Research Article
Amelioration of Nicotine Induced Toxicity by Nanocurcumin in Protein Malnourished Condition
Krishna Chattopadhyay1*, Somashree Biswas2, Subrata Mukhopadhyay1 and Brajadulal Chattopadhyay2
1Department of Chemistry, Jadavpur University, Kolkata-700032, India
2Department of Physics, Jadavpur University, Kolkata-700032, India
*Address for Correspondence: Dr. Krishna Chattopadhyay, Department of Chemistry, Jadavpur University, Kolkata-700032, India, Tel: +919-433-122-559; E-mail: [email protected]
Dates: 06 June 2018; Approved: 03 July 2018; Published: 07 July 2018
Citation this article: Krishna C, Biswas S, Mukhopadhyay S, Chattopadhyay B. Amelioration of Nicotine Induced Toxicity by Nanocurcumin in Protein Malnourished Condition. Sci J Biol. 2018;1(1): 001-008.
Copyright: © 2018 Krishna C, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Antioxidant; Nanocurcumin; Nicotine toxicity; Oxidative stress; Malnourished
Abstract
Topic: Nicotine, the core addictive component presents in a substantial quantity in tobacco. Addiction of nicotine in female populations especially who belong to lower socio-economic status causes many adverse effects, like inducing oxidative stress, genotoxicity and disrupting immune response in the body. The investigation was designed to determine first time the improved efficacy of nanocurcumin against aggravated nicotine-induced toxicity on female system.
Experiments: Experiments were conducted on female albino rats maintained under protein malnourished condition where animals were subcutaneously injected with effective dose of nicotine (2.5 mg kg-1 body weight day-1) and orally supplemented with effective dose of nanocurcumin (4 mg kg-1 body weight day-1) for 21 days. The animals were then sacrificed and various biochemical and histological experiments were performed on serum and different tissues.
Significant Findings: Disruption of liver and kidney functions, alteration of phosphatase enzymes activities, triglycerides and total cholesterol in serum and aggravated lipid peroxidation in serum and liver tissues were observed in nicotine-induced and protein malnourished condition. Nicotine also decreased the antioxidant enzymes activities and disrupted severely the structural integrity of liver, kidney and ovary tissues in such condition. Supplementation of nanocurcumin restored the normalcy of various biochemical functions and maintained the structural integrity of liver, kidney and ovarian tissues
Conclusions: Nanocurcumin exhibited more effective (P < 0.001) amelioration than curcumin against the deleterious nicotine-induced toxicities and therefore, it can be used as a potential therapeutic blocker for protecting the health of malnourished female population against nicotine.
Introduction
Increasing use of different forms of tobacco products is an alarming danger for human health worldwide. Along with the common tobacco forms like cigarettes and pipes consumed worldwide, the Indian populations are also habituated with smoking of Chutta and Beedi, (locally used handmade smoking elements) and chewing of tobacco leaves [1]. Consumption of different forms of tobacco is also increasing among the women and this has now become the biggest problems in India [2]. The route of administration for nicotine is through a variety of ways including smoking, insufflations, chewing, transdermal and vaporization etc. In addition, depending on the route of administration, the exposure time and dosage, the amount of nicotine enters in the body may vary.
Nicotine is well known to have serious systemic side effects in addition to being highly addictive [3]. It is the most acute acting pharmacological agent and major component of tobacco and plays a significant impact in the development of cardiovascular disorder [4], Chronic Obstructive Pulmonary Disease (COPD) and lung cancer [5] and many other non- communicable diseases [6]. It also aggravates Th1/Th2 cytokine imbalance, alters transcription factor, lipid peroxidation in the liver and other tissues and effects on the activities of antioxidant enzymes in rats [7,8]. Nicotine disrupts antioxidant mechanism by enhancing the production of Reactive Oxygen Species (ROS) and thereby decreases antioxidant level that causes peroxidative tissue damage [8-10]. The chemicals present in tobacco smoke alter the endocrine function, perhaps at the level of the ovary which in turn affects the release of female hormones. This endocrine disruption likely contributes to the reported associations of smoking with adverse reproductive outcomes, including menstrual dysfunction, infertility and early menopause [11].
Nutritional factors play an important role in the metabolism and pharmaco-toxicological activities of various drugs and xenobiotic [12]. The major nutritional disorders which are widely prevalent in all developing countries are due to Protein Energy Malnutrition (PEM) which affects all age groups and more so of poorer segments of the population. However, the repercussions are the strongest in children and women [12]. Low dietary protein possesses a constraint on the biosynthetic activity, disposition and toxicity. Report says that the protein content present in the nicotine induced hepatic cell decides either cell-survival pathway or cytotoxic pathway [13]. Though it is already established that nicotine causes various damage in our body in normal dietary condition but the toxic effects of nicotine particularly in protein restricted dietary situation are still cause of concern.
