Research Article
Effect of Jack Bean Supplement on the Response of Rabbits to In-vivo Experimental Cystitis
Robert M. Levin* , Catherine Schuler, Robert E. Leggett, Chang C. Lan, Wen C. Chen and Alpha DY. Lin
Robert M. Levin1,2*, Catherine Schuler1, Robert E. Leggett1, Chang C. Lan3, Wen C. Chen4 and Alpha DY. Lin5,6
1Stratton VA Medical Center, Albany, NY, USA
2Albany College of Pharmacy and Health Science
3Wellstrong Biotech Ltd.
4Daoyi Biotechnology Co., Ltd.
5Central-Clinic Hospital, Taipei
6Urology Department, National Yang-Ming University, Taiwan
*Address for Correspondence: Robert M. Levin, Senior Research Career Scientist, Stratton VA Medical Center, Albany, NY 12208, USA, Fax: +518-369-0176; E-mail: [email protected]
Dates: 25 August 2017; Approved: 06 September 2017; Published: 06 September 2017
Citation this article: Levin RM, Schuler C, Leggett RE, Lan CC, Chen WC, et al. Effect of Jack Bean Supplement on the Response of Rabbits to In-Vivo Experimental Cystitis. Sci J Biol. 2017;1(1): 001-007.
Copyright: © 2017 Levin RM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Jack bean; LUTS (Lower urinary tract symptoms); Voiding frequency; Voiding urgency; Cystitis; Oxidative stress; Rabbit; Urinary bladder
Abstract
Chemical cystitis results from therapy with drugs such as ketamine, cyclophosphamide, and protamine sulfate. The etiology of cystitis relates to a damaged bladder urothelium. This results in the penetration of substances from the urine into the bladder wall resulting in inflammation and increased permeability. We determined the ability of Jack Bean (JB) extracts (CavatideR) to prevent the pathologies associated with experimental cystitis of the rabbit urinary bladder. 18 male WNZ rabbits were divided into 3 groups of 6 rabbits. Group1 - control rabbits. Group 2 - chemical cystitis was induced by placing 25ml saline containing protamine sulfate + uric acid in the bladder for 30 minutes then washing. Group 3 - were given a suspension of the JB preparation by gavage daily for 2 weeks prior to inducing cystitis and for 2 weeks following cystitis. For all groups, each rabbit received a cystometry prior to entering the study, immediately prior to inducing the cystitis, and at 1 and 2 weeks (end of the study). Statistical Analysis: One way analysis of variance followed by the Tukey test for individual differences was used; a p < 0.05 was required for significance. Chemical cystitis resulted in decreased compliance, and decreased contractile responses. The compliance of the cystitis + JB group was significantly higher than the control cystitis group. The contractility of the cystitis + JB group to all forms of stimulation were significantly greater than for the control-cystitis group. Our conclusion was that JB provided significant protection against the pathophysiological effects of cystitis.
Introduction
Background
Voiding urgency and frequency are symptoms of lower urinary tract dysfunctions in both men and women. In men these symptoms originate from responses to Benign Prostatic Hyperplasia (BPH) [1,2]. In women these symptoms can arise from postmenopausal changes in the urinary bladder, and also from various forms of cystitis [3,4]. Chemical cystitis may result from oral therapy with drugs such as ketamine, cyclophosphamide, protamine sulfate, acetone, and from vesicle instillation of various other chemical agents [5-9]. Cystitis is the most common urological pathology of women. The incidence increases with age, especially following menopause [10-14]. One major hypothesis concerning the etiology of various forms of cystitis or cystitis-like symptoms relates to a defunctionalized and damaged bladder urothelial surface with subsequent penetration of substances in the urine into the bladder wall causing inflammation and increased permeability [15-17]. Ketamine and Protamine sulfate has been used to effectively damage the mucin layer and urothelium of the bladder inducing cystitis [5,18,19]. The addition of uric acid enhances the severity of the cystitis [20,21].
The major etiology of the damage to the urothelium in chemical cystitis is oxidative stress [5,7,22-25]. Natural products showing antioxidant activity in several types of chemical cystitis have been shown to be effective in their treatment [24,25]. Canavalia ensiformis: common name Jack bean (JB) is a legume which is used for human nutrition. Two components of JB are urease and concanavalin A which are mildly toxic. The toxic properties of these two components have been completely eliminated by a proprietary methodology. Our JB preparation was supplied by: Well strong Biotech Co., Taipei Taiwan. JB has been shown to have significant antioxidant activity using a variety of both in-vivo and in-vitro techniques [26,27]. JB preparations have been utilized in traditional Chinese medicine to treat a number of conditions including cancer and diseases associated with oxidative stress with no side effects [28].
