Rosa Scipioni*, Francesco Carubbi, Michele Saltarelli, Valentino Di Tomasso and Marco Petrarca
Rosa Scipioni1*, Francesco Carubbi1, Michele Saltarelli1, Valentino Di Tomasso1 and Marco Petrarca2
1Division of Internal Medicine, Castel Di Sangro Hospital, Via Sangrina, 67031 Castel Di Sangro (AQ), Italy
2Division of Internal Medicine, Giuseppe Mazzini Hospital, Piazza Italia, 64100 Teramo
*Address for Correspondence: Rosa Scipioni, Department of Internal Medicine, Castel Di Sangro Hospital, via Sangrina, 67031 Castel Di Sangro (AQ), Italy. Tel +39-0864-899298; Fax +39.0864.899219; E-mail: rosascip@gmail.com
Dates: Submitted: 01 27 June, 2017; Approved: 14 July, 2017; Published: 15 July, 2017
Citation this article: Scipioni R, Carubbi F, Saltarelli M, Di Tomasso V, Petrarca M. Relation between Thrombosis and Electrophysiological Study: a Case Report. Int J Case Rep Short Rev. 2017;3(2): 019-022.
Copyright: © 2017 Scipioni R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
strong>Keywords: Pulmonary Thromboembolism; Deep Venous Thrombosis; Cardiac Electrophysiologic Techniques
Abstract
Electrophysiological procedures imply multiple pathophysiological repercussions, such as the induction of the blood coagulation and thrombosis and, rarely, potentially life-threatening thromboembolism. In this sense, pulmonary embolism is a probably underestimated occurrence, and might be related to deep thrombosis of the pricked femoral vein. The role of anticoagulation in preventing deep vein thrombosis after electrophysiological procedures is still debated, but various evidences have pointed out a reduction of femoral vein thrombosis by anticoagulant treatment. Moreover, it might be useful to pay attention to the risks related to femoral access, to an individualized anticoagulation, and to consider an ultrasound screening after the catheter removal. We describe the case of a 44-years-old woman in oral contraceptive therapy who developed deep venous thrombosis and subsequently submassive pulmonary embolism after undergoing an electrophysiological study. The patient was effectively treated with enoxaparin and then rivaroxaban, obtaining a complete resolution of thrombosis.
Abbreviations
CT: Computed Tomography; DVT: Deep Venous Thrombosis; EPS: Electrophysiological Study; LMWH: Low Molecular Weight Heparin; PE: Pulmonary Embolism; VTE: Venous Thromboembolism
Introduction
Electrophysiological Studies (EPS) whether or not associated with therapeutic procedures consist of percutaneous introduction of one or more catheters to record the electrical activity of the heart or to pace its cavities. The introduction and manipulation of these sheaths have multiple pathophysiological consequences, and one of the most evident is to activate the coagulation cascade with potential induction of thrombosis [1]. The risk of developing symptomatic Pulmonary Embolism (PE) from EPS has been reported to be 0 to 1.7 percent [2-4]. Despite PE is a rare complication, it is a serious and potentially fatal condition [5]. Its symptoms are nonspecific; therefore, a high knowledge and clinical suspicion are necessary for its diagnosis. The role of anticoagulation both during and after EPS in preventing PE is still unknown. The assessment of any other risk factors for PE is useful to guide its management.
Case presentation
A 44-year-old woman was admitted to our Department for a three-day of cough, mild dyspnea and chest pain on the right side exacerbated by deep inspiration. Her home therapy was bisoprolol 1.25 mg once a day and oral contraceptive for uterine fibroids. Moreover, her medical history was remarkable for intermittent episodes of palpitation, so one week before she had undergone a trans-femoral EPS at another hospital and discharged the day after the procedure. The study showed no functional changes and no post-procedure anticoagulant drug was prescribed.
At admission, the patient was found a febrile (36.5°C), tachycardic (96 bpm), normotensive (130/80 mmHg), slightly tachypneic (20 breaths/min). Physical examination showed normal heart sound without heart murmurs, and diminished breath sounds of the right lung base. A hemogasanalysis performed without administration of oxygen showed: pH 7.48; pCO2 32 mmHg; pO2 70 mmHg; SO2 91.9%; HCO3- 22 mmol/L.
Laboratory tests were normal, except for mild anemia (Hb 114 gr/L), an increase of CRP (152 mg/L), ERS (69 mm/h) and D-dimer (3027 µg/L). The electrocardiogram revealed sinus tachycardia, and the chest X-ray showed a non-specific diffuse opacity in the right basal side.
There were no signs of Deep Venous Thrombosis (DVT) such as unilateral leg swelling or pain. However, based on her medical history (oral contraceptive, recent EPS through femoral vein access), laboratory tests (elevated D-dimer), and symptoms (chest pain and dyspnea), a thoracic Computed Tomography (CT) with intravenous iodine contrast medium wasalso performed, revealing an acute right sub-segmental PE (Figure 1).
