Review Article
Right Ventricular Systolic Function and Pulmonary Pressure: The Weak Correlation in Stable Patients
Paolo Giovanardi*Enrico Tincani, Carlo Cappelli, Paolo Magnavacchi, Cristina Leonardi, Fabrizio Turrini, Guglielmo Stefanelli, Giovanni Pinelli and Stefano Tondi
Paolo Giovanardi1,2*, Enrico Tincani3, Carlo Cappelli2, Paolo Magnavacchi2, Cristina Leonardi2, Fabrizio Turrini3, Guglielmo Stefanelli4, Giovanni Pinelli3 and Stefano Tondi2
1Cardiology Service, Department of Primary Care, Azienda USL Modena, Italy
2Cardiology Division, Nuovo Ospedale S.Agostino–Estense Modena, Italy
3Internal Medicine Division, Nuovo Ospedale S.Agostino–Estense, Modena, Italy
4Department of Cardiology-Cardiothoracic Surgery, Hesperia Hospital, Modena, Italy
*Address for Correspondence: Paolo Giovanardi, Cardiology Service, Department of Primary Care, Azienda USL Modena, Italy, Via del Pozzo N 71, 41100 Modena, Italy, Tel: +39059437410; Fax: +390536886684; Cardiology Division Nuovo Ospedale S. Agostino–Estense Modena, Italy, Via Giardini 1355, 41126 Baggiovara, Modena, Italy, Tel: +390593961111; E-mail: [email protected]
Dates: Submitted: 11 August 2017; Approved: 29 August 2017; Published: 05 September 2017
Citation this article: Giovanardi P, Tincani E, Cappelli C, Magnavacchi P, Leonardi C, et al. Right Ventricular Systolic Function and Pulmonary Pressure: The Weak Correlation in Stable Patients. Int J Clin Cardiol Res. 2017;1(2): 060-066.
Copyright: © 2017 Giovanardi P, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Pulmonary Pressure; Pulmonary Hypertension; Pulmonary Vascular Resistances; Right Ventricular Function, TAPSE
Abstract
Purpose: Right Ventricular (RV) function and Pulmonary Artery Pressure (PAP) play a role in the morbility and mortality of cardiopulmonary diseases and they are commonly considered strictly related parameters. Not enough attention is given to Pulmonary Vascular Resistances (PVR) even though they play a pivotal role in RV afterload. This cohort study consisted of clinically stable patients and was designed to improve knowledge between echocardiographic indices of RV function, PAP, and PVR.
Methods: 529 consecutive patients underwent a complete echocardiographic examination with the evaluation of Tricuspid Annular Plane Systolic Excursion (TAPSE), RV Systolic Peak (RVSyP) and RV Presystolic Peak (RVPrP). Pulmonary Artery Systolic Pressure (PAPs) and Mean Pulmonary Artery Pressure (mPAP) were measured, thus PVR and RV diastolic function were assessed.
Results: Only TAPSE showed a correlation with PAPs (p < 0.001, correlation coefficient -3.131, (r2 0.025), mPAP (p < 0.001, correlation coefficient -1.91, (r2 0.025), and PVR (p < 0.001, correlation coefficient -0.21, r2 0.064). In the stepwise multiple linear regressions only PVR entered. RVSyP and RVPrP were not related to PAPs, mPAP, and PVR. RV diastolic function was related to PVR (p < 0.001), PAPs ((p 0.001), mPAP (p 0.001), and to TAPSE (p 0.001).
Conclusions: The study showed the capacity only of TAPSE, among the considered indexes of tricuspid annular motion, to reflect PAP and PVR in stable patients. RV function indexes and PAP are not closely related in stable patients.
Introduction
Right Ventricular (RV) function and Pulmonary Artery Pressure (PAP) have a clinical and prognostic role in heart failure [1-3], valvulopathies [4,5], ischemic cardiopathy [6], in congenital heart diseases [7,8], and stable cardiovascular diseases [9], as well as in pulmonary diseases, such as pulmonary hypertension, pulmonary embolism, and chronic obstructive pulmonary disease [10-12]. Not enough attention is given to Pulmonary Vascular Resistances (PVR) even though they represent a crucial component of RV afterload [13-15].
Cardiac magnetic resonance imaging and cardiac catheterization are the gold standards for the assessment of RV function, PAP, and PVR, however echocardiographic parameters of RV function are available and a reliable estimation of PAP and PVR can be obtained by measuring pulmonary and tricuspid flows.
It is commonly believed that RV function and PAP are closely related [12-16] but it is possible that low RV afterload cannot influence RV systolic function [17]. The interactions afterload echocardiograhpy indices of RV function and PAP have been previously studied in healthy subjects and in overt cardiac and lung diseases [17-19], but in stable patients are not completely understood. To address such issue, we conducted a cohort study in stable patients with cardiovascular risk factors studying the correlations between three RV function indices derived from tricuspid annular motion, PAP, PVR, and RV diastolic function.
Methods
Patients
All patients referred to the “Echocardiography Lab – Division of Cardiovascular Medicine”, in Modena, Italy, from October 2011 through August 2014 were eligible.
The study was performed in accordance with the Ethical Standards of the 1964 Helsinki Declaration and its later amendments. The study had been approved by the local Ethic Committee (approval number 238/2010); and informed consent was obtained.
Patients’ data pertaining to medical history and cardiovascular risk factors were collected.