Methods
All methods were approved by the IACUC and R&D Committees of the Stratton VA Medical Center, Albany, NY. 18 adult male NZW rabbits were divided into 3 groups of 6 rabbits each.
Group 1 were control rabbits. Each rabbit received cystometry before entering into the study and also at 1 and 2 weeks (end of the study). Group 2 were rabbits given a placebo by gavage daily for 2 weeks prior to inducing cystitis and for 2 weeks following cystitis. Each rabbit received cystometry prior to entering into the study, immediately prior to inducing the cystitis, and at 1 and 2 weeks (end of the study) post cystitis. Group 3 were rabbits given a suspension of the Jack Bean preparation (100 mg/ ml) at 1 ml/ kg by gavage daily for 2 weeks prior to inducing chemical cystitis and for 2 weeks following chemical cystitis. Each rabbit received cystometry prior to entering the study, immediately prior to inducing the cystitis, and at 1 and 2 weeks (end of the study) post cystitis. The dose of JB was chosen based on its use in animal studies and consultation with the manufacturer.
Cystometry
Before chemical cystitis and also at 1 and 2 weeks following chemical cystitis each rabbit was sedated using ketamine/xylazine (25 mg/ 10 mg, im). The bladder was then catheterized under sterile conditions with an 8F Foley catheter and the bladder emptied. A filling cystometrogram was performed using warmed saline at a filling rate of 2ml per minute until a micturition contraction or overflow incontinence occurred. In general, each cystometry took approximately 30 minutes. Each cystometry was set to the volume at micturition = 100% so that compliances could be normalized for differences in volume. The compliance is calculated as the rise in intravesical pressure over 20% of the curve on the plateau region.
Cystitis
Immediately following cystometry, each rabbit was sedated with ketamine/xylazine (25 mg/10 mg, im). Under sterile conditions, the urinary bladder was catheterized with an 8 Fr. Foley catheter, emptied, and then filled with 25 ml of a saline solution containing protamine sulfate (10 mg/ ml) + uric acid (100 mg/ ml). The solution remained in the bladder for 30 minutes; the bladder was drained, and then washed three times with 50 ml of saline. The rabbits were allowed to recover for two weeks.
Contractile studies
After 2 weeks, each rabbit received a final cystometry under sedation. After cystometry, each rabbit was then euthanized and the bladder excised intact. Four full thickness longitudinal strips were then cut from the mid-bladder and placed in individual isolated baths containing 15 ml of an oxygenated tyrodes physiological solution containing 1 mg/ml glucose. During a 30 minute period of equilibrium, 2 grams of tension were placed on each strip and they were then stimulated as follows: field stimulation at 2, 8, and 32 Hz (5 seconds at 80 volts and 1 ms duration), carbachol (20 mM), ATP (1 mM) and KCl (120 mM). After each drug stimulation, the bladder strips were rinsed with oxygenated warmed Tyrodes 3X at 15 minute intervals. Maximal contractile responses were recorded. All contractile responses were recorded using a Grass model D polygraph, and digitized using the Grass Poly view System.
CUPRAC assay for total antioxidants
The CUPRAC assay was utilized to determine the total antioxidant capacity. This assay relies on the electron donating capabilities of antioxidants to reduce the copper ion. The CUPRAC working solution consisted of 10 mM copper (II) chloride dihydrate, 1M ammonium acetate, and 7.5 mM neocuproine. 0.15 mL of the above three solutions were added to 0.15 mL of each sample and allowed to react for 30 minutes at room temperature, after which the absorbance was read at 450 nm in a Hitachi U-2001 spectrophotometer [29,30].
Statistical analysis
Each set of data was analyzed individually. One way analysis of variance was used followed by the TUKEY test for individual differences among the groups; p < 0.05 was required for statistical significance.