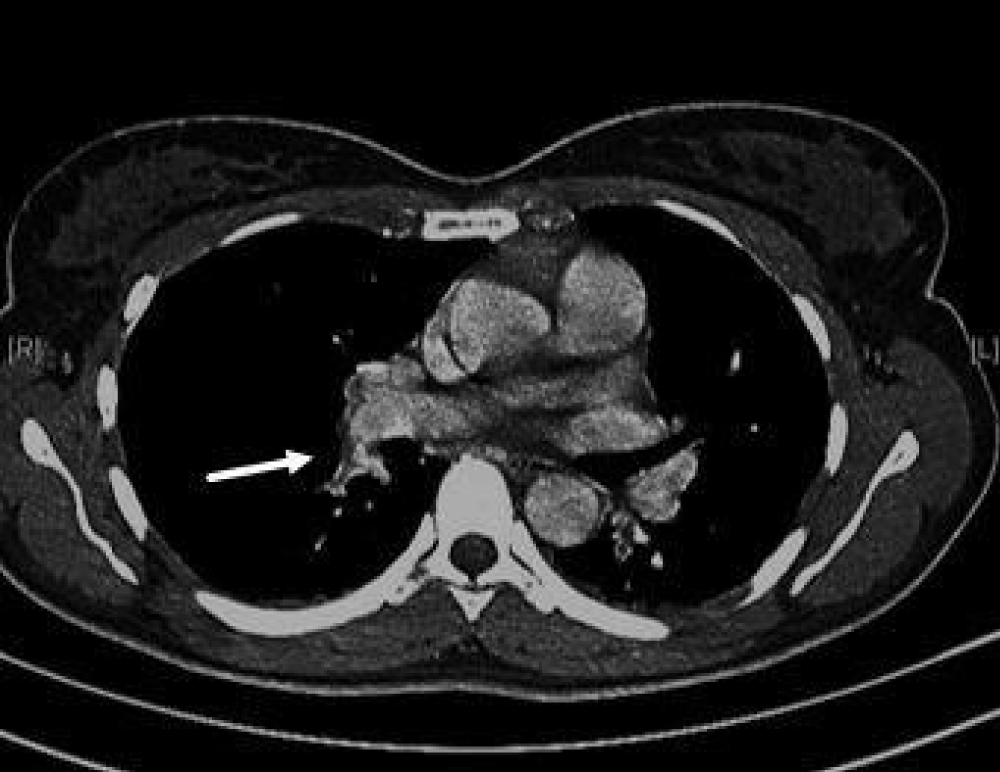 Figure 1: Pulmonary CT with contrast which shows filling defects in the right sub segmental pulmonary artery compatible with local thrombosis.
Figure 1: Pulmonary CT with contrast which shows filling defects in the right sub segmental pulmonary artery compatible with local thrombosis.
Moreover, a duplex ultrasound study of lower limb showed a thrombotic deposit with loss of color-doppler signals from the puncture site to the right external iliac vein (Figure 2). Echocardiography did not show signs of overload or dysfunction of the right ventricule or intracardiac thrombi.
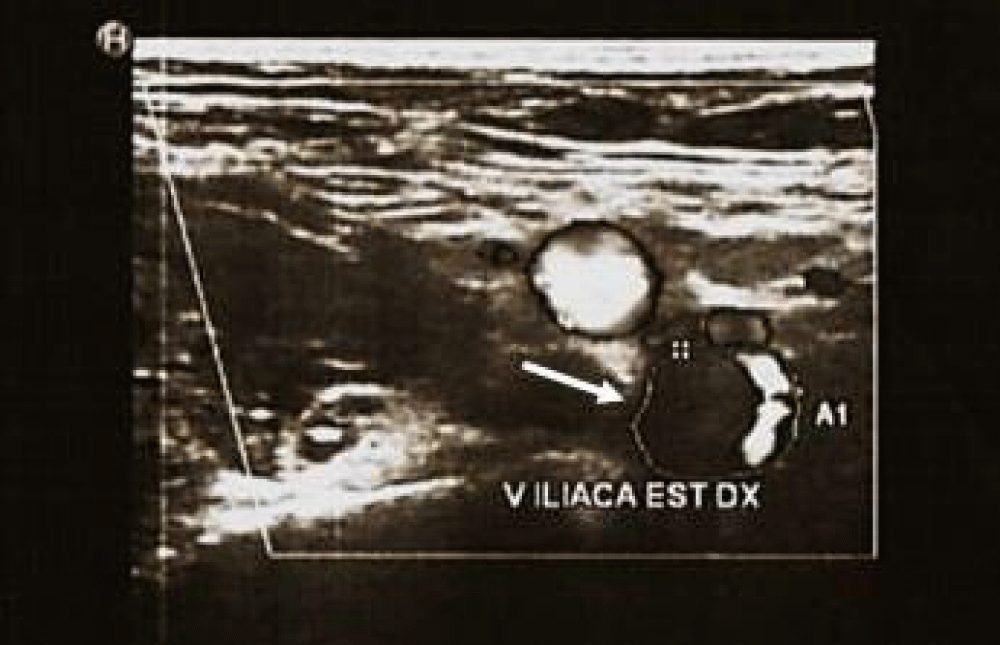 Figure 2: Vascular ultrasound study with transversal view of the right external iliac vein, showing thrombotic deposit.