The study did not include patients suffering from pulmonary embolism, severe pulmonary hypertension (from all causes), congenital heart disease, heart failure decompensation, acute myocardial infarction and chronic ischemic cardiopathy, severe valvular stenosis or regurgitation, and atrial fibrillation in whom heart rhythm, global and regional contractility and filling pressure can’t be accurately estimated.
Patients suffering from chronic obstructive pulmonary disease and chronic lung diseases, prior heart failure decompensation, prior stroke, and stable peripheral atherosclerosis could be enrolled if stable at least12 months, but only in the absence of severe pulmonary hypertension, atrial fibrillation, or severe valvular diseases.
Diabetes was considered as a group of metabolic disorders characterized by hyperglycemia resulting from defect in insulin secretion, insulin action, or both. Systemic arterial hypertension was considered as the increase of systolic blood pressure equal to or above 140 mmHg and/or a diastolic blood pressure equal to or above 90 mmHg. Dyslipidemia was considered as a disorder in lipoprotein metabolism resulting in elevation of the blood concentration of cholesterol and/or triglycerides. Tobacco smoke refers to active exposure to tobacco smoke products.
Subjects with bad acoustic window were also excluded.
Echocardiography
Images were obtained by two operators using two commercially available echocardiographic systems (Sequoia 512 – Acuson Siemens, Mountain View, USA; Vivid E9 – GE Healthcare, Norvalk, USA) both equipped with Doppler Tissue Imaging (DTI) technology and with contemporary electrocardiogram recording.
According to the recommendation of the American Society of Echocardiography/European Association of Cardiovascular Imaging (ASE/EACVI), a complete echocardiographic study was performed in the left lateral decubitus and included M-mode, two-dimensional, pulsed and continuous Doppler spectral recording, and DTI evaluation of mitral and tricuspid annulus [20-22]. Two-dimensional images were obtained in parasternal long and short axis views, in apical 4- and 2-chambers view and in subcostal view.
RV systolic function was assessed utilizing three indices derived from tricuspid free wall annular motion: Tricuspid Annular Plane Systolic Excursion (TAPSE), Right Ventricular Systolic Peak velocity (RVSyP), and Right Ventricular Presystolic Peak velocity (RVPrP).
TAPSE was measured in the apical 4-chambers view determining with M-mode guide the maximal excursion of the RV free wall at the junction of the tricuspid valve plane with the tricuspid free wall from the lowest position to the systolic peak. In compliance with ASE/EACVI recommendations, TAPSE was considered normal if higher than 1.7 cm [23].
RVSyP and RVPrP velocities were measured by DTI of tricuspid free wall annulus with the sample volume placed in the basal segment; according to ASE/EACVI recommendations RVSyP was considered normal if higher than 10 cm/sec [11,21,24,26].
PAP was assessed by determining Pulmonary Artery Systolic Pressure (PAPs) and Mean Pulmonary Artery Systolic Pressure (mPAP).
PAPs was obtained in the absence of pulmonary outflow tract obstruction and/or pulmonary valve stenosis through the application of the simplified Bernoulli equation by measuring the systolic tricuspid regurgitant flow peak and then adding right atrial pressure approximated by inferior cava diameter and collapsibility [27]. PAPs was divided into four classes: normal (PAPs < 35 mmHg), mildly (PAPs 35-44 mmHg), moderately (PAPs 45-55 mmHg), and severely increased (PAPs > 55 mmHg) [28].
mPAP was derived, when pulmonary regurgitation was detected, by applying the simplified Bernoulli equation from the early diastolic peak of pulmonary valve regurgitation [29], or from PAPs [30]. mPAP was defined normal when lower than 25 mmHg.
PVR were calculated from the peak tricuspid regurgitation velocity and right ventricular outflow time-velocity integral placing a pulsed wave sample volume in the right ventricular outflow tract at the level of the pulmonic valve in the parasternal short-axis view [31]; PVR were considered normal when lower than 3 Woods Units.
The evaluation of RV diastolic function was assessed studying transtricuspidal flow and DTI of the lateral tricuspidal annulus. E and A peaks, their ratio, E’ and A’ peaks, their ratio, and E/E’ ratio were calculated. RV diastolic function was then classified, according to these parameters, into three classes: normal, impaired relaxation, and restrictive [32].
The echocardiographic parameters were measured at end-expiration during quiet breathing, and three measurements on consecutive heart cycles were averaged. Special care was taken to obtain an ultrasound beam parallel to the direction of tricuspid annular motion and to tricuspid and pulmonary flow, to optimize focus, gain and compression setting, in order to obtain the most accurate endocardium visualization. If necessary echocardiographic parameters were calculated from multiple views.
Intraobserver and interobserver variability were calculated.
Statistical analysis
Continuous variables are displayed as means ± standard deviation, while categorical data are displayed as frequencies. A two-tailed p value ≤ 0.05 was considered statistically significant.
Pearson’s method was used to assess the correlations between the continuous variables. One-way ANOVA was used to estimate the differences of means between RV diastolic function groups. Simple and multivariable linear regression analyses were constructed to assess the association of PAPs, mPAP, and PVR with TAPSE. Colinearity diagnostics was performed. Intra- and inter-observer variability were tested re-measuring with the two echocardiographer TAPSE, RVSyP, RVPrP, PVR, PAPs, mPAP, and RV diastolic function in 29 enrolled patients. Statistical analyses were performed using SPSS/PC release 2013 (IBM Corp., Armonk, NY, USA).