Results
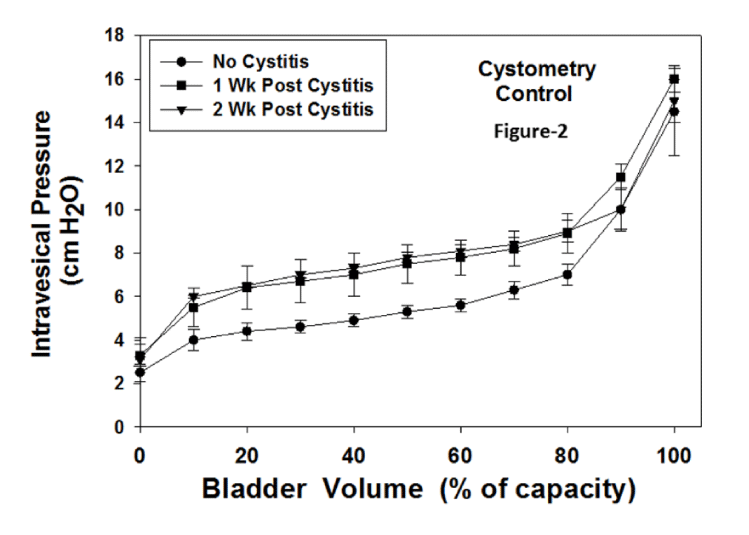
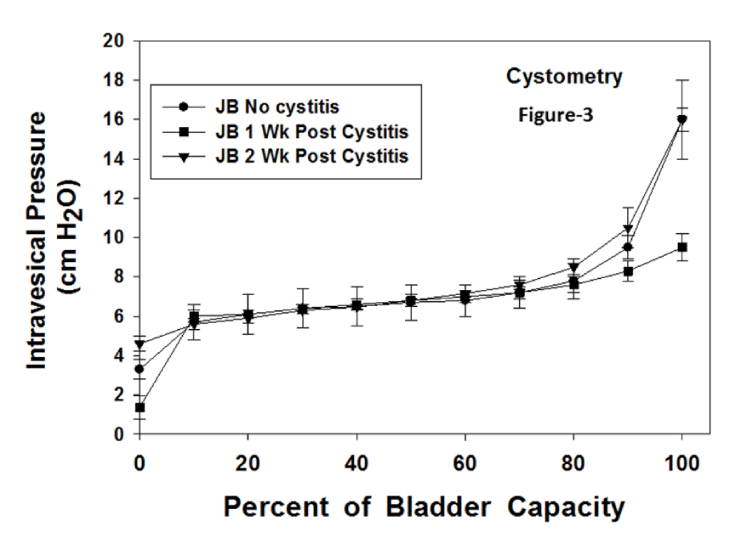
Figure 1 displays the bladder weights for the 3 groups. There were no significant differences among the groups. Figure 2 presents the cystometries for control rabbits (no JB), and at 1 and 2 weeks following cystitis. There were no statistical analyses for these curves. The compliance for each curve was calculated and the statistical differences in compliance are displayed in figure 4. Figure 3 presents the cystometries for no cystitis, and at 1 and 2 weeks following cystitis (+ JB). There were no statistical analyses for these curves. The compliance for each curve was calculated and the statistical differences displayed in figure 4. Figure 4 shows the compliance for all groups. It should be noted that an increase in the compliance number represents an increase in the stiffness of the bladder and thus a decrease in the true compliance. For the no JB group there were significant decreases in compliance at both 1 and 2 weeks post cystitis. Compared to the no JB group, the compliances of the JB group were similar prior to cystitis. However, they were significantly lower (more compliant for both post cystitis groups). Although the compliances were significantly lower than those of no JB, they were slightly but significantly higher than JB without cystitis.
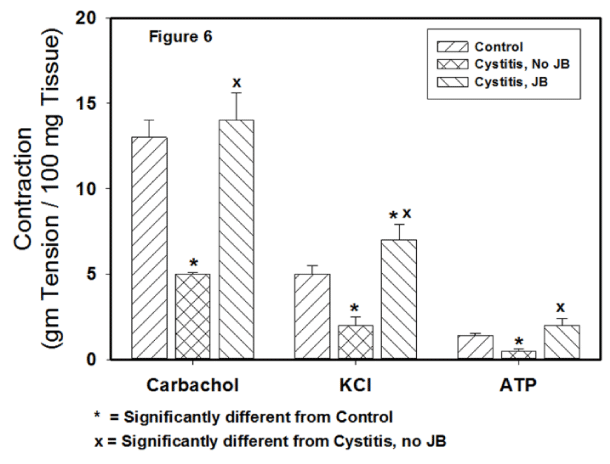
Figures 5,6 displays the contractile responses all forms of stimulation. At 2 weeks post cystitis, the contractile responses to all forms of stimulation were significantly reduced from the no cystitis no JB group. For all forms of stimulation, the cystitis + JB group showed no significant decreases in the contractile responses.