Figure 2: Vascular ultrasound study with transversal view of the right external iliac vein, showing thrombotic deposit.
A thrombophilic work-up was carried out: anticardiolipin antibodies, homocysteine, antinuclear antibodies, antithrombin, antiphospholipid antibodies, lupus anticoagulant, protein S, protein C, factor V Leiden mutation,prothrombin gene mutationand cryoglobulin were all negative. She had no prior history of thromboembolic events and she denied any familial thrombophilic disorder. A conclusive diagnosis of post-electrophysiological iliofemoral vein thrombosis with submassive PE in patient treated with oral contraceptive was made.
The patient received a full therapeutic dose of enoxaparin (6000 IU twice daily subcutaneously) in the day 1, then shifted to oral anticoagulation with rivaroxaban (15 mg twice daily for 3 weeks followed by 20 mg once a day). Oral contraceptives were stopped. After 1 month of anticoagulant therapy, there were a normalization of laboratory tests, chest X-ray and complete resolution of thrombotic deposit.She was asymptomatic at a 3-months follow-up and rivaroxaban was discontinued.
Discussion
PE is a well-known cause of morbidity and mortality and thought to be associated with more than 300 000 deaths per year in Europe alone [6].
Acute PE is the most serious clinical presentation of Venous Thromboembolism (VTE). Since PE is, in most cases, the consequence of DVT, most of the existing data on its epidemiology, risk factors, and natural history derive from studies that have examined VTE as a whole.
There are well established risk factors associated with VTE and subsequent PE such as obesity, immobilization, hospitalization, malignancy, long flights, use of contraceptive pill, and surgery [7].
PE after EPS is rarely seen but is frequently underestimated. Primm, et al. [8], using radionuclide ventilation/perfusion lung scan, reported that 12% of patients undergoing routine right heart catheterization had asymptomatic pulmonary 1 day after these procedures, suggesting that PE may be more common than previously appreciated. This complication might be attributed to DVT of the punctured femoral vein [9]. Similarly, two small studies (27 and 52 patients) [10-11] have reported asymptomatic DVT following venous sheath placement in 44 and 21% of patients, respectively. Recently, Moubarack, et al. [12] showed that asymptomatic femoral DVT occurred in 5% of EPSs and right-heart radiofrequency catheter ablations, particularly when large sheaths are inserted for long periods. On the contrary, Alizadeh, et al. [13] in a large study of 200 patients found no DVT on Doppler examination performed 24 h after procedure, but they detected in situ thrombosis during catheter removal in 20% of cases.
The role of anticoagulation in the prevention and treatment of DVT in patients that have undergone an EPS is controversial.Chen, et al. [11] observed a DVT regression in 92% of patients after 1 week, although antithrombotic treatment was not mentioned. Davutoglu, et al. [10] reported a significant increase in femoral vein thrombosis after the insertion of multiple venous sheaths during EPS, with no use of heparin; the incidence of femoral vein thrombosis was high (62.5%), and Low Molecular Weight Heparin (LMWH) significantly decreased the risk of femoral thrombosis in these patients (18%). Other studies specifically examined the increased risk of DVT after a single ilio-femoral sheath insertion. They reached varying findings and indicated the need both to be aware of the risks related to the femoral route and to individualize the treatment with heparin. Furthermore, some author suggests to consider ultrasound screening of the lower limbs after catheter removal to evaluate the formation of any thrombi at the site of punctured femoral veins [14].
The Virchow’s triad (blood flow stasis, hypercoagulability, and endothelial injury) may explain the mechanism of thrombosis. Indeed, the introduction and manipulation of catheters in arteries, veins and cardiac cavities during EPS (whether or not associated with therapeutic procedures) have multiple pathophysiological consequences, such as the activation of the coagulation cascade with the risk to induce new clots or to mobilize pre-existing ones. Furthermore, withdrawal of catheters could induce hemorrhages if an adequate post-procedure compression bandage of the site of venous or arterial puncture is not performed, and there is a close relationship between EPS and thrombogenesis. On these bases, to reach a balance between risks of thromboembolism and bleeding is mandatory [1].
