Jazar Hassan, Abolfathi Aliakabar and Seyed R. Arefhosseini*
Nutritional Biochemistry, School of Nutrition & Food Sciences, Tabriz University of Medical Sciences, Attar Neishaboori Ave, Golgasht St, Tabriz, 5166614711, Iran
*Address for Correspondence: Seyed R. Arefhosseini, Nutritional Biochemistry, School of Nutrition & Food Sciences, Tabriz University of Medical Sciences, Attar Neishaboori Ave, Golgasht St, Tabriz, 5166614711, Iran; Tel: +984113357580-3; E-mail: [email protected]
Dates: 20 August 2017; Approved: 05 September 2017; Published: 07 September 2017
Citation this article: Hassan J, Aliakabar A, Arefhosseini SR. Study of Variations in SNAP25 Protein Levels in Serum of Diabetic Patients. Sci J Biol. 2017;1(1): 008-010.
Copyright: © 2017 Arefhosseini SR, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Introduction: Type 2 diabetes is a common problem in human society; it is a metabolic and multi-factorial disorder disease that endangers public health and lifestyle play an important role in its onset.
However, SNAP25 Protein is one of the Abnormalities that may play important role in its affection.
Its disruption causes disturbances in the plasma membrane structure and consequently type of insulin secretion in type 2 diabetes mellitus.
Materials and Methods: Ninety subjects, 50 diabetes and 40 healthy volunteers, were studied. Fasting blood glucose, HbA1C and serum SNAP25 were measured by, Immunoturbidimetry and assessed by Auto analyzer Elisa method, respectively.
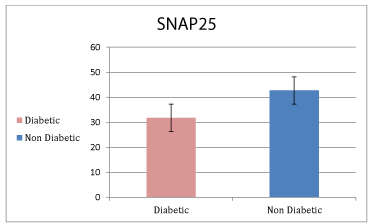
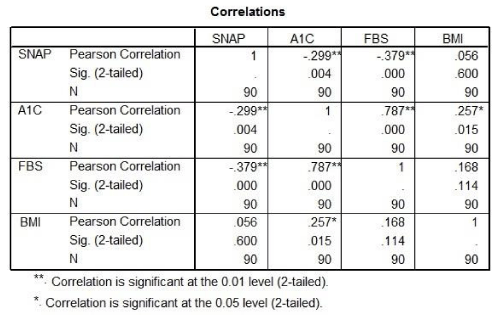
Results: The results showed that the SNAP25 levels were significantly lower in diabetic patients than healthy individuals, and inversely and significant correlation was found with FBS and HbA1C level.
Conclusion: These results showed that the assessment of SNAP25, could be considered as a valuable indicator for Type 2 diabetes. And also could be considerate as an important factor in Etiology of Diabetes Type 2.
Introduction
Synaptosmal-associated protein 25 (Snap25) is a t-SNARE protein that encoded by the SNAP25 gene in humans. SNAP25 is a component of the trans-SNARE complex, which is proposed to account of the specify membranes fusion and to directly execute fusion by forming a tight complex that brings the synaptic vesicle and plasma membranes together. SNAP-25, a Q-SNARE protein, is anchored to the cytosolic face of membranes via palmitoyl side chains covalently bound to cysteine amino acid residues in the middle of the molecule. This means that SNAP-25 does not contain a trans-membrane domain [1-7].
SNAP-25 has been identified in contributing two α-helix to the SNARE complex, a four-α-helix domain complex participates in vesicle fusion, which involves the docking and merging of a vesicle with the cell membrane to bring about an exocytotic event. Synaptobrevin, a protein that is a part of the Vesicle-Associated Membrane Protein (VAMP) family, and syntaxin-1 also help form the SNARE complex by each contributing one α-helix. SNAP-25 assembles with synaptobrevin and syntaxin-1 and the selective binding of these proteins enables vesicle docking and fusion to occur at the correct location [5,8-10].