Curcumin is a natural diphenolic compound derived from turmeric Curcuma longa, and possesses many therapeutic properties like antihepatoxic, antioxidant, anti-inflammatory, antidiabetic, antitumour, hepato-protective, and anti-HIV activities [14,15]. It has proven to be a modulator of intracellular signaling pathways that control cellular inflammation, invasion and apoptosis [13]. Curcumin significantly ameliorates the nicotine-induced toxicity and regulates the imbalance between cell survival and death induced by nicotine [13,14]. Nanocurcumin is a nanoparticles form of curcumin in which the particles of curcumin are more soluble and deliverable in the body [16]. These particles have been shown to be more targeted to the tissue of interest that leads to better drug delivery and faster treatment without any wastage or side effects [17,18]. Our previous results revealed that nanocurcumin under normal dietary condition effectively ameliorated the nicotine-induced toxicities at much lower concentration due to its higher aqueous solubility and more bioavailability [19]. Curcumin in itself is an extremely effective and non-toxic compound for which it is preferred for therapy. But nanoparticles that are used to coat or carry curcumin molecules to the body might be toxic. Thus, recent research is now focusing not only on the formation of a better nanocurcumin medication but also on increasing the safety levels of nanoparticles and reducing the side effects of the drug to a minimum against the aggravated nicotine-induced toxicities under protein malnourished condition.
Materials and Methods
Chemicals
Dichloromethane used as a solvent for the preparation of nanoparticles of curcumin was purchased from Merk, India. Nicotine hydrogen tartrate and curcumin were purchased from the Sigma Chemical Company, USA. All other required chemicals were purchased from Spectrochem Pvt. Ltd. India. All the chemicals and reagents used were of analytical grade.
Animals and diet
Adult female albino rats of Wistar strain (weighing approximately 120-150 g) were taken from Animal Housing Facility of Jadavpur University, Kolkata, India. Prior to use, all animals were acclimatized under standard conditions of temperature and humidity with 12 h light/dark cycles. They were maintained in accordance with the guidelines of the rule of Instructional Animal Ethics Committee of Jadavpur University, Kolkata, India (Reference No. AEC/PHARM/1502/14/2015, Dated: 30th July, 2015). The animals were housed in polypropylene cages in an air-conditioned room. Animals were allowed to protein-restricted diet (5% casein, 83% carbohydrate, 7% fat, 4% salt mixture and 1% vitamin mixture) according to Hawk et al. [20] throughout the experiment and water adlibitum. Twenty four female albino rats were equally divided into four groups of six rats in each and treated as below for 21 days.
• Group-I: Served as Control group in which animals were received no treatment.
• Group-II: Nicotine treated group in which animals were injected with the effective dose of nicotine.
• Group-III: Nicotine and curcumin treated group in which animals were injected with the effective dose of nicotine and received effective dose of curcumin orally.
• Group-IV: Nicotine and nanocurcumin treated group in which animals were injected with the effective dose of nicotine and received effective dose of nanocurcumin orally.
Preparation of nanocurcumin
The preparation and characterization of nanocurcumin was done by modifying the method of Basniwal et al. [16] as already reported [19]. The as prepared nanocurcumin was used in this study.
Mode of treatment
The animals were maintained on their respective dietary regimen well before (1 week) the treatment start to till the completion of the treatment. The mode of treatment and effective doses of nicotine (2.5 mg kg-1 body weight day-1), curcumin (80 mg kg-1 body weight day-1) and nanocurcumin (4 mg kg-1 body weight day-1) were similar to the study of Chattopadhyay et al. [19]. The animals in control group received subcutaneous injection of 0.5 ml physiological saline only at the same time.
Collection of samples
After the last dose of injection received, animals were kept fasting overnight and sacrificed on the following morning after mild anesthesia. Blood was collected from the heart in sterilized with or without anticoagulant (heparin) containing tubes and serum was separated out after centrifugation and stored at -20° C prior to further analysis. Liver, kidney and ovary were dissected, cleaned properly and stored for further investigations. The tissues were kept under ice-cold conditions throughout the experiment.
Biochemical assays
Serum and tissues (liver, kidney and ovary) protein contents were determined by the method of Lowry et al. [21]. The Alkaline Phosphatase (ALP), Alanine-Transaminase (ALT), and Aspartase-Transaminase (AST) activities in the serum were measured by using the standard kit supplied by ARKRAY Healthcare Pvt. Ltd., Surat, India. The other liver function enzyme, Acid Phosphatase (ACP) in the serum was assayed according to the method illustrated by Bergmeyer and Bernt [22] by using para nitrophenyl-phosphate as the substrate. The lipid components such as TC: Total Cholesterol; HDL-C: High Density Lipoprotein-Cholesterol and triglyceride in serum were estimated by using standard kits supplied by Ranbaxy Diagnostic Ltd., Mumbai, India. VLDL-C and LDL-C: Low Density Lipoprotein-Cholesterol were calculated from the values of triglyceride, TC and HDL-C by using the Friedwald and Fredicksons formula [23]. Lipid Peroxidation (LPO) was measured in plasma and liver by the determination of Thiobarbituric Acid-Reactive Substances (TBARS) according to the standard protocol. The amount of MDA was calculated by taking the extinction coefficient of MDA as 1.56 x 105 M-1 cm-1. Antioxidant enzyme activities in liver such as Superoxide Dismutase (SOD) and Catalase (CAT) were determined from rats of all the groups [24]. The Glutathione-Reductase (GSH) and glutathione-peroxidase enzymes activities of the liver tissue were determined by the methods described by Griffith [25]. All the entire biochemical assays were repeated twice and data were averaged (n = 12).