Results
1667 patients were assessed for study eligibility; 529 were enrolled. Of these, 282 (53.3%) were men and 247 (46.7%) were women, with a mean age of 70.52 +/- 5.27 years. Table 1 shows the clinical characteristics of the patients enrolled and exclusion criteria; Table 2 shows the cardiovascular structure and function in the studied cohort revealing the almost complete normality of the echocardiographic parameters.
| Table 1: Clinical characteristics of studied patients. | |
| Number of enrolled patients | 529 § |
| Caucasian | 502 § (94.9%) |
| Body mass index (BMI) | 24.6 +/- 4.3* |
| Mean systolic blood pressure (mmHg) | 130 +/- 7.3* |
| Mean diastolic blood pressure (mmHg) | 85 +/- 4.9* |
| Mean heart rate (beats per minute) | 70 +/- 10.6* |
| Cardiovascular diseases for enrolled patients [Number (percent of patients)]: | |
| Chronic obstructive pulmonary disease and lung diseases | 119 § (22.5%) |
| Prior stroke / transient ischemic attack | 99 § (18.7%) |
| Peripheral atherosclerosis | 53 § (10%) |
| Prior heart failure | 97 § (18.3%) |
| Prevalence of cardiovascular risk factors for enrolled patients [Number / percent of patients]: | |
| Diabetes | 112 § (21.2%) |
| Arterial hypertension | 407 § (76.9%) |
| Dyslipidemia | 350 § (66.2%) |
| Current tobacco use | 151 § (28.5%) |
| Medications for cardiovascular conditions for enrolled patients [Number (percent of patients)]: | |
| Anti platelet agents | 236 § (44.6%) |
| Ace-inhibitors or angiotensin II receptors antagonists | 422 § (79.8%) |
| Beta blockers | 161 § (30.4%) |
| Calcium channel blockers | 216 § (40.8%) |
| Lipid lowering drugs | 303 § (57.3%) |
| Hypoglycaemic agents | 92 § (17.4%) |
| Exclusion criteria [Number of patients]: | |
| Recent heart failure decomposition | 281 § |
| Unstable chronic obstructive pulmonary disease and lung diseases | 263 § |
| Atrial fibrillation | 139 § |
| Acute myocardial infarction and chronic ischemic cardiopathy | 135 § |
| Severe pulmonary hypertension | 77 § |
| Pulmonary embolism | 70 § |
| Severe valvular diseases | 44 § |
| Congenital heart diseases | 39 § |
| Bad acoustic window | 90 § |
| §Number of patients, %percent of patients, *Mean +/- standard deviation | |
| Table 2: Main echocardiographic parameters of studied patients. | |
| Left ventricular ejection fraction (%) | 52.11 +/- 7.21* |
| MAPSE#(cm) | 1.43 +/- 0.74* |
| Left ventricular systolic peak (cm/sec) | 12.06 +/- 4.51* |
| Functional mitral regurgitation [Number (percent of patients)]: | |
| absent | 142 § (26.9%) |
| mild | 297 § (56.1%) |
| moderate | 90 § (17%) |
| Left ventricular diastolic function [Number (percent of patients)]: | |
| normal | 73 § (13.8%) |
| impaired relaxation | 392 § (74.1%) |
| pseudonormal | 51 § (9.6%) |
| restrictive | 13 § (2.5%) |
| TAPSE^ (cm) | 2.42 +/- 0.48* |
| RVSyP^^ (cm/sec) | 17.27 +/- 5.27* |
| RVPrP^^^ (cm/sec) | 19.9 +/- 8.34* |
| PAPs+ (mmHg) | 31.42 +/- 9.62* |
| mPAP++ (mmHg) | 21.16 +/- 5.87* |
| PVR° (Wood Unit) | 1.34 +/- 0.39* |
| Right ventricular diastolic function [Number (percent of patients)]: | |
| normal | 107 § (20.2%) |
| impaired relaxation | 411 § (77.7%) |
| restrictive | 11 § (2.1%) |
| *Mean +/- standard deviation, # Mitral Annular Plane systolic excursion, § Number of patients, %Percent of patients, ^ Tricuspid Annular Plane Systolic Excursion, ^^ Right Ventricular Systolic Peak, ^^^ Right Ventricular Pre systolic Peak, + Pulmonary Artery Systolic Pressure, ++ Mean Pulmonary Artery Pressure, ° Pulmonary Vascular Resistances. | |
No differences were found between the two echocardiographic systems used in M-mode, pulsed and continuous Doppler, and DTI measures. Intra- and inter-observer variability resulted < 1% for RV diastolic function, < 5% for TAPSE, RVSyP, RVPrP, and PVR, and < 7 % for PAPs and mPAP.
PAPs and mPAP were strictly related to PVR (p < 0.001, r 0.675 for both) (Table 3). among the three RV function indices derived from tricuspid annular motion, Pearson’s analysis showed that only TAPSE had a correlation with PAPs (p < 0.001, r 0.166), mPAP (p < 0.001, r 0.166), and PVR (p < 0.001, r 0.256) (Table 3). RVSyP and RVPrP were not related to PAPs (p = 0.326, r 0.043 and p= 0.329, r 0.043 respectively), mPAP (p = 0.326, r 0.043 and p = 0.329, r 0.043 respectively), and PVR (p = 0.481, r 0.032 and p = 0.445, r 0.034 respectively) (Table 3).
