F.A. Benaich1*, M. El Bakkali2and H. Benjellun1
1Unit of Cardiology A, Ibn Sina University Hospital, Rabat- Morocco
2Physiology of Exercise Team (EPE), Faculty of Medicine and Pharmacy, University Mohammed V, Rabat- Morocco
*Address for Correspondence: Fatima Azzahrae BENAICH, Ibn Sina University Hospital, Unit of Cardiology A, Av Lamfadel Cherkaoui, BP 6203, Rabat-Institutes
Dates: Submitted: 01 October, 2016; Approved: 31 October, 2016; Published: 02 November, 2016
Citation this article: Benaich FA, El Bakkali M, Benjellun H. Fibromyalgia and Dysfunction of Autonomic Nervous System. Int J Case Rep Short Rev. 2016;2(1): 004-010
Copyright: © 2016 Benaich FA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Autonomic nervous system; Fibromyalgia; Parasympathetic nervous system; Sympathetic nervous system; Treatment
Abstract
Fibromyalgia syndrome (FMS) has a strong clinical and social impact affecting the personal, family and working life of the sufferer. The first controlled study of FMS was published by Yunus et al. In Europe, FMS has been estimated to affect approximately 4.7% of the general population. We report a case of 52 year old woman suffered from chronic fatigue for 2 years with widespread pain. Physical examination reveals fibromyalgia syndrome. Complementary tests rule out other etiology. She was treated for her physical abnormality with a significant improvement in her quality of life and had a relief from pain. This study may bring the FMS to clinical consciousness.
Introduction
Fibromyalgia syndrome (FMS) as a disorder has more than one sign or symptom, and has the unfortunate misconception among physicians who either believe in the reality of this disorder or not. FMS is characterized by chronic widespread musculoskeletal pain, stiffness and tenderness to palpation at specific tender points [1]. There is no universal known cause, although numerous overlapping risk factors have been identified. Disorder of the autonomic nervous system (ANS) represents one such overlapping risk factor. The first controlled study of FMS was published by Yunus et al. [2]. In Europe, FMS has been estimated to affect approximately 4.7% of the general population [3].
Case Reports
Patient
A 52 year old woman had a history of rheumatoid arthritis treated by Salazopirine, and hypothyroidism treated with the substitutive hormone. She was referred to our unit of ANS by rheumatologist. The patient suffers from chronic fatigue with a widespread pain, stiffness and non-refreshing sleep for at least 2 years. Physical examination revealed the following results: blood pressure while sitting was 127/68 mmHg, decreasing to 94/52 mmHg in standing position; heart rate: 66 beats/min; tenderness in palpation revealed pain on both sides of the body, below and above joint articulation, and along the axial skeleton.
The heart sound was normal. Electrocardiogram showed regular sinus rhythm. Finding of chest radiography was normal. Transthoracic echocardiography was normal. The laboratory investigations on admission were normal. The thyroid hormones were normal. The electrolytes found no stigmata of adrenal insufficiency. Blood count was normal.
Methods
The Autonomic Nervous System ANS was performed, In front of the evocative clinical symptoms of fibromyalgia, showing a dysautonomia nervous system.
Cardiovascular autonomic testing
Patients were initially lied in bed in a quiet room for at least 30 min. Then monitoring of the BP, using a Dynamap (Critikon, 1846 SXP) and the Heart Rate (HR) (screen of posting LCD CS 503 E, HELLIGE, EK 512 E) was done. All the tests were carried out in the morning, at fasting and under no treatment during at least 48 hours. The basal systolic BP (SBP) and HR were measured in both arms at rest of at least 10 min, and then Ewing cardiovascular autonomic tests were performed.
Tests Description:
- The Deep Breathing Test (DB)
This test analyzes the vagal response [4, 5]. The respiratory frequency has an influence on the variation of RR interval on the electrocardiogram (EKG). The procedure was as the following: the patient breathes deeply at a frequency of six breaths/minute [6]. It makes it possible to evaluate the vagal activity which is expressed as a percentage:
(RRmaximal - RRminimal/RRminimal) x 100.
