared to control group
#: p < 0.05 compared to loperamide group.
Effects of MSAE and loperamide on antioxidant enzymes activities
As depicted in figure 2, loperamide intoxication significantly decreased intestinal antioxidant enzyme activities as SOD (A), CAT (B), and GPx (C). While MSAE treatment significantly reversed all loperamide-induced antioxidant enzymes depletion in a dose-dependent manner. Yohimbine, and competitive antagonist of selective α-2 adrenergic receptors, also exhibited the same protection.
 Figure 2: Effects of Malva sylvestris aqueous extract (MSAE) and yohimbine on jejunal antioxidant enzyme activities: SOD (A), CAT (B) and GPx (C) during loperamide intoxication. Animals were treated with various doses of MSAE (100, 200 and 400 mg/kg, b.w., p.o.), reference molecule (yohimbine, 2 mg/kg, b.w., i.p.) or vehicle (NaCl 0.9%) after loperamide (Lop, 3 mg/kg b.w., p.o.) intoxication.
*: p < 0.05 compared to control group
#: p < 0.05 compared to loperamide group.
Figure 2: Effects of Malva sylvestris aqueous extract (MSAE) and yohimbine on jejunal antioxidant enzyme activities: SOD (A), CAT (B) and GPx (C) during loperamide intoxication. Animals were treated with various doses of MSAE (100, 200 and 400 mg/kg, b.w., p.o.), reference molecule (yohimbine, 2 mg/kg, b.w., i.p.) or vehicle (NaCl 0.9%) after loperamide (Lop, 3 mg/kg b.w., p.o.) intoxication.
*: p < 0.05 compared to control group
#: p < 0.05 compared to loperamide group.
Effects of MSAE and loperamide on sulfhydryl groups and reduced glutathione levels
Loperamide-induced intoxication and oxidative stress resulted in decreased jejuna sulfhydryl groups and reduced glutathione levels in comparison with normal. Treatment with M. sylvestris aqueous extract (100, 200 and 400 mg/ kg, b.w. p.o) for 5 days produced a significant increase in intestinal –SH groups and GSH levels compared to loperamide intoxicated group. Rats treated with yohimbine (2 mg/kg, b.w. p.i.) also showed the significant protection (Figure 3).
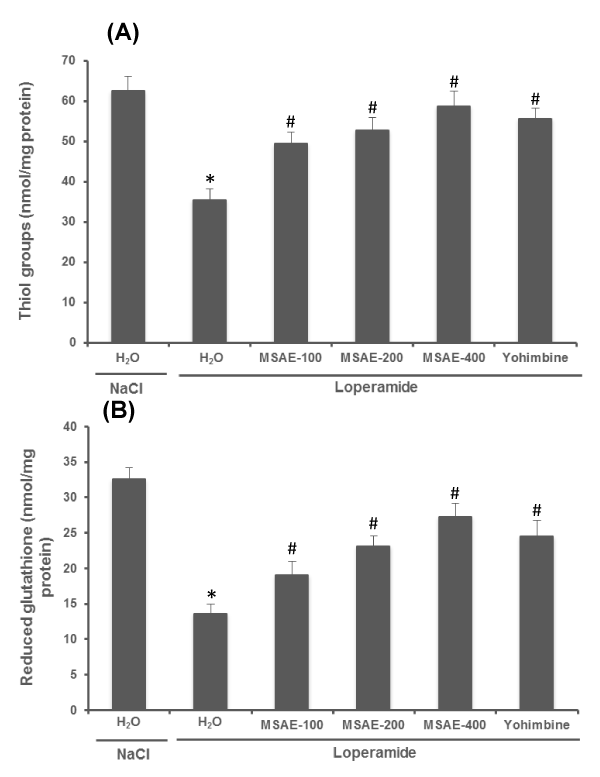 Figure 3: Effects of Malva sylvestris aqueous extract (MSAE) and yohimbine on jejunal sulfhydryl groups (A) and reduced glutathione (B) levels during loperamide intoxication. Animals were treated with various doses of MSAE (100, 200 and 400 mg/kg, b.w., p.o.), reference molecule (yohimbine, 2 mg/kg, b.w., i.p.) or vehicle (NaCl 0.9%) after loperamide (Lop, 3 mg/kg b.w., p.o.) intoxication.
*: p < 0.05 compared to control group
#: p < 0.05 compared to loperamide group.
