Case Report
Aprepitant in Emesis Induced by Leptomeningeal Carcinomatosis: Case Report and Rationale
Lamberti G1*, Minichillo S2, Zamagni C3and Biasco G1
1Department of Experimental, Diagnostic, and Specialty Medicine, S. Orsola-Malpighi University Hospital, Bologna, Italy
2Azienda Unita Sanitaria Locale - IRCCS Institute of Neurologic Sciences, Bologna, Italy
3Medical Oncology "Addarii" Oncologia Medica Addarii Policlinico S. Orsola Malpighi, Bologna, Italy
*Address for Correspondence: Lamberti G, Department of Experimental, Diagnostic, and Specialty Medicine, S. Orsola-Malpighi University Hospital, Bologna, Italy
Dates: Submitted: 10 December, 2016; Approved: 30 December, 2016; Published: 05 January, 2017
Citation this article: Lamberti G, Minichillo S, Zamagni C, Biasco G. Aprepitant in Emesis Induced by Leptomeningeal Carcinomatosis: Case Report and Rationale. Int J Case Rep Short Rev. 2017;3(1): 001-004
Copyright: © 2016 Lamberti G, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Aprepitant; Vomiting; Emesis; Leptomeningeal Carcinomatosis; Emetic Circuitry; NK1 Receptor
Abstract
Introduction: We report the case of a young woman affected by emesis refractory to antiemetic drugs, but that temporarily responded to aprepitant, and the possible biological rationale.
Case Report: A 37-year-old woman affected by breast cancer presented with worsening pulsating headache. Leptomeningeal metastases were diagnosed and she rapidly developed nausea and vomiting. Vomiting was unresponsive to antiedemigen and specific antiemetic treatment. Patient underwent chemotherapy administration and aprepitant was given as off-label CINV prevention therapy. The following 6 days were characterized by complete control of vomiting (no episodes, no rescue therapy). After that time vomiting resumed.
Discussion: We hypothesize that the emesis-free period in the patient was due to aprepitant action at a level in emetic circuitry different from other drugs previously administered that had proved ineffective. Our hypothesis is supported by a biological, anatomic and functional rationale in preclinical and clinical studies, which are presented, and by another analogue report in literature.
Conclusion: Aprepitant might have a role in management of emesis from causes other than CINV, within both the palliative and supportive care setting. Further proof-of-concept investigation is needed.
Introduction
Clinical trials on emesis control in Medical Oncology are focused almost exclusively on Chemotherapy Induced Nausea and Vomiting (CINV). Despite great improvement in CINV management in the last 20 years, less is known about emesis induced by other causes. The etiological-based approach in the choice of the anti-emetic drug is a useful overall framework and remains the recommended practice in most current guidelines [1]. However, patients with Central Nervous System (CNS) metastases causing symptoms, such as vomiting, are systematically excluded from clinical trials, in order to avoid biases. All of these contribute to a generalized lack of evidence that results in the impossibility of making an aware therapeutic choice [2-4]. Aprepitant is an orally administered highly selective antagonist of Substance P (SP) on Neurokinin-1 (NK1) receptor. It is registered for use in CINV prevention in chemotherapy regimens with high and moderate emetogenic potential. It is given orally at 125 mg on the first day of therapy (with 12 mg Dexamethasone and 8 mg Ondansetron), and 80 mg on the two subsequent days (with 8 mg Dexamethasone). Randomized placebo-controlled phase III trials showed that aprepitant protected from acute and delayed CINV: this effect is more evident starting from 12-18 hours from chemotherapy administration and lasts until the fifth day from chemotherapy [2-4]. Aprepitant is also approved in some countries as 40 mg oral administration before abdominal surgery for the prevention of Post-Operative Nausea and Vomiting (PONV).
Here is a brief case report on a woman with breast cancer in which emesis was not controlled, except for a few days following the administration of aprepitant, which was given as off-label CINV premedication.