- The Isometric Contraction or Hand Grip Test (HG)
During three minutes the patient performs a manual pressure of 50% of the maximum with assistance of a dynamometer. The muscular contraction involves a rise in BP related to an increase of sympathetic nerve activity at the muscular level that is effort-dependent and time-dependent [7, 8]. The peripheral alpha sympathetic nerve response is given by the increase degree of the BP.
Alpha peripheral sympathetic response (alpha PS):
= (BPafter the test - BPbefore the test/ BPbefore the test) x 100.
- The Mental Stress Test (SM)
The patient performs mental arithmetic calculations by removing the number 7 successively from 200. The result is an increase in BP and in HR by activation of the central sympathetic nerve [6]. In mental stress, the central sympathetic nerves activities "α" was evaluated by measuring the variations of BP as showing in the following formula [7, 8]:
Alpha central sympathetic response (alpha SC):
= (BPafter stimulation - BPbefore stimulation/BPbefore stimulation) x 100.
The "β" central sympathetic nerves activities was evaluated by measuring the variations of HR as showing in the following formula [7, 8] :
Beta central sympathetic response (beta SC):
= (HRafter stimulation - HRbefore stimulation/HRbefore stimulation) x 100.
- The Orthostatic Test (OT)
The OT is a simple, non invasive and reproducible test included among the cardiovascular ANS tests, involving the measurement of the BP and the HR variation during the upright posture [9]. The basal SBP and HR were measured in both arms after a rest of at least 10 minutes in supine position. Then we proceeded to the OT. Orthostatic SBP (ortho SBP) was recorded for 10 minutes at the rhythm of 3 measurements per minute.
The alpha peripheral adrenergic sympathetic response (Alpha PAS) obtained during OT was evaluated by measuring the variations of BP as showing in the following formula:
Alpha peripheral adrenergic sympathetic response (alpha PS):
= (BP orthostatic - BP supine position/BP supine position) x 100.
Figure 1
Heart rate During the postural orthostatic test performed on 06-08-2012 (Before treatment with fludro-cortisone), reflecting a beta peripheral adrenergic deficiency.
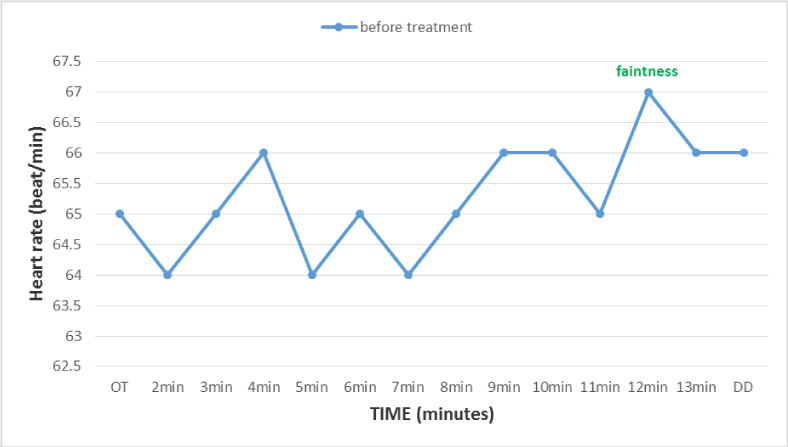
Figure 2
Evolution of blood pressure during the postural orthostatic test performed on 06-08-2012 (Before treatment with fludro-cortisone), reflecting peripheral sympathetic adrenergic alpha deficiency explained by s orthostatic hypotension.