| Table 3: Pearson’s correlation between the continuous echocardiography parameters. | ||||||
| > | >RVSyP ^ | >TAPSE ^^ | >RVPrP ^^^ | >PVR* | >PAPs+ | >mPAP++ |
| >RVSyP | > | >r0.119# p 0.007§ |
>r0.741 p < 0.001 |
>r0.032 p 0.481 |
>r0.043 p 0.326 |
>r0.043 p 0.323 |
| >TAPSE | >r0.119 p 0.007 |
> | >r0.02 p 0.659 |
>r0.256 p < 0.001 |
>r0.166 p < 0.001 |
>r0.166 p < 0.001 |
| >RVPrP | >r0.741 p < 0.001 |
>r0.02 p 0.659 |
> | >r0.034 p 0.445 |
>r0.043 p 0.329 |
>r0.043 p 0.33 |
| >PVR | >r0.032 p 0.481 |
>r0.256 p < 0.001 |
>r0.034 p 0.445 |
> | >r0.675 p < 0.001 |
>r0.675 p < 0.001 |
| >PAPs | >r0.043 p 0.326 |
>r0.166 p < 0.001 |
>r0.043 p 0.329 |
>r0.675 p < 0.001 |
> | >r0.96 p < 0.001 |
| >mPAP | >r0.043 p 0.326 |
>r0.166 p < 0.001 |
>r0.043 p 0.329 |
>r0.675 p < 0.001 |
>r0.96 p < 0.001 |
> |
| ^Right Ventricular Systolic Peak, ^^Tricuspid Annulus Plane Systolic Excursion, ^^^Right Ventricular Presystolic Peak, *Pulmonary Vascular Resistances, +Pulmonary Artery Systolic Pressure, ++Mean Pulmonary Artery Pressure, #r =Pearson’s correlation value, § p= p value, correlation is significant at the 0.05 level (2-tailed). | ||||||
Simple regression analysis revealed a linear correlation between TAPSE and PAPs (p < 0.001, correlation coefficient -3.131, r2 0.025) (Figure 1), mPAP (p < 0.001, correlation coefficient -1.91, r2 0.025) (Figure 2), and PVR (p < 0.001, correlation coefficient -0.21, r2 0.064) (Figure 3). In the stepwise multiple linear regressions only PVR entered (p < 0.001, correlation coefficient -0.312).
The One way ANOVA revealed that RV diastolic function was related to TAPSE (p = 0.001), PVR ( p < 0.001), PAPs (p = 0.001), and mPAP (p = 0.001). No association was found between RV diastolic function, RVSyP, and RVPrP (p = 0.276 and p = 0.062 respectively).
The colinearity statistic showed tolerance 1.0 and VIF 1.0 excluding multi colinearity.
Discussion
RV has a complex geometry, with a larger volume and a reduced mass with respect to the Left Ventricle (LV) [33]. RV free wall is made up of two muscle layers: a thin circumferential layer and a deeper longitudinal layer. RV contraction in mainly longitudinal reaching 80% of total RV function (as opposed to LV where radial contraction is predominant) and RV indexes derived from tricuspid annular motion well reflect RV global systolic function [34]. RV has a smaller mass, but pumps the same stroke volume as the LV facilitated by the low resistance and the high capacitance of the pulmonary circulation which is capable of accommodating significant increases in RV stroke volume [35].
RV systolic function depends on RV preload (determined by RV stroke volume, tricuspid and pulmonary regurgitation), RV afterload (determined by the forces that oppose RV output and a reflection of PAP and PVR), and ventricular interdependence (mediated through septum, circumferential muscle fibers, and pericardium) [16-19]. Conversely PAP is determined by cardiac output, properties of the vasculature (resistance, capacitance, and impedance), and left atrial filling pressure [34-36].
RV function and PAP are related, since most of the clinical manifestations of pulmonary hypertension are determined by ventricular adaptation however, the mechanism underlying these relationships are complex and not fully understood [16]. It is well known that PAP elevation lead rapidly to an increase in RV contractility and to RV hypertrophy, but chronically increased after load cause RV dilation and failure [36].
The assessment of RV afterload highlights the fascinating role played by PVR: with respect to systemic circulation, PVR are comparatively lower (one-tenth of systemic vascular resistances) and closely related to pressure changes [37].
Echocardiography is the most simple procedure to evaluate the above mentioned parameters in spite of the fact that previous studies showed limitations pertaining to patients suffering from severe pulmonary diseases and severe pulmonary hypertension (excluded from this study) [38,39]. Furthermore PAP is influenced by age, diabetes, and body mass index [40,41] while RV function indices and PAP are more closely related to clinical state when measured during exercise testing [14].
The interest in these parameters is not merely academic. In fact, the clinical and prognostic importance of RV function and PAP has increasingly gained attention [42]. Today, RV function and PAP are considered predictors of outcome in most cardiovascular diseases, in lung diseases, in pulmonary embolism, and are therefore recommended as part of the standard echocardiographic evaluation [1-12]. Also PVR gained attention and are now considered one of the most important variables to decide the surgical time in congenital heart disease, to evaluate critically ill patients, and represent one of the therapeutic targets in pulmonary hypertension [13-15].
This study was designed to evaluate the relationships among three RV indices derived from tricuspid annular motion (TAPSE, RVSyP, RVPrP), PAPs, mPAP, PVR, and RV diastolic function in stable patients with cardiovascular risk factors and without severe pulmonary hypertension. These patients have not been enclosed in previous studies mostly conducted on healthy or unstable patients.
vAs expected a close relationship between PAP and PVR was observed but unexpectedly only one of the three considered indices of RV function had a correlation with PAP, PVR, and RV diastolic function with a low (p value and a low Pearson’s correlation coefficient = ((r2). Moreover only TAPSE was found to have, in the simple regression analysis, a linear correlation with PAPs (p < 0.001, correlation coefficient -3.131, (r2 0.025) (Figure 1), mPAP (p < 0.001, correlation coefficient -1.91, r2 0.025) (Figure 2), and PVR (p < 0.001, correlation coefficient -0.21, r2 0.064) (Figure 3) and displayed an association with PVR in the stepwise multiple linear regression (p < 0.001, correlation coefficient -0.312).