Histological study
Cleaned tissues of liver and kidney were fixed by using Bouin’s fluid. After fixation, the tissues were washed several times by different graded alcohol to remove excess fluid and then embedded in paraffin. Using of rotary microtome the embedded tissues were sliced. The paraffin sections were then attached on the slide and washed by xylol before staining. The tissue sections were then stained by using haematoxylin and eosin staining. Histological study of liver, kidney and ovary tissues were accomplished by using Leica DFC450C microscope at 20X by using CFP filter.
Statistical analysis
The experimental setup was repeated twice and all data were averaged over n = 12 animals, and given mean ± S.D. Significance levels were determined by using ANOVA, where * implied significant (p < 0.01) and ** implied more significant (p < 0.001) of the data when compared with the nicotine treatment. Similarly, # implied significant (p < 0.01) and ## implied more significant (p < 0.001) of the data when compared with the nicotine plus curcumin treatments.
Results
The as prepared nanocurcumin is shown in figure 1. The most sensitive and widely used liver enzymes i.e., AST and ALT activities in the serum were significantly increased due to nicotine treatment in protein malnourished condition (Table 1). The levels of activities of those two enzymes were decreased significantly (P < 0.01) in curcumin supplementation and more significantly (P < 0.001) in nanocurcumin supplementation as seen in table 1. Similar results were observed for ACP and ALP liver function enzymes activities (Table 1). Urea and creatinine levels in the serum of nicotine exposed animals were significantly elevated compared to the control group animals (Table 2). The supplementation of nanocurcumin showed much more ameliorative effects than curcumin by decreasing the levels of urea and creatinine respectively in the serum. Nicotine caused significant alterations of cholesterol, triglyceride, LDL-C, and VLDL-C concentrations and decreased HDL-C concentration in the serum under protein malnourished condition (Table 3). However, administration of nanocurcumin to the nicotine-induced animals showed more significant ameliorative effects by normalizing almost the concentrations of those parameters in the serum of the rats. The results of lipid peroxidation both in the serum and liver tissue of rats as shown in table 4 similarly suggested that supplementation of nanocurcumin was more effective than curcumin in protein malnourished rats. The degenerative effects of the antioxidant enzymes (SOD, CAT, GSH and GPx) due to nicotinic stress and ameliorative effects caused by curcumin and nanocurcumin are shown in table 5. Nanocurcumin was found to be comparatively more active against nicotine-induced effect on antioxidant enzymes particularly in protein malnourished condition. Estimation of protein content in different tissues is given in table 6.
| Table 1: Effect of nanocurcumin on hepatic enzymes in protein malnourished condition. | ||||
| Enzymes | Control | Nicotine | Groups Nicotine + Curcumin | Nicotine + Nanocurcumin |
| ACP (m mol/h/100 ml) | 1.36 ± 0.10 | 1.94 ± 0.11(42.6↑) | 1.52 ± 0.12**(11.8↑) | 1.42 ± 0.10** ##(4.4↑) |
| ALP (m mol/h/100 ml) | 9.30 ± 0.60 | 19.20 ± 0.30(106.5↑) | 12.95 ± 0.30* (39.2↑) | 10.42 ± 0.23** ##(12.0↑) |
| AST (IU/L) | 9.80 ± 1.60 | 17.50 ± 4.00(78.6↑) | 11.32 ± 0.70** (15.5↑) | 10.35 ± 0.90** ##(5.61↑) |
| ALT (IU/L) | 37.40 ± 2.60 | 71.35 ± 1.61(90.77↑) | 42.91 ± 3.49** (14.7↑) | 39.99 ± 2.86** ##(6.9↑) |
| The experimental setup was repeated twice and all data were averaged over n = 12 animals, and given mean ± S.D. Significance levels were determined by using ANOVA, where * implied significant (P < 0.01) and ** implied more significant (P < 0.001) of the data when compared with the data of nicotine treatment. Similarly, # implied significant (P < 0.01) and ## implied more significant (P < 0.001) of the data when compared with the data of nicotine + curcumin treatments. The data within the parenthesis represent the average percentage of increase (↑) or decrease (↓) relative to the control. | ||||
| Table2: Effect ofnanocurcumin on renal function parameters in protein malnourished condition. | ||||
| Parameter | Control | Nicotine | Groups Nicotine +Curcumin | Nicotine +Nanocurcumin |