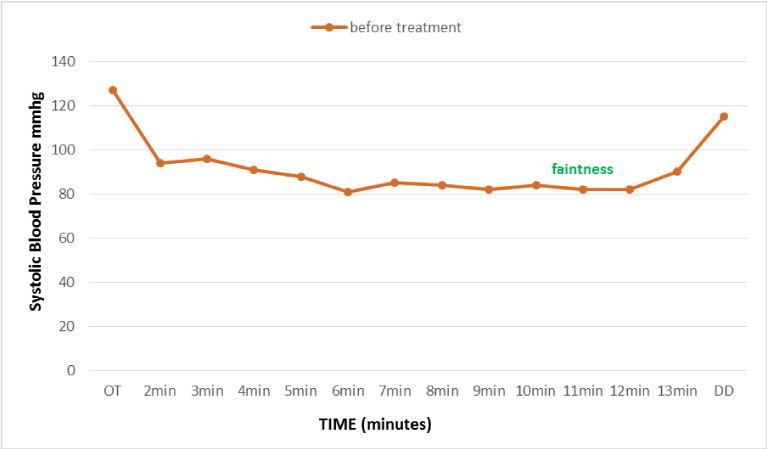
Figure 3
Alpha and beta Central sympathetic response during mental stress test performed on 06-08-2012(Before treatment with fludro-cortisone).
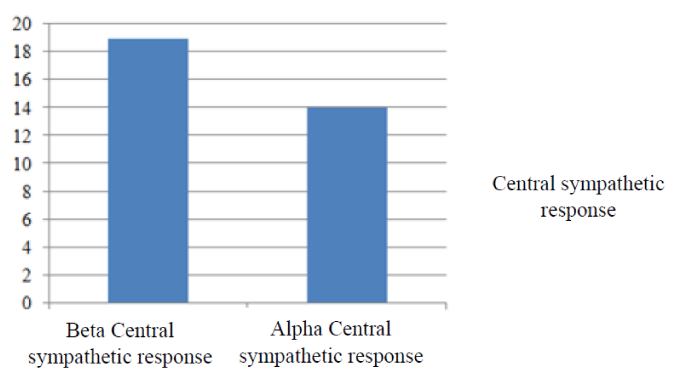
Figure 4
Heart rate during the postural orthostatic test done after 7 months of treatment with fludro-cortisone, reflecting an improvement in beta peripheral adrenergic response.
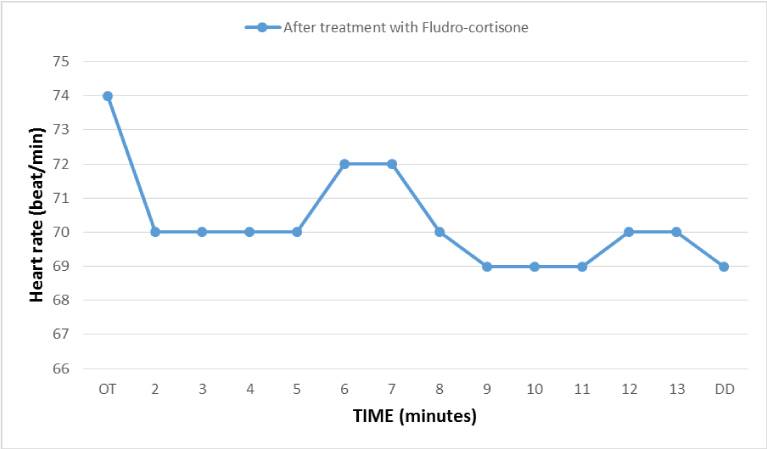
Figure 5
Evolution of blood pressure during the postural orthostatic test done after 7 months of treatment with fludro-cortisone, reflecting an improvement in the sympathetic alpha adrenergic response (late onset of orthostatic hypotension).
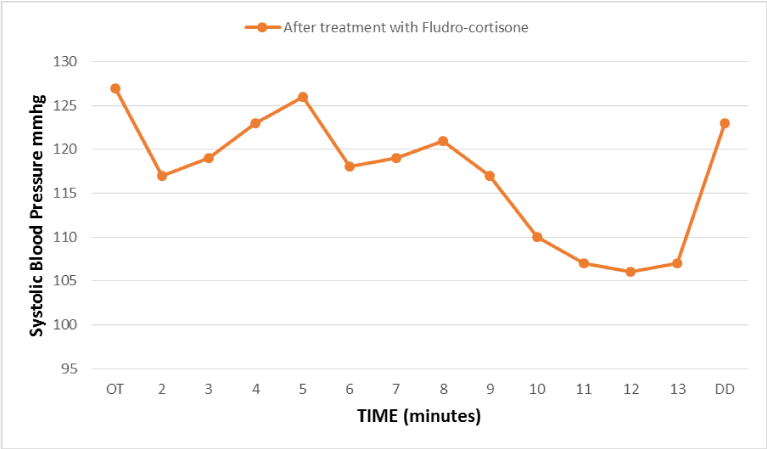
Results
The patient was put under vienotonic with low peripheral venous contention, fludrocortisone and Maprotiline an inhibitor of serotonin and dopamine.