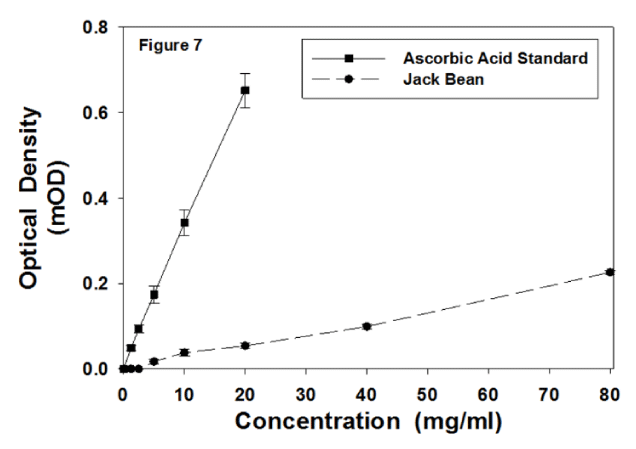
Figure 7 displays the total antioxidant activity for the ascorbic acid standard and for the JB preparation. Both curves were linear. Figure 8 displays the antioxidant values for ascorbic acid and JB. The ascorbic acid standard had approximately 10 times the antioxidant activity of the JB preparation per 10 mg.
These studies clearly demonstrate that our hypothesis that JB would be protective against chemical cystitis was proven to be true [10].
Discussion
Cystitis relates to the inflammation of the bladder. Most commonly, the inflammation is caused by a bacterial infection (Urinary Tract Infection - UTI). A bladder infection can be painful and annoying, and it can become a serious health problem if the infection spreads to your kidneys. Other forms of cystitis occur as a reaction to certain drugs, radiation therapy or irritants within the bladder. Cystitis of all etiologies is one of the most common medical problems [10,31]. 3.6% of adults over 20 self-reported having cystitis in the US. This indicates that 6.2 million adults self-reported having cystitis in the US (prevalence and incidence of cystitis, right diagnosis.com).
As mentioned previously, one of the major hypothesis concerning the etiology of virtually all forms of cystitis relates to a damaged bladder urothelial surface, and subsequent penetration of caustic substances in the urine into the bladder wall with a resultant inflammation, increased permeability, decreased compliance, and decreased contractility [15-17].
The symptoms of cystitis include: Bladder (Abdominal) pain, painful urination, frequent and difficulty in urination, urinary urgency (feeling that you need to urinate) (prevalence and incidence of cystitis, right diagnosis.com). In rabbits, there is an increased frequency of urination and a decreased volume per urination. Using in-vivo cystometry, there is also a decreased compliance (increased bladder stiffness) and a decreased volume at micturition. In addition, there is a decreased contractile response of the bladder smooth muscle to various forms of stimulation [32-34].
Although rats and mice have been extensively used in research because of the amenability of genetic manipulations [35-37], they are not the best species to study the effects of chemical cystitis in the lower urinary tract because of the extremely different micturition mechanisms in rats and mice compared to those of rabbits and humans. Rabbit bladder capacity is between 50 and 100 ml. Bladder compliance can be evaluated in vivo by cystometry using an 8 Fr. Foley catheter to catheterize the rabbit bladder through the urethra. The cystometric curve of the rabbit is similar in shape to that of humans: the bladder fills at low intravesical pressure until capacity is reached at which time a micturition contraction occurs (Functional Capacity). The rabbit urinates approximately 4-6 times per day which is similar to humans and very different from mice or rats [38-40]. Similar to man, bladder emptying occurs during the tonic phase of the contractile response of the bladder (Unlike the Mouse and Rat where Emptying occurs during the Phasic Contraction) [39,41]. The rabbit has been used extensively as a model for Benign Prostatic Hyperplasia (BPH), obstructive bladder dysfunction, and ischemic bladder dysfunction [41-44,,45,46].