Case Report
A 37-year-old woman presented in July 2015 with worsening pulsating headache in the previous few days. She suffered from chronic sinusitis, but reported this headache to be different from her usual headache. She had been diagnosed with Her-2 positive cancer of her left breast two years before. Last evaluation by mean of Computed Tomography (CT) scans in January 2015 was negative for local and distant disease recurrence.
On physical examination, Brudzinski's sign was positive, without focal neurological deficits, fever, vomiting or nausea, suggesting meningeal involvement. A contrast-enhancing lesion was present in the left Cerebellar hemisphere in brain CT scans.
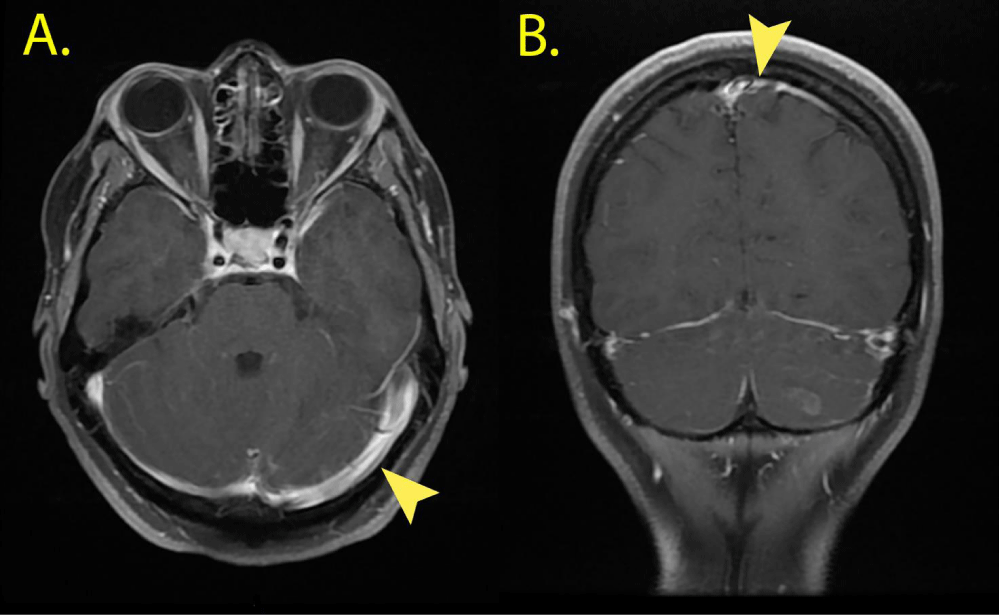
A contrast magnetic resonance imaging of the brain confirmed the subcortical cerebellar lesion characterized by ring-enhancement and mild surrounding edema. There was also remarkable leptomeningeal enhancement over the left cerebral hemisphere and the cerebellar folia, suggesting meningeal carcinomatosis (Figure 1). The diagnosis was confirmed through cytologic analysis of liquor. No other disease localizations were shown by total body CT scans.
Antiedemigen Intravenous therapy with mannitol 18% and high-dose Dexamethasone was started. Despite therapy and improvement of headache, there was a fast worsening of patient's general conditions due to severe lower limbs asthenia, prostration and recurring episodes of vomiting, especially in early morning hours, sometimes preceded by nausea and not associated with food assumption.
Antiemetic therapy with intravenous methoclopramide was started, but it was then refused by the patient due to a not better defined sensation of general and psychological malaise, possibly due to a psychogenic component. Intravenous ondansetron was prescribed as rescue therapy, without benefit.
In agreement with her Oncologist, patient underwent first dose of first-line chemotherapy with docetaxel, trastuzumab and pertuzumab with palliation intent. The patient had been and still was experiencing nausea and several vomiting episodes per day, and, as mentioned above, she was intolerant to methoclopramide and already assuming dexamethasone and ondansetron with poor benefit on emesis. Oral aprepitant was given off-label for CINV prevention (125 mg on day 1 and 80 mg on days 2 and 3), even though the chemotherapy regimen chosen had low emetogenic potential. Antiedemigen therapy was continued, but the steroid dose was reduced first to 16 mg daily, and then to 12 mg daily because of a psychotic episode.