Verification carried 04 months and 07 months after treatment showed a significant improvement on the quality of life with correction of postural hypotension.
Discussion
FMS is a chronic disease that is relatively new on the medical scene [1]. In Europe, FMS has been estimated to affect approximately 4.7% of the general population [3] with a high prevalence in females, sex ratio 10:1 [10, 11]. FMS is a disabling condition with patients experiencing high levels of widespread persistent pain, fatigue and sleep disturbance that makes it difficult to engage in everyday activities [11]. FMS has been classified as primary and concomitant [12]. Primary FMS indicates that there is no underlying or concomitant medical condition that might have contributed to a patient's pain. FMS is considered concomitant if another condition such as osteoarthritis, rheumatoid arthritis, systemic lupus erythematosus or hypothyroidism is present, and, in turn contributes to a patient's pain. However, there are no specific differences that exist between primary FMS and concomitant. The diagnosis criteria of FMS has been refined over 21 years. The basic symptoms of FMS include persistent (= 3 months) widespread pain, along with stiffness, fatigue and many other unexplained symptoms. FMS is commonly associated with significant disability [13]. These pain criteria were developed by the American college of rheumatology ACR in their determination of the diagnostic classification of FMS table N°1. An example of the current diagnosis questions/criteria taken from the fibromyalgia Network is found in figure 6.
FMS still a diagnosis of exclusion. The pathophysiology of FMS remains to be "central nervous system hypersensitivity". There are a number of abnormalities that have been found to be associated with this disorder. But the main problem is central in origin. It is the trigger. The 2 branches of ANS have antagonistic effects. ANS is under tonic inhibitory control of the parasympathetic branch via the myelinated vague nerve. Parasympathetic maintenance promotes calm engagement, whereas vagal withdrawal facilitates quick escape from danger [14]. FMS are linked to higher baseline sympathetic activity or predominance [14-17], and lower baseline parasympathetic activity [17]. Exaggerated parasympathetic decline in response to safe stimuli is associated with hypersensitivity to environmental danger and various reactive emotional disorders such as poorer general physical and mental functioning, poorer sleep and greater chronic pain. The severity of symptoms is depending on individual difference [18].
The treatment plan of FMS must be individualized and flexible, and it is considered long-term management. In general, non-medication treatment may be helpful, but many patients may not stick to them. Currently, three drugs pregabalin, duloxetine and milnacipran have been approved by the FDA for the treatment of FMS. The goal of pharmacological treatment is symptom amelioration, and medications should be started "low and slow", with small doses increased gradually. Tramadol appears to inhibit reuptake of norepinephrine and serotonin, and it is also a receptor agonist. Tramadol has been shown to provide pain relief that is superior to placebo [19]. Beneficial effects for multidisciplinary treatment are found for those patients compared to monodisciplinary treatment programs [20]. Multimodal treatment based on light physical exercise, cognitive behavioral therapy, patient education, biofeedback relaxation and medication, are recommended [21, 22]. However, there is a clear need for continued research into both the diagnostic and therapeutic approach in FMS.
Conclusion
Still, FMS may be a diagnosis of exclusion, and other etiology must be ruled out. However, it is a frequent disorder that affects typically young women. A variety of treatment strategies are available for patients with FMS, ranging from monotherapy to multidisciplinary treatment. The multidisciplinary approach remains recommended.
References
1.Wolfe F, et al., Social security work disability and its predictors in patients with fibromyalgia Arthritis Care Res, 2014. 66 p. 1354-1363.
2.Yunus MB, Masi AT, and Calabro JJ et al, Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched normal controls. Semin Arthritis Rheum, 1981. 11: p. 151-171.
3.Branco JC, et al., Prevalence of fibromyalgia: a survey in five European countries. Semin Arthritis Rheum, 2010. 39: p. 448-53.