The bladder is composed of two major structures, the urothelium, which is specific to the urinary bladder, and is highly elastic. The urothelium consists of approximately 4 cell layer and is covered by a layer of protective [47,48]. The urothelium along with the glycosaminoglycan layer represents the first line of defense against both the attachment of bacteria (Anti-Bladder Infection) and is generally impermeable to the contents of the urine, providing a protective surface against irritants and materials that can induce inflammation [49-51]. Both the anti-adherence and permeability barrier of the urothelium can be damaged by both ischemia and cystitis which can be caused by infection and chemicals within the urine [49-51].
The second structure is the bladder smooth muscle wall, which provides the coordinated contraction required for urination [52-54]. There is excellent evidence that the urothelium can communicate with the bladder smooth muscle via stretch (Bladder Filling) through the release of intracellular mediators from the urothelium which can influence smooth muscle tension and contraction, especially via interacting with the autonomic nerves and synapses [50,55-57].
Two intracellular organelles play major roles (but Different Roles) in the physiology and pathophysiology of both the urothelium and smooth muscle components of the bladder. They are the mitochondria, and sarcoplasmic reticulum.
Sarcoplasmic reticular control of calcium storage and release in the urothelium plays a major role in the process of angiogenesis stimulation by ischemia [58-61]. Although most of these studies were not performed using the urothelium, there is considerable data showing that ischemia of the urothelium induces a highly significant stimulation of angiogenesis [62].
In the current study, we evaluated JB extracts (CavatideR) in its ability to reduce or prevent the dysfunctions induced by chemical cystitis. We induced the chemical cystitis by placing 25ml saline containing protamine sulfate (10 mg/ ml) + uric acid (100 mg/ ml) in the bladder via 8 F catheter for 30 minutes [20,21]. Rabbits were treated for 2 weeks prior to cystitis and for two weeks following cystitis with JB. The control group had no treatment. Cystitis induced a significant decrease in compliance in the control group at both 1 and 2 weeks, there were no changes in the compliance in the JB group at either 1 or 2 weeks following cystitis.
Another natural product that has beneficial effects in the treatment of cystitis are (Cyperaceae) which is a medicinal herb traditionally used to treat various clinical conditions such as diarrhea, diabetes, and cystitis [63]. Cranberry juice has long been used to treat or prevent cystitis, although most studies are clinical trials and the data does not show detailed physiological or urological responses as demonstrated in the current study [64,65].
In regard to the contractility studies, control cystitis induced significant decreases in the responses to all forms of stimulation. Field stimulation requires the following sequence of events:
1. Stimulation of postsynaptic membranes to release acetylcholine and ATP [66-70].
2. Diffusion across the synaptic cleft and stimulation of the postsynaptic muscarinic cholinergic and purinergic receptors.
3. Stimulated release of Ca++ from the sarcoplasmic reticulum and from extracellular sites through calcium channels into the smooth muscle cell.
4. Activation of the smooth muscle components to contract.
5. Both muscarinic receptor activation and smooth muscle contraction require energy from the breakdown of ATP to ADP + P. Interference with any of these 5 factors would result in a decrease in the contractile force.
At 2 weeks post cystitis, the contractile responses to all forms of stimulation in cystitis+ JB group showed no decreased contractile responses. Thus the six mechanisms related to contractile responses were not affected by cystitis in the JB treated group.
These studies support fully the use of this JB extract to protect the urinary bladder from the pathophysiologies associated with chemical cystitis. In addition JB has significant antioxidant activity to support the idea that this protection is due to its antioxidant activity. Future studies are being designed to study whether JB can be used to treat chemical cystitis rather than protect the bladder from the cystitis.
Acknowledgements
These studies were based upon work supported in part by Wellspring Biotech Company, Beijing Tong Ren Tang Chinese medicine Co., LTD, the Office of Research and Development Department of the Veterans Affairs, and by the Capital Region Medical Research Foundation.