For the six days following chemotherapy administration, the patient's general conditions were stable, but she was free from emesis for the first time from hospitalization: no vomiting occurred and no rescue therapy was needed.
On the seventh day from chemotherapy, vomiting relapsed and later the same day the patient started palliative whole brain irradiation. In particular, subsequent episodes occurred when shifting from clinostasis to seated position and vice-versa and during her transportation to the Radiotherapy department.
In a further attempt to control relapsed emesis, intravenous 8 mg Ondansetron was administered every 8 hours; after a few days, due to poor results in emesis control and onset of constipation, Ondansetron was replaced by intravenous 50 mg Alizapride three times a day.
Patient was discharged at home a few days after she had completed scheduled radiotherapy; all her symptoms improved but emesis, which persisted with one to two vomiting episodes per day. She died three months after discharge.
Figure 1
Contrast brain MR Scans. Leptomeningeal thickening and remarkable contrast enhancement (Arrows) over the Cerebellar folia (A. Axial Scans) and the left Cerebral hemisphere (B. Coronal Scans).
Discussion
Leptomeningeal Carcinomatosis (LC) is common in breast cancer patients treated with anti-Her-2 therapies in the adjuvant setting [5]. This can be ascribed to prolonged survival with therapies targeting Her-2 receptor together with the poor penetration of such drugs (i.e. Trasuzumab) through the Blood-Brain Barrier (BBB).
Frequent presenting symptoms of LC are headache, pain, signs of intracranial hypertension, e.g. nausea vomiting and dizziness, and of meningeal irritation [6]. Diagnosis is based on high clinical and/or imaging suspicion and is confirmed by liquor cytology.
In our patient, the cause of emesis was LC rather than the brain metastasis, given the limited brain parenchyma involvement, the mild surrounding edema and the scarce response to steroids and mannitol. Nausea and vomiting are also more frequently associated with LC than brain metastases. [7, 8].
In our patient, the cause of emesis was LC rather than the brain metastasis, given the limited brain parenchyma involvement, the mild surrounding edema and the scarce response to steroids and mannitol. Nausea and vomiting are also more frequently associated with LC than brain metastases. [7, 8].
Multiple observations suggest a role for aprepitant on the control of emesis in the patient. First of all, the temporal correlation between drug administration and vomiting response for the following six days. Duration of vomiting response was comparable to the five-day emesis protection granted by aprepitant in phase III clinical trials [2]. It was also the last antiemetic drug administered, while steroids dose was already being reducing.
Resolution of headache with steroids and mannitol can be considered as a sign of lowering intracranial hypertension, so persistence of symptoms may be due to other factors.
Response to chemotherapy is another possible cause for the temporary vomiting response. However, it has to be considered that other symptoms didn't improve (i.e. pain, lower limb asthenia, prostration) until radiotherapy started, the short delay between chemotherapy administration and symptoms relief, poor BBB crossing by chemotherapy drugs, and subsequent survival. As reported in literature, in fact, median overall survival in LC patients unresponsive to treatment is 2.0 to 4.0 months and is similar to the survival of our patient of only 3 months, while breast cancer responsive patients had a reported median overall survival of 7.0 to 7.5 months [9,10]. Hence, we considered that chemotherapy may have contributed but it was unlikely to be the main responsible for vomiting temporary resolution.
Neither D2 (Methoclopramide and Alizapride) nor type 3 5-hydroxytryptamine (5HT3)(Ondansetron) receptors blockade was effective in relieving significantly patient's symptoms. This could be because of their mechanism of action. The targets of these drugs, in fact, are in the Chemoreceptor Trigger Zone (CTZ), which is located in the area postrema of the fourth ventricle floor. Its neurons have dopamine D2 receptors, 5HT3 receptors and NK1 receptors and are functionally outside the BBB so that this area is activated by emetogenic substances in peripheral blood [11].
Since localization of disease and ineffectiveness of other peripherally acting antiemetic drugs, it seems legitimate to think that the main emetogenic stimulus was acting beyond the BBB.