| Urea (mg/100 ml) | 38.30 ± 2.15 | 56.80 ± 2.60(48.3↑) | 47.00 ± 2.78*(22.7↑) | 39.23 ± 2.06**## (2.4↓) |
| Creatinine (mg/100 ml) | 1.35 ± 0.12 | 1.85 ± 0.21(37.0↑) | 1.56 ± 0.50*(15.6↑) | 1.30 ± 0.10**## (3.7↓) |
| The experimental setup was repeated twice and alldata were averaged over n = 12 animals, and given mean ± S.D. Significance levels were determined by usingANOVA, where * implied significant (P <0.01) and ** implied more significant (P <0.001) of the data when compared with the data of nicotine treatment.Similarly, # implied significant (P <0.01) and ## implied more significant (P <0.001) of the data when compared with the data of nicotine + curcumin treatments. The data within the parenthesisrepresent the average percentage of increase (↑)or decrease (↓) relative to the control. | ||||
| Table 3: Effect of nanocurcumin on Lipid profile in protein malnourished condition. | ||||
| Parameters | Control | Nicotine | Groups Nicotine + Curcumin | Nicotine + Nanocurcumin |
| Triglyceride(mg/dl) | 105.6 ± 8.4 | 185.8 ± 9.1 (75.9↑) | 109.5 ± 8.6** (3.7↑) | 106.4 ± 2.5**## (0.8↑) |
| Cholesterol (mg/dl) | 107.3 ± 7.9 | 138.7 ± 9.0 (29.3↑) | 115.0 ± 5.6** (7.2↑) | 109.3 ± 2.4**## (1.9↑) |
| HDL (mg/dl) | 39.5 ± 7.0 | 29.8 ± 4.0 (24.6↓) | 31.7 ± 1.0* (19.7↓) | 33.5 ± 1.5*# (15.2 ↓) |
| VLDL (mg/dl) | 21.1 ± 1.6 | 36.8 ± 0.7 (74.4↑) | 30.7 ± 1.8* (45.5↑) | 21.5 ± 0.4**## (1.03↑) |
| LDL (mg/dl) | 46.1 ± 2.1 | 81.8 ± 4.1 (75.9↑) | 64.79 ± 1.2* (40.5↑) | 43.5 ± 1.5**## (5.6↓) |
| The experimental setup was repeated twice and all data were averaged over n = 12 animals, and given mean ± S.D. Significance levels were determined by using ANOVA, where * implied significant (P < 0.01) and ** implied more significant (P < 0.001) of the data when compared with the data of nicotine treatment. Similarly, # implied significant (P < 0.01) and ## implied more significant (P < 0.001) of the data when compared with the data of nicotine + curcumin treatments. The data within the parenthesis represent the average percentage of increase (↑) or decrease (↓) relative to the control. | ||||
| Table 4: Effect of nanocurcumin on lipid peroxidation in protein malnourished condition. | ||||
| MDA level | Control | Nicotine | Groups Nicotine + Curcumin | Nicotine + Nanocurcumin |
| Serum (n mol/ml) | 5.61 ± 0.6 | 7.98 ± 1.3(42.2↑) | 6.30 ± 0.5**(12.3↑) | 6.32 ± 1.1** (12.7↑) |
| Liver (n mol/mg protein) | 14.98 ± 0.4 | 22.92 ± 0.5(53.0↑) | 16.01 ± 2.96**(6.9↑) | 15.50 ± 0.9**# (3.5↑) |
| The experimental setup was repeated twice and all data were averaged over n = 12 animals, and given mean ± S.D. Significance levels were determined by using ANOVA, where * implied significant (P < 0.01) and **implied more significant (P < 0.001) of the data when compared with the data of nicotine treatment. Similarly, # implied significant (P < 0.01) and ## implied more significant (P < 0.001) of the data when compared with the data of nicotine + curcumin treatments. The data within the parenthesis represent the average percentage of increase (↑) or decrease (↓) relative to the control. | ||||
| Table 5: Effect of nanocurcumin on antioxidant enzymes in protein malnourished condition. | ||||
| Enzymes | Control | Nicotine | Groups Nicotine + Curcumin | Nicotine + Nanocurcumin |
| SOD(n mol/O2 decomposed/ min/100 mg protein) | 9.02 ± 0.2 | 4.09 ± 0.1(54.7↓) | 6.72 ± 0.14*(25.5↓) | 7.15 ± 0.5**#(20.7↓) |
| CAT (n mol/H2O2 decomposed/ min/mg protein) | 40.21 ± 1.0 | 29.22 ± 1.0(27.3↓) | 32.9 ± 0.5*(18.2↓) | 35.50 ± 1.0**#(11.7↓) |
| GSH (µg/mg protein) | 18.75 ± 1.76 | 12.02 ± 1.13 | 14.00 ± 1.41* | 16.00 ± 1.41**# |
| GPx (n mol/min/mg protein) | 150.52 ± 2.5 | 135.11 ± 2.9(10.2↓) | 144.34 ± 2.2*(4.1↓) | 149.50 ± 2.5**#(0.6↓) |
| The experimental setup was repeated twice and all data were averaged over n = 12 animals, and given mean ± S.D. Significance levels were determined by using ANOVA, where * implied significant (P < 0.01) and **implied more significant (P < 0.001) of the data when compared with the data of nicotine treatment. Similarly, # implied significant (P < 0.01) and ## implied more significant (P < 0.001) of the data when compared with the data of nicotine + curcumin treatments. The data within the parenthesis represent the average percentage of increase (↑) or decrease (↓) relative to the control. | ||||