References
- Macey MR, Raynor MC. Medical and Surgical Treatment Modalities for Lower Urinary Tract Symptoms in the Male Patient Secondary to Benign Prostatic Hyperplasia: A Review. Semin Intervent Radiol. 2016; 33: 217-223. https://goo.gl/uCkvzr
- Chughtai B, Forde JC, Thomas DD, Laor L, Hossack T, Woo HH, et al. Benign prostatic hyperplasia. Nat Rev Dis Primers. 2016; 2:16031. https://goo.gl/pdQmKs
- Boyd K, Hilas O. α-adrenergic blockers for the treatment of lower-urinary-tract symptoms and dysfunction in women. Ann Pharmacother. 2014; 48: 711-722. https://goo.gl/3P7ufs
- Hashim H, Abrams P. Pharmacological management of women with mixed urinary incontinence. Drugs. 2006; 66: 591-606. https://goo.gl/T2TKwN
- Akin Y, Bozkurt A, Erol HS, Halici M, Celebi F, Kapakin KA, et al.Impact of Rho-Kinase Inhibitor Hydroxyfasudil in Protamine Sulphate Induced Cystitis Rat Bladder. Low Urin Tract Symptoms. 2015; 7: 108-114. https://goo.gl/DtSjLJ
- Uysal E, Yılmaz HR, Ugan Y, Altuntas A, Dogru A, Kutlucan A, et al. Protective Effects of Caffeic Acid Phenethyl Ester on Cyclophosphamide-Induced Hemorrhagic Cystitis in Rats. J Biochem Mol Toxicol, 2015; 29: 559-63. https://goo.gl/uQ7Puq
- Liu KM, Chuang SM, Long CY, Lee YL, Wang CC, Lu MC, et al. Ketamine-induced ulcerative cystitis and bladder apoptosis involve oxidative stress mediated by mitochondria and the endoplasmic reticulum. Am J Physiol Renal Physiol. 2015; 309: 318-331. https://goo.gl/LAHdDS
- Saitoh C, Yokoyama H, Chancellor MB, de Groat WC, Yoshimura N. Comparison of voiding function and nociceptive behavior in two rat models of cystitis induced by cyclophosphamide or acetone. Neurourol Urodyn. 2010; 29: 501-505. https://goo.gl/cCnP2u
- Kato K, Kitada S, Longhurst PA, Wein AJ, Levin RM. Time-course of alterations of bladder function following acetone-induced cystitis. J Urol. 1990; 144: 1272-1276. https://goo.gl/rzfaeF
- French L, Phelps K, Pothula NR, Mushkbar S. Urinary problems in women. Prim Care. 2009; 36: 53-71. https://goo.gl/BdaQxA
- Sen A. Recurrent cystitis in non-pregnant women. BMJ Clin Evid. 2008: 2008. https://goo.gl/N56vGZ
- Wechsler A. Recurrent cystitis in non-pregnant women. Clin Evid. 2003: 2210-2218.
- Cooper B, Jepson R. Recurrent cystitis in non-pregnant women. Clin Evid. 2002:1764-1771.
- Drake MJ, Nixon PM, Crew JP. Drug-induced bladder and urinary disorders. Incidence, prevention and management. Drug Saf. 1998; 19: 45-55. https://goo.gl/X125mQ
- Ruggieri MR, Levin RM, Hanno PM, Witkowski BA, Gill HS, Steinhardt GF. Defective antiadherence activity of bladder extracts from patients with recurrent urinary tract infection. J Urol. 1988; 140: 157-159. https://goo.gl/DVR4cj
- Teichman JM, Moldwin R. The role of the bladder surface in interstitial cystitis/painful bladder syndrome. Can J Urol. 2007; 14: 3599-3607. https://goo.gl/KQuHT9
- Parekh MH, Chichester P, Lobel RW, Aikawa K, Levin RM. Effects of castration on female rabbit bladder physiology and morphology. Urology. 2004; 64: 1048-1051. https://goo.gl/TNMHCy
- Choi BH, Jin LH, Kim KH, Han JY, Kang JH, Yoon SM. Mast cell activation and response to tolterodine in the rat urinary bladder in a chronic model of intravesical protamine sulfate and bacterial endotoxin-induced cystitis. Mol Med Rep. 2014; 10: 670-676. https://goo.gl/eRyztA
- Starkman JS, Martinez-Ferrer M, IturreguiJ M, Uwamariya C, Dmochowski RR, Bhowmick NA. Nicotinic signaling ameliorates acute bladder inflammation induced by protamine sulfate or cyclophosphamide. J Urol. 2008; 179: 2440-2446. https://goo.gl/fCeeRF