Figure 3: Effects of Malva sylvestris aqueous extract (MSAE) and yohimbine on jejunal sulfhydryl groups (A) and reduced glutathione (B) levels during loperamide intoxication. Animals were treated with various doses of MSAE (100, 200 and 400 mg/kg, b.w., p.o.), reference molecule (yohimbine, 2 mg/kg, b.w., i.p.) or vehicle (NaCl 0.9%) after loperamide (Lop, 3 mg/kg b.w., p.o.) intoxication.
*: p < 0.05 compared to control group
#: p < 0.05 compared to loperamide group.
Effects of MSAE and loperamide on intestinal H2O2 and free iron levels
In the present study, we also examined the effect of loperamide and MSAE on jejunal H2O2 (Figure 4A) and free iron (Figure 4B) levels. In fact, these two compounds are the constituents of the Fenton reaction, who is involved in the hydroxyl radical production. Loperamide per se significantly increased H2O2 and free iron levels in the jejunal tissues. While MSAE and yohimbine treatment significantly and does-dependently protected against loperamide-induced intracellular mediator disturbances.
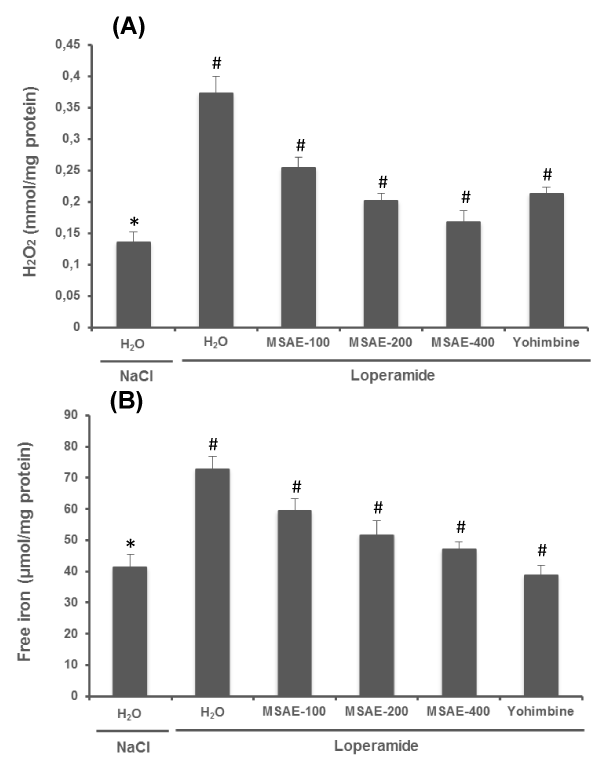 Figure 4: Effects of Malva sylvestris aqueous extract (MSAE) and yohimbine on jejunal H2O2 (A) and free iron (B) levels during loperamide intoxication. Animals were treated with various doses of MSAE (100, 200 and 400 mg/kg, b.w., p.o.), reference molecule (yohimbine, 2 mg/kg, b.w., i.p.) or vehicle (NaCl 0.9%) after loperamide (Lop, 3 mg/kg b.w., p.o.) intoxication.
*: p < 0.05 compared to control group
#: p < 0.05 compared to loperamide group.
Figure 4: Effects of Malva sylvestris aqueous extract (MSAE) and yohimbine on jejunal H2O2 (A) and free iron (B) levels during loperamide intoxication. Animals were treated with various doses of MSAE (100, 200 and 400 mg/kg, b.w., p.o.), reference molecule (yohimbine, 2 mg/kg, b.w., i.p.) or vehicle (NaCl 0.9%) after loperamide (Lop, 3 mg/kg b.w., p.o.) intoxication.
*: p < 0.05 compared to control group
#: p < 0.05 compared to loperamide group.
Discussion
The aim of the present study is to evaluate the effect of loperamide on jejunal redox status, as well as the protective effect of mallow aqueous extract.