| Table 6: Protein content of the tissues in nicotine stress and nanocurcumin supplemented condition of rats under protein malnourished condition. | ||||
| Protein content(mg/g wet tissue) | Control | Nicotine | Groups Nicotine + Curcumin | Nicotine + Nanocurcumin |
| Liver | 15.00 ± 0.70 | 7.12 ± 1.59 | 12. 85 ± 0.91* | 13.77± 0.70**# |
| Kidney | 15.5 ± 2.12 | 7.5 ± 1.41 | 11.50 ± 2.53* | 14.00 ± 1.4**## |
| Ovary | 18.75 ± 1.76 | 12.02 ± 1.13 | 14.00 ± 1.41* | 16.00 ±1.41**# |
| The experimental setup was repeated twice and all data were averaged over n = 12 animals, and given mean ± S.D. Significance levels were determined by using ANOVA, where * implied significant (P < 0.01) and ** implied more significant (P < 0.001) of the data when compared with the data of nicotine treatment. Similarly, # implied significant (P < 0.01) and ## implied more significant (P < 0.001) of the data when compared with the data of nicotine + curcumin treatments. The data within the parenthesis represent the average percentage of increase (↑) or decrease (↓) relative to the control. | ||||
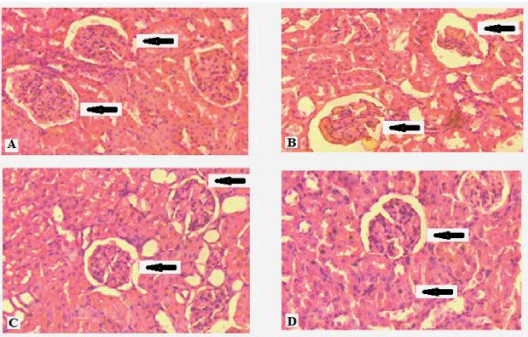
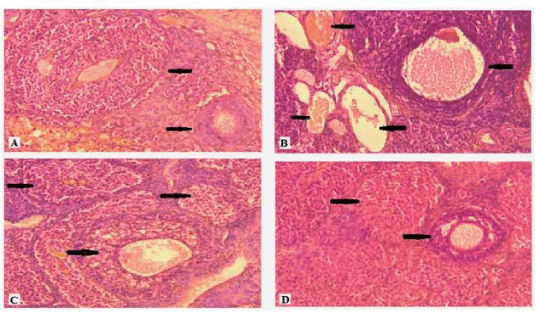
The histological study of the liver tissue of control group animals is shown in figure 2A. The distorted central vein (marked by arrow head) and irregular cell arrangement of the nicotine exposed liver are shown in figure 2B. The photographs of curcumin and nanocurcumin supplemented rat liver are shown in figure 2C and 2D respectively. The histological studies of kidney tissues of the control, nicotine treated, curcumin and nanocurcumin supplemented groups are presented in figure 3A to 3D respectively. Here destruction of Bowmann’s capsule and cell walls are seen in nicotine treated condition (Figure 3B) which almost restored in nanocurcumin supplementation as seen in figure 3D. The toxic effect on ovary by nicotine (Figure 4A and 4B) and the corresponding protective effects of curcumin (Figure 4C) and nanocurcumin (Figure 4D) similarly shown in figure 4.
 Figure 2: Histological section of Liver (20X)
A: Control Liver
B: Nicotine treated Liver
C: Nicotine and curcumin treated Liver
D: Nicotine and Nanocurcumin treated Liver
Figure 2: Histological section of Liver (20X)
A: Control Liver
B: Nicotine treated Liver
C: Nicotine and curcumin treated Liver
D: Nicotine and Nanocurcumin treated Liver
Discussion
Though several studies in animal models has demonstrated the effective amelioration of curcumin against nicotine-induced toxicities, the undesirable pharmacokinetic properties restricts the clinical efficacy of curcumin in human subjects [26]. Nanocurcumin is aqueous soluble and more bio-available than curcumin [27]. Scientists are thus trying to use nanocurcumin against various diseases to achieve its clinical benefits [17,18]. We have recently reported the effectiveness of orally supplemented nanocurcumin against the nicotine-induced toxicities in rats under normal protein-diet condition [19]. This study furnishes the useful application of nanocurcumin against the aggravated toxic effects that are induced by nicotine in rats under protein malnourished status.
The oxidative stress generated by nicotine increases the activities of ACP and ALP in the serum. The increased oxidative stress causes tissue injury which is related to the ROS generation [27]. In protein malnourished condition, the levels of ACP and ALP were elevated drastically showing more aggravated effect of nicotine on the tissues (Table 1). The supplementation of nanocurcumin showed more significant (P < 0.001) effect on the antioxidant status of protein malnourished female rats due to which the ACP and ALP activities were normalized. Nicotine also damages the cell membranes of liver tissues causing liver injury. The activities of AST and ALT are thus increased due to loss of functional integrity of liver cells [28]. It is already reported that curcumin can normalize the levels of the liver enzymes to some extent in normal protein condition [19,29]. The situation became more serious in protein malnourished condition because aggravated liver injury was observed in such condition. Our results suggest that nanocurcumin is better than curcumin to nullify the nicotinic toxicity in liver (Table 1). The smaller dimension of nanocurcumin is more bio-available than curcumin which may explain these findings.