4.Ewing DJ, et al., The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care, 1985. 8: p. 491-3.
5.Ewing DJ, et al., Cardiovascular responses to sustained handgrip in normal subjects and in patients with diabetes mellitus: a test of autonomic function. Clin Sci Mol Med, 1974. 46(3): p. 295-306.
6.Low P A, Laboratory evaluation of autonomic function," in Clinical Autonomic Disorders. Evaluation and Managment, Low PA. Ed., pp. 179-208, Lippincott-Raven, Philadelphia, Pa, USA, 2nd edition. 1997.
7.Coghlan H C, Orthostatic intolerance: mitral valve prolapse, in Primer on the Autonomic Nervous System, D. Robertson, P. A. Low, and J. Polinsky, Eds., pp. 283-286, Academic Press, San Diego, Calif, USA, . 1996.
8.Johansen T L, Kambskar G, and Mehlsen J, Heart rate variability in evaluation of the autonomic nervous system. Ugeskr Laeger, 1997. 159: p. 6666-6671.
9.Low PA and Pfeifer MA, Standardization of autonomic function. In : Clinical Autonomic Disorders. 2nd edition, Ed PA Low, Lippincott-Raven 1997: p. 287-295.
10.Yunus MB, The role of gender in fibromyalgia syndrome. CurrRheumatolRep, 2001. 3: p. 128-34.
11.Wolfe F, et al., The American College of Rheumatology 1990 Criteria for the classification of Fibromyalgia. Report of the MulticenterCriteriaCommittee. Arthritis Rheum, 1990. 33: p. 160-72.
12.Wolfe F, et al., The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum, 1995. 38: p. 19-28.
13.Bennett RM, Fibromyalgia and the disability dilemma. A new era in understanding a complex, multidimensional pain syndrome. Arthritis Rheum, 1996. 39(10): p. 1627-1634.
14.Porges SW, The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication, and Self-Regulation.W. W. Norton & Company, New York (2011). 2011.
15.Reyes del Paso GA, et al., Autonomic cardiovascular control and responses to experimental pain stimulation in fibromyalgia syndrome. J Psychosom Res, 2011. 70 p. 125-134.
16.Staud R, Heart rate variability as a biomarker of fibromyalgia syndrome. Future, 2008. 3: p. 475-483.
17.Chervin RD, et al., Objective measures of disordered sleep in fibromyalgia. JRheumatol, 2009. 36: p. 2009-2017.
18.Tory A, Eisenlohr-Moul. Parasympathetic Reactivity in Fibromyalgia and Temporomandibular Disorder: Associations With Sleep Problems, Symptom Severity, and Functional Impairment. The Journal of Pain, 2015. 16(3): p. 247-257.
19.Yunus MB, Masi AT, and Aldag JC, Short term effects of ibuprofen in primary fibromyalgia syndrome: a double blind, placebo controlled trial. J Rheumatol, 1989. 16: p. 527-532.
20.Scascighini L, et al., Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford), 2008. 47: p. 670-678.
21.De Miquel C, Garci'a-Campayo J, and Toma's-Flo'rez M, Documento de Consensointerdisciplinar para el tratamiento de la fibromialgia. Actas Espanolas de Psiquiatria, 2010. 38(2): p. 108-120.
22.Parra-Delgado M and Miguel Latorre-Postigo J, Effectiveness of Mindfulness-Based Cognitive Therapy in the Treatment of Fibromyalgia: A Randomised Trial. CognTher Res, 2013. 37: p. 1015-1026.
23.Gary WJ and R. LB, Fibromyalgia. Disease-a-Month. 2015. 61(3): p. 66-111.
24.Wolfe F, Clauw DJ, and Fitzcharles MA et al, Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. JRheumatol, 2011. 38(6): p. 1113-1122.
25.Bennett R, Friend R, and Marcus D et al, Criteria for the diagnosis of fibromyalgia: validation of the modified 2010 preliminary ACR criteria and the development of alternative criteria. Arthritis Care Res, 2014. 4.
Authors submit all Proposals and manuscripts via Electronic Form!