The importance of SNAP25 in secretion of Insulin from Islet cells has been reported in Animal models. S. Nagamatus and his Colleagues have found that decreased levels of t-SNARE, Syntaxin1 and SNAP25 in Pancreatic beta cells of Islets in involved in impartments of Insulin secretion from diabetic Gk rat pancreas. Since nowadays there is a special attention to SNAP25 protein in type II Diabetes [11-16]. So, we tried expatiating whether the circulation level of the protein could be varied in Diabetic patients?
Results
Table 1 show that the age of our subjects were between 31-56 years old and diabetes patients with control were matched respect to age and sex. Fasting blood glucose and %HbA1c in diabetic patents compared with control were significantly high (p < 0.05) but there was no difference in BMI between two groups.
| Table 1: Age and Sex distribution of case and control subjects. | |||
| Non diabetic | Diabetic | ||
| 40 | 50 | No | |
| 39 ± 85 | 40 ± 16 | ||
| 26 | 26 | Male | |
| 14 | 24 | Female | |
| 89.5 ± 16 | 225 ± 11 | (mg/dl (FBS |
|
| 5.5 ± 1 | 8.9 ± 9 | A1C (%) | |
| 25.3 ± 3 | 27.3 ± 4.6 | BMI (kg/ m2) | |
| 42.7 ± 18.3 | 31.83± 9.9 | SNAP25 ng/ ml) | |
As shown in figure 1 blood level of SNAP25 was significantly lower in diabetic patents compared with healthy subjects.
SNAP25 inversely correlated with FBS and HbA1c level. But there was no significantly correlation between BMI and SNAP25 (Table 2).
Discussion
SNAP25 (Synaptosmal Associated Protein 25) as a subunit from SNARE protein, is encoded by the SNAP25 membrane protein in humans.SNAP25 in a component of the trans – SNARE complex is proposed to account for the specificity of membrane fusion and to directly execute fusion by forming tight complex that beings the synaptic vesicle and membrane together [5,17].
SNAP25 a Q SNARE protein is anchored to the cytosolic face of membrane via palmitoyl acid chains of the covalently bound to cysteine amino acid residues in middle of the molecule. It means that SNAP25 does not contain a transmembrane domain. SNAP25 has been identified in contributing two α-helixes to SNARE complex a four α-helixes domain complex. The SNARE complex participates in vesicle fusion which involves the docking and a vesicle with cell membrane to perform the exocytolic process [7,14].
It is now accepted that default of Insulin secretion in response to high blood glucose is one of the cause of insulin physiologic proper function in diabetes mellitus. It is also postulated that SNARE protein are a large protein super family of biological membrane protein which mediate vesicle fusion and are responsible for insulin secretion, although it is still not understood exactly how their performance. SNARE proteins can be divided into two categories: vesicle or V-SNAREs incorporated into membranes of vesicle during buddling and target or t-SNAREs are located in the membranes of target component [7,9]. The SNARE complex participates in vesicle fusion which involve in the docking and merging of a vesicle with cell membrane to bring about the exocytolic event. The core SNARE complex is formed by four α-helixes contributed by Synaptobrevin, syntaxin and SNAP25 [11]. SNAP25 is a t-SNRE protein that is encoded by the SNAP25 gene in humans. SNAP25 does not contain a transmembrane domain and is anchored to the cytosolic face of membranes via palmitoyl acid chains that covalently bound to amino acid cysteine residues. SNAP25 modulate Reveal process apart from the actual fusion event including the activity of potassium voltage gated (KV) 2.1 channels [2]. Kv2.1 current repolarizes β-cell action potential upon exposure to glucose to limited Ca2+ entry and insulin secretion. So, SNAP25 polymorphism have been play a role in metabolic disorders in T2DM patient [16] Our finding significantly showed that there is reduction in circulating SNAP25 in diabetic patients compared with control Healthy subjects. Blood level of SNAP25 Negatively correlated with FBS and percent of HbA1C, but it was not correlated with BMI. These results show the importance of SNAP25 in diabetes and will be interestingly to explore whether circulating SNAP25 could be an important factor in evaluation do diabetes.