Normally, oral fluid intakes exceed fecal losses, the digestive tract behaving in a resultant manner with respect to the internal hydroelectrolytic movements, as an absorption system. Several pumps (cation and anions exchangers) and secretion proteins exist on the apical or basolateral surface of the enterocytes. Various agonists and antagonists of these pumps direct the resultant of the exchanges towards the secretion. This leads to a state of intestinal hypersecretion [1]. Several drugs have been used in this case, the best known is loperamide. Indeed, loperamide is an antidiarrheal drug; it is a structural analog of opiates. It has an antisecretory activity by increasing the hydro-electrolytic flow of the intestinal lumen towards to the plasma pole of the enterocyte, with reverse flow reduction. It also causes a slowing of the colonic transit with an increase in segmental contractions [5-7,28,29]. However, loperamide is a double-edged a weapon, to be used with caution. Possible side effects are usually mild and temporary. Some people may have constipation, drowsiness, abdominal discomfort, dizziness, tiredness, dry mouth, nausea and vomiting [30-32]. In this context, we have shown in this study that taking loperamide for 5 days causes an jejunal oxidative stress state.
In fact, loperamide intoxication has influenced the jejunal redox balance by inducing lipid peroxidation which is manifested by increased levels of MDA, decrease in non-enzymatic antioxidants levels such as sulfhydryl groups and reduced glutathione as well as deleterious effects on the antioxidant enzymes activity such as superoxide dismutase, catalase and glutathione peroxidase. The induction of intestinal oxidative stress was chemically caused by several agents, like aspirin [33], castor oil [34], acetic acid [35] and ethanol [36].
A state of oxidative stress is characterized by an imbalance between the production of Reactive Oxygen Species (ROS) and the level of antioxidant defense systems of the cell, in favor of ROS [37]. ROS may have different cellular sources, the most important of which is mitochondria [38]. The excessive production of free radicals causes direct lesions of biological molecules (oxidation of DNA, carbohydrates, lipids, proteins), but also secondary lesions due to the cytotoxic and mutagenic character of the metabolites released especially during lipids oxidation [38,39]. However, MSAE treatment significantly backed all loperamide-induced jejunal oxidative stress to near control levels. The most of the antioxidant defenses are the micronutrients that oppose the action of the ROS and participate in the recycling of endogenous antioxidants, and which represent cofactors essential for the proper functioning of enzymatic systems such as glutathione peroxidase or superoxide dismutase [40].
In the other hand, we have shown that taking loperamide for five days leads to iron and hydrogen peroxide overload in the jejunum tissues. In addition, the Fenton reagent (a mixture of Fe2+ and H2O2) is one of the most active systems for the oxidation of organics in water. This reactivity is due to the generation of hydroxyl radicals [41,42]. The hydroxyl radicals are the most damaging ROS of oxidative stress, due to their extreme reactivity. Hydroxyl radicals attack all biological materials (DNA, proteins, lipids...). They are powerful oxidants which react according to three modes of action: either by pulling off an electron, or by tearing off a hydrogen atom, or by adding to the double bonds of the biomolecules [37]. However, M. sylvestris aqueous extract has strongly inhibited the jejunal overload of hydrogen peroxide and free iron, which results in inhibition of the hydroxyl radical production. The MSAE free iron chelation and H2O2 scavenging activities can be attributed to its richness in antioxidants molecules such as delphinidin, apigenin, malvidin, malvin, myricetin, quercetin and kaempferol [4,13].
Conclusion
This study has shown that aside from the known adverse effects on loperamide, It also acts negatively on the intestinal oxidative balance by causing of oxidative damages that have been attenuated by Malva sylvestris aqueous extract, due to its antioxidant properties.
Ethical Consideration
All procedures on animals in this study were compiled with the NIH recommendations for the use and care of animals.
Acknowledgements
Financial support of the Tunisian Ministry of Higher Education and Scientific Research is gratefully acknowledged. Financial disclosures: none declared.
Competing interests
The authors declare that they have any competing interests.