Urea and creatinine levels were significantly elevated in the serum of nicotine exposed protein malnourished group compared to the control group (Table 2) which could be related to the progressive kidney failure as described by Addo et al. [30]. Usunobun et al. [31] have reported that some heavy metals in tobacco (e.g., Cadmium, Mercury, Lead etc.) may play some role in tobacco-induced renal damage. This finding suggests that nanocurcumin supplementation is a better restorative phenomenon compared to curcumin for the healing of kidney damage.
The increment of triglyceride (75.9%), VLDL-C (74.4%), VLDL-C (75.9%) and cholesterol (29.3%) levels and decrement of HDL-C (24.6%) level in the serum of rats under nicotine treatment clearly revealed that nicotine affected the lipid profile severely in protein malnourished condition (Table 3). This result is in agreement with the finding of Chattopadhyay et al. [10]. Balakrishnan and Menon [28] have shown that nicotine-stimulated catecholamine synthesis lipolysis adipose tissue which increased the serum cholesterol level. The absorption of total cholesterol and increase CYP7A1 gene expression (which is a rate limiting enzyme in the biosynthesis of bile acid from cholesterol) by curcumin helps to reduce the total cholesterol level in the serum [32]. The multiple inductions of fatty acid catabolism may lower the triglyceride level by curcumin. The increased lipid peroxidation in the plasma and liver tissues due to nicotinic stress in protein malnourished condition (Table 4) corroborates with the earlier findings [10,19]. The significant amelioration of lipid peroxidation by nanocurcumin was observed in this study. It may be inferred that due more bioavailability and higher intake of nanocurcumin into the cells, the above-mention beneficial effects of curcumin become more prominent for nanocurcumin in protein malnourished condition.
Nicotine interrupts the mitochondrial respiratory chain and causes increased generations various free radical ions (e.g., super oxide ions, hydrogen peroxide ions etc.) and enhances lipid peroxidation on human circulating lymphocytes [33]. The primary role of antioxidant enzymes is to take part in defense mechanism for protecting the cells and cellular organelles from oxidative damage. It was noted that the activities of two main antioxidant enzymes (SOD and CAT) decreased significantly in the nicotine-treated rats compared to the control group (Table 5). Dispose of the free radicals, production of hydrogen peroxide or inactivation of the enzyme proteins by ROS generation and depletion of the enzyme substrates and/or down regulation of transcription and translation processes due to nicotine toxicity are the possible causes for the decreased activities of those scavenging enzymes [34]. Similarly, depletion of GSH and GPx levels not only weakens cell defense system but also enhances the oxidative stress that damages various tissues and organs [27]. The significant ameliorative effect of nanocurcumin proved its scavenging free radicals activity and maintained the cell membrane interiority and functions through inhibiting membrane lipid peroxidation. Nicotine also causes reduction in the protein concentration of liver, kidney and ovarian tissues due to its toxic effect. The increased concentration of the protein in different tissues suggests that nanocurcumin can protect the tissues in a better way against nicotine-induced damage as seen from table 6.
Significant histological changes were observed in hepatocytes of protein malnourished female rats under nicotine exposure. The regular hepatic cells arrangement and normal central vein (marked by arrow head) of the liver tissue of control group rats were noted in figure 2A. Nicotine abuse affected the liver tissues for which distorted cell arrangement and the enlarged central vein filled with sinusoidal fluid was observed in figure 2B under protein malnourished condition. This was in agreement with the observation of Bateman [35] who suggested that the histological changes might be preferred as standard for the assessment of liver cell damage. Nicotine stress caused damage of liver cells by enhancing the levels of liver enzymes (AST and ALT) into the blood which indicated the liver disease. Curcumin and nanocurcumin appeared to reduce the levels of those liver enzymes and thus prevented liver damages by maintaining the structural integrity of the liver cell membrane as seen from figures 2C and 2D respectively. Histological studies of kidney tissues (Figure 3A to 3D) of the protein malnourished animals were similarly revealed the effect of nicotine and corresponding amelioration of curcumin and nanocurcumin. Nicotine exposure distorted the normal arrangement of the glomerulus (marked by arrow head), the bowman space and the proximal/distal tubules of kidney tissue which were more significantly (P < 0.001) restored by supplementation of nanocurcumin (Figure 3D) in comparison to curcumin (Figure 3C). In the ovary of female rats maintained with protein malnourished diet, the regression of graafian follicles with disrupted cellular structures forming vacuoles were seen due to nicotine treatment (Figure 4B). This was probably due to the synergistic effect of nicotine toxicity and protein malnourished stress which decreased the production of ovarian hormones in such condition as reported by Sinha et al. [8]. Curcumin (Figure 4C) and nanocurcumin (Figure 4D) both restored the graafian follicular structures but the effect of nanocurcumin was more prominent in restoration of the normalcy of ovarian structure than curcumin.