References
- Cohen, P. The twentieth century struggle to decipher insulin signalling. Nat Rev Mol Cell Biol. 2006; 7: 867-873. https://goo.gl/tpfHgw
- Taylor SI. Deconstructing Type 2 Diabetes. Cell; 1999; 97: 9-12. https://goo.gl/w4UqY9
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001; 414: 799-806. https://goo.gl/JX1E7n
- Ostenson CG, Khan A, Abdel-Halim SM, Guenifi A, Suzuki K, Goto Y, et al. Abnormal insulin secretion and glucose metabolism in pancre-atic islets from the spontaneously diabetic GK rat. Diabetologia; 1993; 36: 3-8. https://goo.gl/TPdvgA
- Nagamatsu S, Nakamichi Y, Yamamura S, Watanabe T, Ozawa S, Furukawa H, et al. Decreased expression of t-SNARE syntaxin-1 and SNAP25 in pancreatic β-cells is involved in impaired insulin secretion from diabetic GK rat islets; restoration of decreased t-SNARE proteins improves impaired insulin secretion. Diabetes. 1999; 48: 2367-2373. https://goo.gl/MKokNd
- Gerich JE. The genetic basis of type 2 diabetes mellitus: impaired insulin secretion versus impaired insulin sensitivity. Endocer Rev. 1998; 19: 491-503. https://goo.gl/5DmS9q
- Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNARE Es. Proc Natl Acad Sci USA. 1998; 95: 15781-15786. https://goo.gl/xQpzzR
- Rizo J, Sudhof TC. Snares and Munc 18 in synaptic vesicle fusion. Nat Rev Neurosci. 2002; 3: 641-653. https://goo.gl/m5K39a
- Garcia EP, McPherson PS, Chilcote TJ, Takei K, De Camilli P. rbSec1A and B colocalize with syntaxin 1 and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. J. Cell Biol. 129,105-120. https://goo.gl/JcMsqw
- Diefenbach RJ, Diefenbach E, Douglas MW, Cunningham AL. The heavy chain of conventional kinesin interacts with the SNARE proteins SNAP25 and SNAP23”. Biochemistry. 2002; 41: 14906-14915. https://goo.gl/sKLFEA
- Chapman ER, An S, Barton N, Jahn R. SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils. J Biol Chem. 1994; 269: 27427-27432. https://goo.gl/rKUonw
- Ostenson CG. The pathophysiology of type 2 diabetes mellitus: An overview. Acta Physiol Scand. 2001; 171: 241-247. https://goo.gl/RzQQVX
- Gaisano HY, Ostenson CG, Sheu L, Wheeler MB, Efendic S. Abnormal expression of pancreatic islet exocytotic SNARE proteins in GK rats is normalized by phlorizin treatment. Submitted for publication. 2002; 143: 4218-4226. https://goo.gl/rKbcm2
- Ostenson CG, Khan A, Abdel-Halim SM, Guenifi A, Suzuki K, Goto Y, et al. Abnormal insulin secretion and glucose metabolism in pancreatic islets from the spontaneously diabetic GK rat. Diabetologia. 1993; 36: 3-8. https://goo.gl/DtHWgm
- Abdel-Halim SM, Guenifi A, Khan A, Larsson O, Berggren PO, Ostenson CG Efendic S. Impaired coupling of glucose signal to the exocytotic machinery in diabetic GK rats: A defect ameliorated by cAMP. Diabetes. 1996; 45: 934-940. https://goo.gl/xsJHNw
- Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med. 1976; 119: 85-90. https://goo.gl/Axa45L
- Goto Y, Kakizaki M, Masaki N. Spontaneous diabetes produced by selective breeding of normal Wistar rats. Proc. Japan Acad. 1975; 51: 80-85. https://goo.gl/daNHy3
Authors submit all Proposals and manuscripts via Electronic Form!