The assessment of any other risk factors is useful guide the management. In our report, the patient had one known risk factor (oral contraceptive) that may have increased the hypercoagulable state.
Conclusions
Evidences from our case and others [5,14-15] suggest that, although rare, VTE and PE should be suspected and treated in patients who develop respiratory symptoms after a right-heart procedure, and LMWH prophylaxis should be considered according to the individual patient’s risks factors.
Contribution
RS: Manuscript writing, search for reference; RS, FC, MS and VDT: Case management; VDT: Acquisition of images; MP: Manuscript review.
References
- Blanc JJ, Almendral J, Brignole M, Fatemi M, Gjesdal K, Gonzalez Torrecilla E, et al. Consensus document on antithrombotic therapy in the setting of electrophysiological procedures. Europace. 2008; 10: 513-527. https://goo.gl/BLpJE3
- Dimarco JP, Garan H, Ruskin JN>. Complications in patients undergoing cardiac electrophysiologic procedures. Ann Intern Med. 1982; 97: 490-3. https://goo.gl/VN5AZc
- Horowitz LN, Kay HR, Kutalek SP, Discigil KF, Webb CR, Greenspan AM, et al. Risks and complications of clinical cardiac electrophysiologic studies: a prospective analysis of 1,000 consecutive patients. J Am Coll Cardiol. 1987; 9: 1261-68. https://goo.gl/QtK4Fc
- Hockstad E, Gornick CC. Mildly Symptomatic Pulmonary Emboli Associated With Electrophysiologic Procedures. Indications for Anticoagulant Use. Chest. 1994; 106: 1908-11. https://goo.gl/NajPSZ
- Bauer MP, Vliegen HW, Huisman MV. Massive pulmonary embolism with cardiac arrest after an intracardiacelectrophysiologic study: a strong case for venous thromboprophylaxis. Blood Coagul Fibrinolysis. 2006; 17: 57-8. https://goo.gl/iUkxDo
- Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbility and mortality. Thromb Haemost. 2007; 98: 756-764. https://goo.gl/zxLq4H
- Kroegel C, Reissig A. Principle mechanism underlying venous thromboembolism: epidemiology, risk factors, pathophysiology and pathogenesis. Respiration. 2003; 70: 7-30. https://goo.gl/QJTcxk
- Primm RK, Segall PH, Alison HW, Singh PR, Logic JR, Russell RO JR, et al. Incidence of new pulmonary perfusion defects after routine cardiac catheterization. Am J Cardiol. 1979; 43: 529-32. https://goo.gl/Z7cCYp
- Marijon E, Albenque JP, Boveda S, Jacob S, Schmutz M, Bortone A, et al. Feasibility and safety of same-day home discharge after radiofrequency catheter ablation. Am J Cardiol 2009; 104: 254-8. https://goo.gl/zPP86G
- Davutoglu V, Kervancioglu S, Dinckal H, Soydinc S, Turkmen S, Akdemir I, et al. High incidence of occult femoral vein thrombosis related to multiple venous sheaths during electrophysiological studies. Heart. 2004; 90: 1061–1062. https://goo.gl/WZhnoi
- Chen JY, Chang KC, Lin YC, Chou HT, Hung JS. Safety and outcomes of short-term multiple femoral venous sheath placement in cardiac electrophysiological study and radiofrequency catheter ablation. Jpn Heart J. 2004; 45: 257-64. https://goo.gl/CEV7Lc
- Moubarak G, Bonhomme S, Vedrenne G, Bouleti C, Ollitrault J, Priollet P, et al. Femoral vein thrombosis after right-sided electrophysiological procedures. J Interv Card Electrophysiol. 2013; 38: 155-8. https://goo.gl/tGmk6q
- Alizadeh A, Yazdi AH, Kafi M, Rad MA, Moradi M, Emkanjoo Z. Predictors of local venous complications resulting from electrophysiological procedures. Cardiol J. 2012; 19: 15-9. https://goo.gl/QBcES5
- Hung CY, Lin TC, Hsieh YC, Lee WL, Huang JL, Chang WC, et al. Acute massive pulmonary embolism after radiofrequency catheter ablation: A rare complication after a common procedure. J Chin Med Assoc. 2012; 75: 409-12. https://goo.gl/1giVdn
- Nasrin S, Cader FA, Salahuddin M, Nazrin T, Iqbal J, Shafi MJ. Pulmonary embolism as a complication of an electrophysiological study: a case report. J Med Case Rep. 2016; 10: 89. https://goo.gl/rNqtTt
Authors submit all Proposals and manuscripts via Electronic Form!




