References
- CDU-HGE - Editions Elsevier-Masson. The fundamentals of digestive pathology. 22 October 2014. https://goo.gl/7ftiwF
- Sundaram U, Coon S, Wisel S, West AB. Corticosteroids reverse the inhibition of Na-glucose co transport in the chronically inflamed rabbit ileum. Am J Physiol. 1999; 276: G211-8. https://goo.gl/6UjQw8
- Thiesen A, Wild GE, Keelan M, Clandinin MT, Agellon LB, Thomson AB. Locally and systemically active glucocorticosteroids modify intestinal absorption of lipids in rats. Lipids. 2002; 37: 159-66. https://goo.gl/DgvKfa
- Jabri MA, Wannes D, Hajji N, Sakly M, Marzouki L, Sebai H. Role of laxative and antioxidant properties of Malva sylvestris leaves in constipation treatment. Biomed Pharmacother. 2017; 89: 29-35. https://goo.gl/ky2zDv
- Van Nueten JM, Janssen PA, Fontaine J. Loperamide (R18553) a novel type of antidiarrheal agent. III In vitro studies on the peristaltic reflex and other experiments on isolated tissues. Arzneim Forsch. 1974; 24: 1641-5. https://goo.gl/a8Awsn
- Hughes S, Higgs NB, Turnberg LA. Antidiarrhoeal activity of loperamide: studies of its influence on ion transport across rabbit ileal mucosa in vitro. Gut. 1982; 23: 944-9. https://goo.gl/sAeaga
- Hughes S, Higgs NB, Turnberg LA. Loperamide has antisecretory activity in the human jejunum in vivo. Gut. 1984; 25: 931-5. https://goo.gl/un4Qp2
- Favier A. Le stress oxydant : Oxidative stress: conceptual and experimental interest in the understanding of disease mechanisms and therapeutic potential. L'Act Chim. 2003; 270 :108-115. https://goo.gl/vTzPmg
- Beaudeux JL, Delattre J, Therond P, et al. Oxidative stress in the atherosclerotic process. Immuno. Anal Biol Spe. 2006; 21: 144–150.
- Hurtado-Nedeleca M, Dang PMC, Renato C. Monteiro, Monteiroa RC. Physiology of human neutrophils. Revue Francophone des Laboratoires. 2014; 462: 25-38. https://goo.gl/Q4zeen
- Barros L, Carvalho AM, Ferreira IC. Leaves, flowers, immature fruits and leafy flowered stems of Malva sylvestris: a comparative study of the nutraceutical potential and composition. Food Chem Toxicol. 2010; 48: 1466–1472. https://goo.gl/4Rcgrq
- Ballero M, Poli F, Sacchetti G, Loi MC. Ethnobotanical research in the territory of Fluminimaggiore (south-western Sardinia). Fitoterapia. 2001; 72: 788-801. https://goo.gl/mkLYPM
- Gasparetto JC, Martins CA, Hayashi SS, Otuky MF, Pontarolo R. Ethnobotanical and scientific aspects of Malva sylvestris L.: a millennial herbal medicine. J Pharm Pharmacol. 2012; 64: 172-89. https://goo.gl/YgnvEi
- Guarrera PM. Food medicine and minor nourishment in the folk traditions of central Italy (Marche, Abruzzo and Latium). Fitoterapia. 2003; 74: 515-544. https://goo.gl/7hXbqP
- Idolo M, Motti R, Mazzoleni S. Ethnobotanical and phytomedicinal knowledge in a long history protected area, the Abruzzo Lazio and Molise National Park (Italian Apennines). J Ethnopharmacol. 2010; 127: 379–395. https://goo.gl/SqmE8B
- Ishtiaq M, Hanif W, Khan MA, Ashraf M, Butt AM. An ethnomedicinal survey and documentation of important medicinal folklore food phytonims of flora of Samahni Valley (Azad Kashmir) Pakistan. Pak J Biol Sci. 2007; 10: 2241–2256. https://goo.gl/YeUhhs
- National Research Council. Guide for the care and the use of laboratory animals, vol. 20. Bethesda: National Institute of Health; 1985: 85.