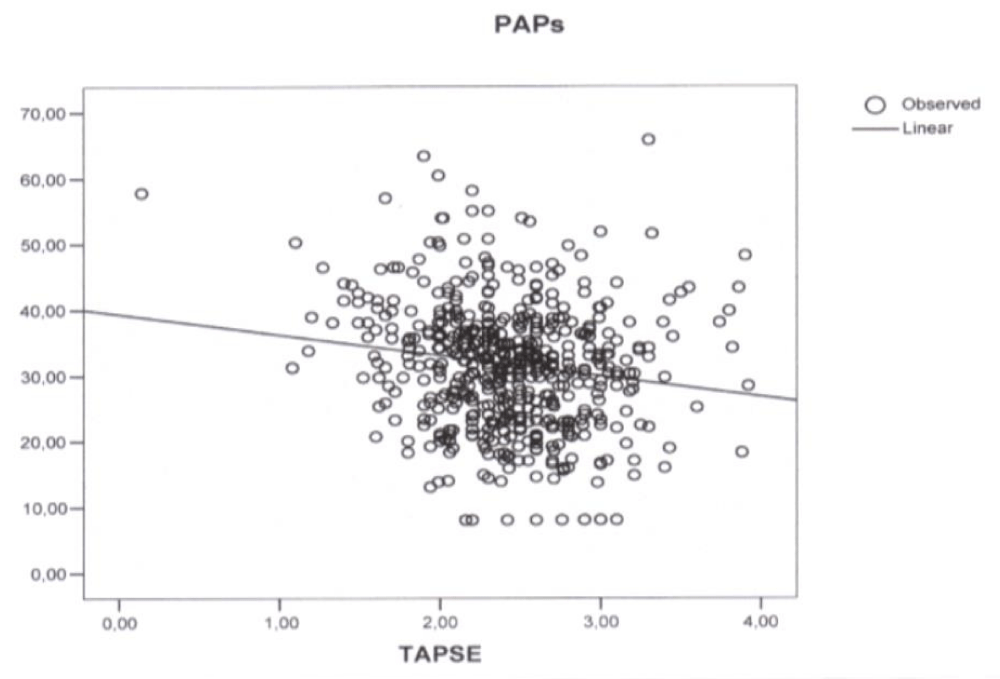 Figure 1: Plot of simple regression analysis between Tricuspid Annular Plane Systolic Excursion (TAPSE) and Pulmonary Artery Systolic Pressure (PAPs) (p < 0.001, correlation coefficient -3.131, r2 0.025).
Figure 1: Plot of simple regression analysis between Tricuspid Annular Plane Systolic Excursion (TAPSE) and Pulmonary Artery Systolic Pressure (PAPs) (p < 0.001, correlation coefficient -3.131, r2 0.025).
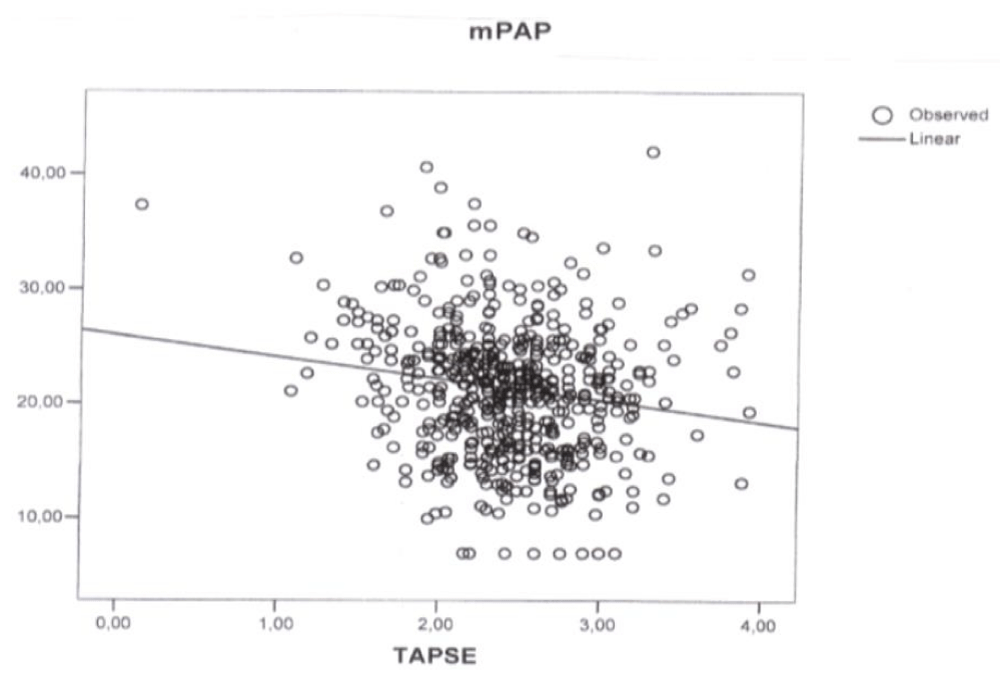 Figure 2: Plot of simple regression analysis between Tricuspid Annular Plane Systolic Excursion (TAPSE) and Mean Pulmonary Artery Pressure (mPAP) (p < 0.001, correlation coefficient -1.91, r2 0.025).