Conclusion
The living organisms depend essentially on proteins, which directly or indirectly regulate the biochemical processes. Protein malnutrition induces marked changes in the functioning form, leading to several biochemical defects, structural disruption and altered physiological functions. Thus the detrimental effects of nicotine are more pronounced in protein deficient condition. The undesirable complications of inorganic nanomaterial can be overcome by using the protein-based nanomaterial or herbal-nanomaterial as they exhibit less cytotoxicity [36,37]. Our study demands that nanocurcumin more effectively ameliorates nicotine-induced toxicities in protein malnourished condition by normalizing the hepato-enzymes activities, kidney function parameters, lipid profiles, anti-oxidant status and also maintaining the structural integrity of different tissues under nicotine stress condition. The safer and more effective nanocurcumin may be used as better therapeutic agent to protect the health of female population who are suffering both from protein malnutrition and nicotine-induced complications.
Acknowledgment
The financial support obtained from Women Scientist-B scheme, Department of Science and Technology (Sanctioned no. DST/Disha/SoRF-PM/013/2015(G), dated; 08/03/2016) is gratefully acknowledged. Authors are also grateful for the experimental and technical supports provided by the Biophysics Laboratory, Department of Physics, Jadavpur University. The permission of work on animal model provided by the Animal Ethics Committee of Jadavpur University is also gratefully acknowledged.
References
- Rahman M, Sakamoto J, Fukui T. Bidi smoking and oral cancer: A meta-analysis. Int J Cancer. 2003; 106: 600-604. https://goo.gl/Fn88wm
- Mishra GA, Pimple SA, Shastri SS. An overview of the tobacco problem in India. Ind J Med Pediatr Oncol. 2012; 33: 139-145. https://goo.gl/wvsjrL
- Mishra A, Chaturvedi P, Datta S, Sinukumar S, Joshi P, Garg A. Harmful effects of nicotine. Ind J Med Pediatr Oncol. 2015; 36: 24-31. https://goo.gl/bSpPRj
- Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016; 26: 515-523. https://goo.gl/HQ2bVE
- Laniado Laborin R. Smoking and Chronic Obstructive Pulmonary Disease (COPD). Parallel epidemics of the 21st Century. Int J Environ Res Public Health. 2009; 6: 209-224. https://goo.gl/rv2R7w
- Thakur JS, Garg R, Narain JP, Menabde N. Tobacco use: A major risk factor for non-communicable diseases in South-East Asia region. Ind J Pub Health. 2011; 55: 155-160. https://goo.gl/3KPPye
- Maiti M, Chattopadhyay K, Verma M, Chattopadhyay BD. Curcumin protects against nicotine-induced stress during protein malnutrition in female rat through immunomodulation with cellular amelioration. Mol Biol Rep. 2015; 42: 1623-1637. https://goo.gl/J1AMTf
- Sinha S, Maiti M, Chattopadhyay K, Chattopadhyay BD. Potential amelioration of curcumin against nicotine-induced toxicity of protein malnourished female rats. J Pharmacol Toxicol. 2012; 7: 166-180. https://goo.gl/X9WgRA
- Chattopadhyay K, Chattopadhyay BD. Effect of nicotine on lipid profile, per oxidation and antioxidant enzymes in female rats with restricted dietary protein. Ind J Med Res. 2008; 127: 571-576. https://goo.gl/efwALn
- Chattopadhyay K, Mondal S, Chattopadhyay BD. Ameliorative effect of sesame lignans on nicotine toxicity in rats. Food Chem Toxicol. 2010; 48: 3215-3220. https://goo.gl/61jRfw
- Dechanet C, Anahory T, Mathieu Daude JC, Quantin X, Reyftmann L, Hamamah S, et al. Effects of cigarette smoking on reproduction. Human Reprod Update. 2011; 17: 76-95. https://goo.gl/1FiSA3
- Krishnaswamy K. Drug/Xenobiotic-Metabolism, Disposition and Toxicity in Malnutrition. Def Sci J. 1987; 37: 133-142. https://goo.gl/2GPRSD
- Banerjee S, Chattopadhyay K, Chhabra JK, Chattopadhyay B. Protein dependent fate of hepatic cells under nicotine induced stress and curcumin ameliorated condition. Eur J Pharmacol. 2012; 684: 132-145. https://goo.gl/6g53LM
- Bandyopadhyaya G, Sinha S, Chattopadhyay BD, Chakraborty A. Protective role of curcumin against nicotine induced genotoxicity on rat liver under restricted dietary protein. Eur J Pharmacol. 2008; 588: 151-157. https://goo.gl/FWUrRV