- Wu D, Wang X, Zhou J, Yuan J, Cui B, An R, et al. Traditional Chinese formula, lubricating gut pill, improves loperamide-induced rat constipation involved in enhance of Cl- secretion across distal colonic epithelium. J Ethnopharmacol. 2010; 130: 347-53. https://goo.gl/RBCtMS
- Hartree EF. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972; 48: 422-427. https://goo.gl/v99sLp
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990; 186: 421-431. https://goo.gl/g2rq61
- Sedlak J, Lindsay RH. Estimation of total protein bound, and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968; 25: 192–205. https://goo.gl/p9xnwG
- ELLMAN GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82: 70-77. https://goo.gl/LM4hDV
- Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984; 105: 114-121. https://goo.gl/xRakm1
- Misra HP, Fridovich I. The role of superoxide anion in autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972; 247: 3170-3175. https://goo.gl/s5hwFN
- Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105: 121-126. https://goo.gl/JY1hDr
- Leardi A, Caraglia M, Selleri C, et al. Desferrioxamine increases iron depletion and apoptosis induced by ara-C of human myeloid leukaemic cells. British Journal of Haematology. 1998; 102: 746–752. https://goo.gl/42pwr5
- Dingeon B, Ferry JP, Roullet A. Automatic assay of blood sugar by Trinder’s method. Ann Ann Biol Clin (Paris). 1975; 33: 3-13. https://goo.gl/dE3e9R
- Schiller LR, Santa Ana CA, Morawski SG, Fordtran JS. Mechanism of the antidiarrheal effect of loperamide. Gastroenterology. 1984; 86: 1475-1480. https://goo.gl/uwxSbZ
- Fioramonti J, Buéno L. Effects of loperamide hypochloride on experimental diarrhea and gastro-intestinal myoelectrical activity in calves. Am J Vet Res. 1987; 48: 415-419. https://goo.gl/VM1YJf
- Wandel C, Kim R, Wood M, Wood A. Interaction of morphine, fentanyl, sufentanil, alfentanil, and loperamide with the efflux drug transporter P-glycoprotein. Anesthesiology. 2002; 96: 913-920. https://goo.gl/Zbpjr1
- Lifschitz AL, Virkel GL, Sallovitz JM, Pis A, Imperiale FA, Lanusse CE. Loperamide modifies the tissue disposition kinetics of ivermectin in rats. J Pharm Pharmacol. 2003; 55: 1-7. https://goo.gl/kj4VM5
- Crowe A, Wong P. Potential roles of P-gP and calcium channels in loperamide and diphenoxylate transport. Toxicol Appl Pharmacol. 2003; 193: 127-137. https://goo.gl/DG452S
- Yin H, Pan X, Wang S, et al. Protective effect of wheat peptides against small intestinal damage induced by non-steroidal anti-inflammatory drugs in rat. J Integr Agr. 2014; 13: 2019-2027. https://goo.gl/4x89jd
- Jabri MA, Rtibi K, Ben-Said A, Aouadhi C, Hosni K, Sakly M, et al. Antidiarrheal, antimicrobial and antioxidant effects of myrtle berries (Myrtus communis L.) seeds extract. J Pharm Pharmacol. 2016; 68: 264-274. https://goo.gl/c5wNQp
- Kais Rtibi, Haifa Tounsi, Karim Hosni, Abdelaziz Souli, Jamel El-Benna, Lamjed Marzouki, et al. Myrtle berries seeds aqueous extract inhibits in vitro human neutrophils myeloperoxidase and attenuates acetic acid-induced ulcerative colitis in rat. RSC Adv. 2015; 5: 64865-64877. https://goo.gl/tE7Rwp
- Jabri MA, Rtibi K, Tounsi H, Hosni K, Marzouki L, Sakly M, et al. Fatty acids composition and mechanism of protective effects of myrtle berries seeds aqueous extract against alcohol-induced peptic ulcer in rat. Can J Physiol Pharmacol. 2017; 95: 510-521. https://goo.gl/zXCrTP
- Monique Gardes-Albert, Dominique Bonnefont-Rousselot, Zohreh Abedinzadeh, Daniel Jore. Reactive Oxygen Species: How Can Oxygen Become Toxic? L'Act Chim. 2003 ; 11-12 ; 91-96. https://goo.gl/DQpfa
- Rigoulet M, Yoboue ED, Devin A. Mitochondrial ROS generation and its regulation: mechanisms involved in H2O2 signaling. Antioxid Redox Signal. 2011; 14: 459–68. https://goo.gl/jFJjDm
- Hulsmans M, Van Dooren E, Holvoet P. Mitochondrial reactive oxygen species and risk of atherosclerosis. Curr Atheroscler Rep. 2012; 14: 264–76. https://goo.gl/97ZSPc
- Reimund JM. Oxidative stress in chronic inflammatory syndromes. Nutr Clin Metab. 2002; 16: 275–284.
- Platon N, Siminiceanu I, Nistor ID. Fe-pillared clay as an efficient Fenton-like heterogeneous catalyst for phenol degradation. Revista de Chimie. 2011; 62: 676–679. https://goo.gl/vYutm5
- Neyens E, Baeyens J. A review of classic Fenton's peroxidation as an advanced oxidation technique. J Hazard Mater. 2003; 98: 33-50. https://goo.gl/ikKU6T
Authors submit all Proposals and manuscripts via Electronic Form!




