Aprepitant crosses BBB and it is proved that it owes its antiemetic effect to this pharmacokinetic characteristic [12-17] and to the binding of its target located in the brain stem, as showed by Positron Emitting Tomography studies [13,18]. In particular, NK1 receptors and Substance P (P stands for "powder" [13]) are highly concentrated in the Nucleus Tractus Solitarius (NTS) [19]. Nucleus Tractus Solitarius is located in the brain stem, ventrally to the area postrema and is a converging site for projections arising from outside (vagal afferents from the upper gastrointestinal tract) and from inside (upper cortical centers, vestibular afferents, area postrema) the CNS. Although there is no anatomic defined "Vomiting Center", NTS is strongly thought to be the final common converging site of multiple afferents which triggers neurons of a central pattern generator responsible for the sequence of behaviors that eventually lead to vomiting [19].
Several studies showed that interfering with NK1 receptor-SP interaction in the NTS prevents vomiting from both peripheral and central stimuli and that the blockade of central NK1 receptors is predominant in comparison to that on NK1 receptors located on vagal terminals in the gut, not excluding a possible contribution from these peripheral sites in vivo [4,12-16, 20-26].
Given the aforementioned considerations, we hypothesized that LC was triggering vomiting in our patient with a mechanism that was not intercepted by other drugs, and that aprepitant was responsible for the patient temporary relief from the symptom because it performs its action at a different level in the emetic circuitry, possibly in the NTS.
In support of our hypothesis, Lowery, et al. [27] reported a similar case of efficacy of aprepitant in controlling refractory nausea and vomiting in a woman affected by meningeal metastases from breast cancer. As in our case, the patient was a young woman whose nausea and vomiting were unresponsive to several antiemetic drugs and who obtained a complete and rapid response to aprepitant. In the case reported by Lowery, the patient took aprepitant without any reported side effect and sustained benefit for a longer period of time than our patient did, who only had six days of complete response, which was compatible with the drug half-life and the reported duration of effect with the usual three-day schedule [4].
Conclusion
In conclusion, our observation is just anecdotal but it is not the only one reported in literature and has a biological rationale. This evidence allows us to speculate that NK1 receptor and SP might be involved in a pathway which is hierarchical higher in generating vomiting. Aprepitant target site may be at a common end transmission way of diverse emetogenic stimuli or, at least, it may have a specific efficacy in nausea and vomiting induced by centrally acting stimuli, such as Leptomeningeal Carcinomatosis. Further proof-of-concept investigation is needed. If demonstrated, the antiemetic effect of aprepitant should be investigated in settings diverse from CINV.
Our experience is not uncommon in advanced cancer patient setting, and also highlights the lack of quality studies aimed to produce valuable evidence in the management of emesis other than CINV. This profoundly affects physician and health professionals involved in cancer patient care in the setting both of the supportive care and palliative care.
References
- Harris DG. Nausea and vomiting in advanced cancer. Br Med Bull. 2010; 96: 175-185.
- Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, et al. Addition of the Neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003; 97: 3090-3098.
- Warr DG, Hesketh PJ, Gralla RJ, Muss HB, Herrstedt J, Eisenberg PD, et al. Efficacy and tolerability of Aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005; 23: 2822-2830.
- Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin-the Aprepitant Protocol 052 Study. Gr. J Clin Oncol. 2003; 21: 4112-4119.
- Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, et al. Central nervous system metastases in women who receive Trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003; 97: 2972-2977.
- Kaplan JG, DeSouza TG, Farkash A, Shafran B, Pack D, Rehman F et al. Leptomeningeal metastases: Comparison of clinical features and laboratory data of solid tumors lymphomas and leukemias. J Neurooncol. 1990; 9: 225-229.
- Yamanaka R, Koga H, Yamamoto Y, Yamada S, Sano T, Fukushige T. Characteristics of patients with brain metastases from lung cancer in a palliative care center. Support Care Cancer. 2011; 19:467-473.
- Oh SY, Lee SJ, Lee J, Lee S, Kim SH, Kwon HC, et al. Gastric Leptomeningeal Carcinomatosis: Multi-Center retrospective analysis of 54 cases. World J Gastroenterol. 2009; 15: 5086-5090.