- Ruby AJ, Kuttan KD, Babu KN, Rajasekharan R, Kuttan R. Antitumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995; 94: 79-83. https://goo.gl/mokevu
- Bhawana, Basniwal RK, Buttar HS, Jain VK, Jain N. Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J Aggri Food Chem. 2011; 59: 2056-2061. https://goo.gl/a5s11s
- Danafar H. Study of the Composition of Polycaprolactone/Poly (Ethylene Glycol)/Polycaprolactone Copolymer and Drug-to-Polymer ratio on drug loading efficiency of curcumin to nanoparticles. Jundishapur J Nat Pharm Prod. 2017; 12: 34179. https://goo.gl/KRGs4S
- Nosrati H, Sefidi N, Sharafi A, Danafar H, Manjili HK. Bovine Serum Albumin (BSA) coated iron oxide magnetic nanoparticles as biocompatible carriers for curcumin-anticancer drug. Bioorg Chem. 2018; 76: 501-509. https://goo.gl/HXWqF4
- Chattopadhyay K, Samant A, Mukhpadhyay S, Chattopadhyay BD. Potential amelioration of nicotine-induced toxicity by nanocurcumin. Drug Dev Res. 2018; 79: 119-128. https://goo.gl/b8rBnn
- Hawk PB, Oser BL, Summerson WH. Practical Physiological Chemistry. 13th ed. New York: Mc Graw Hill Book Comp; 1954. https://goo.gl/6SSF9E
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193: 265-275. https://goo.gl/eEQA2K
- Bergmeyer HU, Bernt E. Glutamate-pyruvate transaminase. In: HU Bergmeyer ed. Methods of enzymatic analysis. New York: Academic Press Inc; 1963.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18: 499-502. https://goo.gl/FxCkUA
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971; 44: 276-287. https://goo.gl/1zsLz3
- Griffith OW. Determination of glutathione and glutathione sulfide using glutathione reductase and 2-vinyl pyridine. Anal Biochem. 1980; 106: 207-212. https://goo.gl/UTa8xg
- Gupta SC, Sung B, Kim JH, Prasad S, Li S, Aggarwal BB. Multitargeting by turmeric, the golden spice: From kitchen to clinic. Mol Nutr Food Res. 2013; 57: 1510-1528. https://goo.gl/hR4SHS
- Sreekala S, Indira M. Effects of exogenous selenium on nicotine-induced oxidative stress in rats. Biol Trace Element Res. 2009; 130: 62-71. https://goo.gl/fFZe7W
- Balakrishnan A, Menon VP. Antioxidant properties of hesperidin in nicotine-induced lung toxicity. Fund Clin Pharmacol. 2007; 21: 535-544. https://goo.gl/fyUtQE
- Salahshoor M, Mohamadian S, Kakabaraei S, Roshankhah S, Jalili C. Curcumin improves liver damage in male mice exposed to nicotine. J Tradit Comp Med. 2015; 6: 176-183. https://goo.gl/H4f6YU
- Addo MA, Gbadago JK, Affum HA, Adom, T, Ahmed K, Okley GM. Mineral profile of ghanaian dried tobacco leaves and local snuff: A comparative study. J Rad Nucl Chem. 2008; 277: 517-524. https://goo.gl/XCrTjR
- Usunobun U, Adegbeg J, Ademuyiwa O, Okugbo TF, Evuen U, Osibemhe M, et al. N-Nitrosodimethylamine (NDMA), liver function enzymes, renal function parameters and oxidative stress parameters: a Review. British J Pharm Toxicol. 2012; 3: 165-176.
- Kim M, Kim Y. Hypocholesterolemic effects of curcumin via up-regulation of cholesterol 7a-hydroxylase in rats fed a high fat diet. Nutr Res Pract. 2010; 4: 191-195. https://goo.gl/3G3nwf
- Miró O, Alonso JR, Jarreta D, Casademont J, Urbano-Márquez A, Cardellach F. Smoking disturbs mitochondrial respiratory chain function and enhances lipid peroxidation on human circulating lymphocytes. Carcinogenesis. 1999; 20: 1331-1336. https://goo.gl/XoFWVh
- Basha KK, Vani M, Poornima PS, Vijayudu B, Venkatramudu M, Ravi B, et al. Interaction of red grape extract and leaf extract on nicotine induced oxidative stress in the lung tissue of male albino rat. Int J Pharmaceut Sci Health. 2018; 2. https://goo.gl/URb6gA
- Bateman AC. Patterns of histological change in liver disease: my approach to ‘medical’ liver biopsy reporting. Histopathol. 2007; 51: 585-596. https://goo.gl/kmqLp9
- Milano F, Mari L, van de Luijtgaarden W, Parikh K, Calpe S, Krishnadath KK. Nano-curcumin inhibits proliferation of esophageal adenocarcinoma cells and enhances the T cell mediated immune response. Front Oncol. 2013; 3: 137. https://goo.gl/8Hckzw Khosropanah MH, Dinarvand A, Nezhadhosseini A, Haghighi A, Hashemi S, Nirouzad F, et al. Analysis of the Antiproliferative Effects of Curcumin and Nanocurcumin in MDA-MB231 as a Breast Cancer Cell Line. Iran J Pharm Res. 2016; 15: 231-239. https://goo.gl/w24y3h
Authors submit all Proposals and manuscripts via Electronic Form!