Figure 2: Plot of simple regression analysis between Tricuspid Annular Plane Systolic Excursion (TAPSE) and Mean Pulmonary Artery Pressure (mPAP) (p < 0.001, correlation coefficient -1.91, r2 0.025).
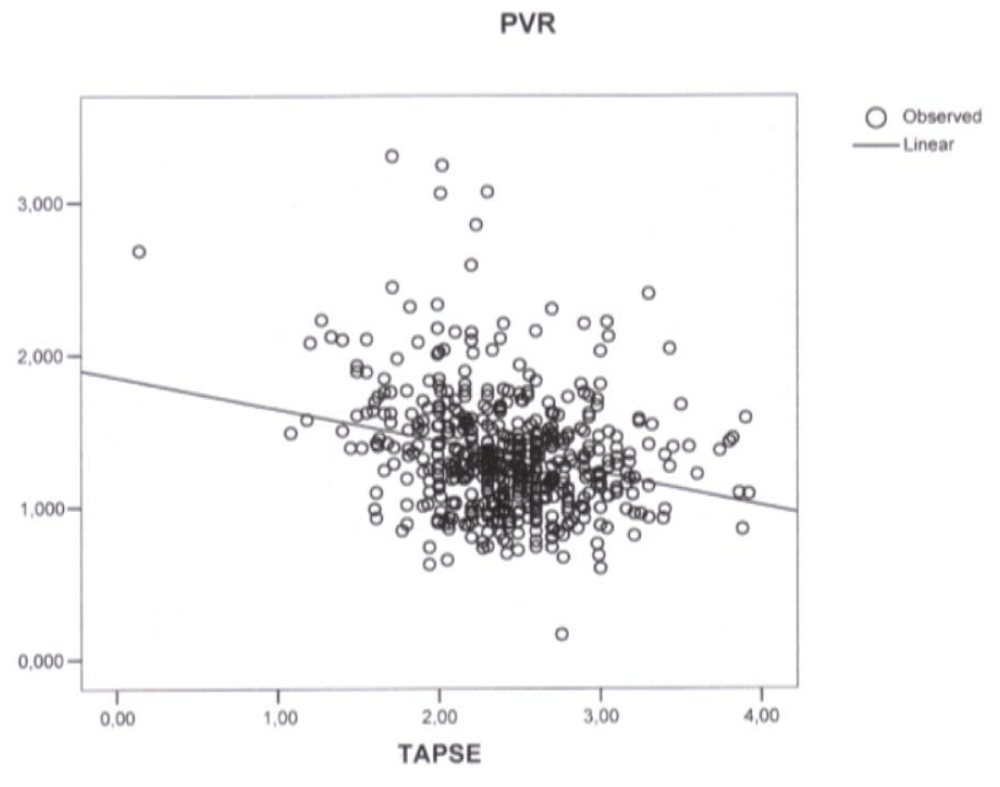 Figure 3: Plot of simple regression analysis between Tricuspid Annular Plane Systolic Excursion (TAPSE) and Pulmonary Vascular Resistances (PVR) (p < 0.001, correlation coefficient -0.21, r2 0.064).
Figure 3: Plot of simple regression analysis between Tricuspid Annular Plane Systolic Excursion (TAPSE) and Pulmonary Vascular Resistances (PVR) (p < 0.001, correlation coefficient -0.21, r2 0.064).
Previous studies evaluated pulmonary hemodynamic in healthy subjects showing that PAPs was associated with LV echocardiographic parameters (posterior wall thickness, ejection fraction, E/E’ ratio) and with systemic vascular stiffness [39,40].
Recently Ferrara, et al. [17] in a large cohort of normal subjects explored the physiologic correlates of TAPSE showing in the univariate analysis a correlation with LV stroke volume, RV basal and longitudinal dimensions, and PVR, and in the multivariable analysis a correlation with cardiac output and RV size. These results are consistent with other reports in which TAPSE seemed more influenced by RV preload [43], but as Ferrara argued it is possible that the low afterload of healthy subjects without pulmonary hypertension is not crucial enough to influence TAPSE [17].
Our study seems halfway between studies performed on healthy subjects and on advanced diseases showing the growing impact of PAP increase on TAPSE [10,11,17].
PAP and PVR were not related to DTI derived indices RVSyP and RVPrP even if Lòpez-Candales, et al. [44] observed that pulmonary hypertension caused a decreased systolic time interval between RVSyP and RVPrP and between the LV mitral presystolic and systolic peaks. The distinctive features between RV function indices are reinforced by evidence that RV diastolic function was correlated with PAP, PVR, and only to TAPSE.
TAPSE represents RV longitudinal and global function and it is a simple test that has been used for a long period time due to its unique characteristics [45]. TAPSE has shown to be influenced also by LV systolic and diastolic function [46] and by ventricular interdependence since it has a pivotal position among LV and RV function indices [47]. TAPSE has a prognostic role in early and advanced cardiovascular diseases [1-12] and in this cohort of stable patients reflects RV afterload more than DTI-derived indices. All RV function indices have distinctive features and we believe that the multi parameter evaluation is required to improve the clinical and prognostic value of RV function.