- Chamberlain MC. Leptomeningeal Metastases: A review of evaluation and treatment. J Neurooncol. 1998; 37: 271-284.
- Gauthier H, Guilhaume MN, Bidard FC, Pierga JY, Girre V, Cottu PH, et al. Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol Off J Eur Soc Med Oncol. 2010; 21: 2183-2187.
- Pleuvry BJ. Physiology and pharmacology of nausea and vomiting. Anaesth Intensive Care Med. 2012; 13: 598-602.
- Diemunsch P, Grelot L. Potential of substance P antagonists as antiemetics. Drugs. 2000; 60: 533-546.
- Hargreaves R, Ferreira JCA, Hughes D, Brands J, Hale J, Mattson B, et al. Development of aprepitant, the first neurokinin-1 receptor antagonist for the prevention of chemotherapy-induced nausea and vomiting. Ann N Y Acad Sci. 2011; 1222: 40-48.
- Rupniak NM, Tattersall FD, Williams AR, Rycroft W, Carlson EJ, Cascieri MA, et al., In vitro and in vivo predictors of the anti-emetic activity of tachykinin NK1 receptor antagonists. Eur J Pharmacol. 1997; 326 :201-209.
- Gardner CJ, Bountra C, Bunce KT, Dale T, Twissell, D et al. Anti-emetic activity of Neurokinin NK1 receptor antagonists is mediated centrally in the ferret [abstract]. Br J Pharmacol. 1994; 112: 516.
- Davidson JS, Oland L, Boissonade F. The effects of centrally injected NK1 receptor antagonists on emesis in the ferret. Gastroenterology. 1995; 108: A589.
- Tattersall FD, Rycroft W, Francis B, Pearce D, Merchant K, MacLeod AM, et al. Tachykinin NK1 receptor antagonists act centrally to inhibit emesis induced by the chemotherapeutic agent cisplatin in ferrets. Neuropharmacology. 1996; 35: 1121-1129.
- Bergstrom M, Hargreaves RJ, Burns HD, Goldberg MR, Sciberras D, Reines SA, et al. Human positron emission tomography studies of brain neurokinin 1 receptor occupancy by aprepitant. Biol Psychiatry. 2004; 55: 1007-1012.
- Hornby PJ. Central neurocircuitry associated with emesis. Am J Med. 2001; 111:106-112.
- Carpenter DO, Briggs DB, Strominger N. Peptide-induced emesis in dogs. Behav Brain Res. 1984; 11: 277-281.
- Andrews PL, Bhandari P. Resinferatoxin, an ultrapotent capsaicin analogue, has anti-emetic properties in the ferret. Neuropharmacology. 1993; 32: 799-806.
- Matsuki N, Toyoda M, Saito H. Role of Substance P in emesis. Folia Pharmacol Jpn. 1996; 108: 133-138.
- Shiroshita Y, Koga T, Fukuda H. Capsaicin in the 4th ventricle abolishes retching and transmission of emetic vagal afferents to solitary nucleus neurons. Eur J Pharmacol. 1997; 339: 183-192.
- Grelot L, AD Miller. A Vomiting- Its Ins and Outs. News Physiol Sci. 1994; 9: 142-147.
- Watson JW, Gonsalves SF, Fossa AA, McLean S, Seeger T, Obach S, et al. The anti-emetic effects of CP-99,994 in the ferret and the dog: Role of the NK1 receptor. Br J Pharmacol. 1995; 115: 84-94.
- Amin AH, Crawford TB, Gaddum JH. The distribution of substance P and 5-hydroxytryptamine in the central nervous system of the dog. J Physiol. 1954; 126: 596-618.
- Lowery L, Andrew I, Gill S, Lee M. The use of aprepitant in a case of refractory nausea and vomiting. Palliat Med. 2014; 28: 990-991.
Authors submit all Proposals and manuscripts via Electronic Form!




