Conclusions
The study showed, in stable patients, the capacity only of TAPSE to reflect PAP among the considered echocardiographic indexes of tricuspid annular motion. In stable patients RV function indexes of tricuspid motion, PAP, and PVR are not closely related.
Limitations
Echocardiographic parameters of RV function, PAP, PVR, and RV diastolic function were calculated at rest. The cohort was not divided in subgroups based on age and body mass index. The study comprised only stable patients without unstable cardiovascular and pulmonary diseases and without severe pulmonary hypertension, specially for the well known limits of echocardiography in these subjects.
Compliance with Ethical Standards
No funding has been received for this study. The authors declare that they have no conflicts of interest. All procedures performed in the study are in accordance with the Ethical Standards of the 1964 Helsinki Declaration and its later amendments and has been approved by the local Ethic.
Acknowledgments
In loving memory of our colleagues Maria Rita Vandelli MD and PierLuigi Pedrazzi MD. Thanks to Martina Adani for her translation suggestions.
References
- Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CS, Weston SA, et al. Pulmonary pressure and death in heart failure; a community study. J Am Coll Cardiol. 2012; 59: 222-31. https://goo.gl/FFus7r
- Ghio S, Temporelli PL, Klersky C, Simioniuc A, Girardi B, Scelsi L, et al. Prognostic relevance of a non-invasive evaluation of right ventricular function and pulmonary artery pressure in patients with chronic heart failure. Eur J Heart Fail. 2013; 15: 408-14. https://goo.gl/DE7BTG
- Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachièry JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016; 37: 942-54. https://goo.gl/z73aVk
- Nagel E, Stuber M, Hess OM. Importance of the right ventricle in valvular heart disease. Eur Heart J. 1996; 17: 829-36. https://goo.gl/hcENgD
- Kusunose K, Agarwal S, Marwick TH, Griffin BP, Popovic ZB. Decision making in asymptomatic aortic regurgitation in the era of guidelines: incremental values of resting and exercise cardiac dysfunction. Circ Cardiovasc Imaging. 2014; 7: 352-62. https://goo.gl/2yc2Jz
- Samad BA, Alam M, Jensen-Urstad K. Prognostic impact of right ventricular involvement as assessed by tricuspid annular motion in patients with acute myocardial infarction. Am J Cardiol. 2002; 90: 778-81. https://goo.gl/qAWPVi
- Van Straten A, Vliegen HW, Hazekamp MG, Bax JJ, Schoof PH, Ottenkamp J, et al. Right ventricular function after pulmonary valve replacement in patients with tetralogy of Fallot. Radiology. 2004; 233: 824-9. https://goo.gl/Zfuir1
- Rentzsch A, Abd El Rahman MY, Hui W, Helweg A, Ewert P, Gutberlet M, et al. Assessment of myocardial function of the systemic right ventricle in patients with D-transposition of the great arteries after atrial switch operation by tissue Doppler echocardiography. Z Kardiol. 2005; 94: 524-31. https://goo.gl/HqfoYT
- Giovanardi P, Tincani E, Rossi R, Agnoletto V, Bondi M, Modena MG. Right ventricular function predicts cardiovascular events in outpatients with stable cardiovascular diseases: preliminary results. Intern Emerg Med. 2012; 7: 251-6. https://goo.gl/YWvyoy
- Vizza CD, Lynch JP, Ochoa LL, Richardson G, Trulock EP. Right and left ventricular dysfunction in patients with severe pulmonary disease. Chest. 1998; 113: 576-83. https://goo.gl/Lp73q1
- Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002; 39: 1214-9. https://goo.gl/JSz1Vd
- Luscher TF. Pulmonary embolism and pulmonary hypertension: two issues often neglected in cardiology. Eur Heart J. 2015; 36: 581-3. https://goo.gl/vitavG
- Braunwald E, Colucci WS. Vasodilator therapy of heart failure. Has the promissory note been paid ? N Engl J Med. 1984; 310: 459-61. https://goo.gl/rvTueY
- Bidart CM, Abbas AE, Parish JM, Chaliki HP, MorenoCA, Lester SJ. The noninvasive evaluation of exercise-induced changes in pulmonary artery pressure and pulmonary vascular resistance. J Am Soc Echocardiogr. 2007; 20: 270-5. https://goo.gl/bE425U
- Rajagopalan N, Simon MA, Suffoletto MS, Shah H, Edelman K, Mathier MA, et al. Noninvasive estimation of pulmonary vascular resistance in pulmonary hypertension. Echocardiography. 2009; 26: 489-94. https://goo.gl/hFGVHZ
- Naeije R, Huez S. Right ventricular function in pulmonary hypertension: physiological concepts. Eur Heart J Suppl 2007; 9: H5-H9. https://goo.gl/7WNDxn
- Ferrara F, Rudski LG, Vriz O, Gargani L, Afilalo J, D’Andrea A, et al. Physiologic correlates of tricuspid annular plane systolic excursion in 1168 healthy subjects. Int J Cardiol. 2016; 223: 736-743. https://goo.gl/owkxnG
- Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, et al. National Heart, Lung, and Blood Institute Working Group on Cellular Molecular Mechanism of Right Heart Failure. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006; 114: 1883-91. https://goo.gl/B1BHx2
- Matthews JC, McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev. 2008; 4: 49-59. https://goo.gl/dwpfUL
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. American Society of Echocardiography's Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification. Eur J Echocardiogr. 2006; 7: 79-108. https://goo.gl/yGAeAj
- Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Doppler Quantification task force of the Nomenclature and Standards Committee of the American Society of Echocardiography. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 2002; 15: 167-84. https://goo.gl/Z37rKz
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015; 28: 1-39.e14. https://goo.gl/PXYVC3
- Kaul S, Tei C, Hopkins JM, Shah PM. A ssessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984; 107: 526-31. https://goo.gl/jJF7KD
- Saxena N, Rajagopalan N, Edelman K, Lòpez-Candales A. Tricuspid annular systolic velocity: a useful measurement in determining right ventricular systolic function regardless of pulmonary artery pressures. Echocardiography. 2006; 23: 750-5. https://goo.gl/tZcnkg
- Vogel M, Schmidt MR, Kristiansen SB, Cheung M, White PA, Sorensen K, et al. Validation of myocardial accelleration during isovolumic contraction as a novel noninvasive index of right ventricular contractility: comparison with ventricular pressure-volume relations in an animal model. Circulation. 2002; 105: 1693-9. https://goo.gl/nJeZMY
- Giovanardi P, Tincani E, Stefanelli G, Turrini F, Magnavacchi P, Sansoni S, et al. Right ventricular presystolic peak velocity represents right ventricular function in stable patients. Right ventricular presystolic peak velocity represents right ventricular function in stable patients. Minerva Cardioangiol. 2017; 65: 134-139. https://goo.gl/23UQXi
- Yock PG, Popp RL.Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984; 70: 657-62. https://goo.gl/GY2oqc
- Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009; 30: 2493-537. https://goo.gl/GnGZM3
- Bossone E, D’Andrea A, D’Alto M, Citro R, Argiento P, Ferrara F, et al. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr. 2013; 26: 1-14. https://goo.gl/riUnzk
- Chemla D, Castelain V, Humbert M, Hébert JL, Simonneau G, Lecarpentier Y, et al. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest. 2004; 126: 1313-7. https://goo.gl/gNyhvv
- Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance J Am Coll Cardiol. 2003; 41: 1021-7. https://goo.gl/q7YkQh
- Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010; 23: 685-713; quiz 786-8. https://goo.gl/nwmSE5
- Moceri P, Baudouy D, Chiche O, Cerboni P, Bouvier P, Chaussade C, et al. Imaging in pulmonary hypertension: focus on the role of echocardiography. Arch Cardiovasc Dis. 2014; 107: 261-71. https://goo.gl/yAqD2c
- D’Alto M, Scognamiglio G, Dimopoulos K, Bossone E, Vizza D,Romeo E, et al. Right heart and pulmonary vessels structure and function. Echocardiography. 2015; 32 Suppl 1: S3-10. https://goo.gl/ir7PKG
- Pristera N, Musarra R, Schilz R, Hoit BD. The role of echocardiography in the evaluation of pulmonary arterial hypertension. Echocardiography. 2016; 33: 105-16. https://goo.gl/2JTnYv
- Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, et al. Right heart adaptation to pulmonary arterial hypertension. J Am Coll Cardiol. 2013; 62: D22-33. https://goo.gl/F9TMkt
- 37Granstam SO, Bjoruklund E, Wikstrom G, Roos MW. Use of echocardiographic pulmonary acceleration time and estimated vascular resistance for the evaluation of possible pulmonary hypertension. Cardiovasc Ultrasound. 2013; 11: 7. https://goo.gl/riKGUx
- Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011; 97: 612-22. https://goo.gl/hPTyHq
- Huez S, Retailleau K, Unger P, Pavelescu A, Vachiéry JL, Derumeaux G, et al. Right and left ventricular adaptation to hypoxia: a tissue Doppler imaging study. Am J Physiol Heart Circ Physiol. 2005; 289: H1391-8. https://goo.gl/6mjc7r
- Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population Circulation. 2009; 119: 2663-70. https://goo.gl/CrnYJ3
- D’Andrea A, Naeije R, Grunig E, Caso P, D’Alto M, Di Palma E, et al. Echocardiography of the pulmonary circulation and right ventricular function: exploring the physiologic spectrum in 1,480 normal subjects. Chest. 2014; 145: 1071-1078. https://goo.gl/joZRGC
- 42 Markley RR, Ali A, Potfay J, Paulsen W, Jovin IS. Echocardiographic evaluation of the right heart. J Cardiovasc Ultrasound. 2016; 24: 183-190. https://goo.gl/GTdJ5k
- Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006; 174: 1034-41. https://goo.gl/MBHPV2
- Lòpez-Candales A, Edelman K, Gulyasy B, Candales MD. New annular tissue Doppler markers of pulmonary hypertension. Echocardiography. 2010; 27: 969-76. https://goo.gl/5mg4ES
- Aloia E, Cameli M, D’Ascenzi F, Sciaccaluga C, Mondillo S. TAPSE: an old but useful tool in different diseases. Int J Cardiol. 2016; 225: 177-183. https://goo.gl/CJVsJa
- Lòpez-Candales A, Rajagopalan N, Saxena N, Edelman K, Gulyasy B, Edelman K, et al. Right ventricular systolic function is not the sole determinant of tricuspid annular motion. Am J Cardiol. 2006; 98: 973-7. https://goo.gl/uJkrr2
- Giovanardi P, Stefanelli G, Turrini F, Sarti L, Lami F, Scarlini S, et al. Interactions between commonly used left and right ventricular function indexes in stable patients. Minerva Cardioangiol. 2014; 62: 335-41. https://goo.gl/5An1T8
Authors submit all Proposals and manuscripts via Electronic Form!




