- Soler R, Bruschini H, Freire MP, Alves MT, Srougi M, Ortiz V. Urine is necessary to provoke bladder inflammation in protamine sulfate induced urothelial injury. J Urol. 2008; 180: 1527-1531. https://goo.gl/h7SCUk
- Rajasekaran M, SteinP, Parsons CL. Toxic factors in human urine that injure urothelium. Int J Urol. 2006; 13: 409-414. https://goo.gl/uxtfGi
- Ener K, Keske M, Aldemir M, Ozcan MF, Okulu E, Ozayar A. Evaluation of oxidative stress status and antioxidant capacity in patients with painful bladder syndrome/interstitial cystitis: preliminary results of a randomised study. Int Urol Nephrol. 2015; 47: 1297-1302. https://goo.gl/noVnqY
- Malone L, Schuler C, Leggett RE, Levin RM. Effect of estrogen and ovariectomy on response of the female rabbit urinary bladder to two forms of in vitro oxidative stress. Int Urogynecol J. 2014; 25: 791-798. https://goo.gl/6UZS2K
- Bae WJ, Ha US, Kim S, Kim SJ, Hong SH, Lee JY. Reduction of oxidative stress may play a role in the anti-inflammatory effect of the novel herbal formulation in a rat model of hydrochloric acid-induced cystitis. Neurourol Urodyn. 2015; 34: 86-91. https://goo.gl/hSjXQj
- Oka M, Fukui T, Ueda M, Tagaya M, Oyama T, Tanaka M. Suppression of bladder oxidative stress and inflammation by a phytotherapeutic agent in a rat model of partial bladder outlet obstruction. J Urol. 2009; 182: 382-390. https://goo.gl/3Q119d
- Aguilera Y, Diaz MF, Jiménez T, Benítez V, Herrera T, Cuadrado C, Martín Pedrosa M, Martín Cabrejas MA. Changes in nonnutritional factors and antioxidant activity during germination of nonconventional legumes. J Agric Food Chem. 2013; 61: 8120-8125. https://goo.gl/vrEczM
- Yao Y, Cheng X, Wang L, Wang S, Ren G. Biological potential of sixteen legumes in China. Int J Mol Sci. 2011; 12: 7048-7058. https://goo.gl/5DzMFa
- Lu HC. Traditional Chinese Medicine: An Authoritive and Comprehensive Guide. 2005: Basic Health Publications. 73.
- Callaghan CM, Leggett RE, Levin RM. A Comparison of Total Antioxidant Capacities of Concord, Purple, Red, and Green Grapes Using the CUPRAC Assay. Antioxidants (Basel). 2013; 2: 257-264. https://goo.gl/cD1KP8
- Bean H, Radu F, De E, Schuler C, Leggett RE, Levin RM. Comparative evaluation of antioxidant reactivity within obstructed and control rabbit urinary bladder tissue using FRAP and CUPRAC assays. Mol Cell Biochem. 2009; 323: 139-42. https://goo.gl/gMRfPV
- Azzarone GS, Liewehr, Connor KO, Cystitis. Pediatr Rev. 2007; 28: 474-476.
- Bayrak O, Seckiner I, Solakhan M, Karakok M, Erturhan SM, Yagci F. Effects of intravesical dexpanthenol use on lipid peroxidation and bladder histology in a chemical cystitis animal model. Urology. 2012; 79: 1023-1026. https://goo.gl/N7Tgse
- Amaro JL, Balasteghin KT, Padovani CR, Montenegro R. Structural alterations of the bladder induced by detrusor instability. Experimental study in rabbits. Int Braz J Urol. 2005; 31: 579-585. https://goo.gl/4sxwtU
- Mayo ME, Hinman F. Structure and function of the rabbit bladder altered by chronic obstruction or cystitis. Invest Urol. 1976; 14: 6-9. https://goo.gl/LrgxJk
- Loeser RF. Aging processes and the development of osteoarthritis. Curr Opin Rheumatol. 2013; 25: 108-113. https://goo.gl/XoVRwP
- Lee Y, Dawson VL, Dawson TM. Animal models of Parkinson's disease: vertebrate genetics. Cold Spring Harb Perspect Med. 2012; 2.
- Anisimov VN, Zabezhinski MA, Popovich IG, Pliss GB, Bespalov VG, Alexandrov VA, et al. Rodent models for the preclinical evaluation of drugs suitable for pharmacological intervention in aging. Expert Opin Drug Discov. 2012; 7: 85-95. https://goo.gl/bYtFyL
- Levin RM, et al. Factors that modulate the initiation of micturition. Scand J Urol Nephrol Suppl. 1995; 175: 3-10. https://goo.gl/LsHP8f
- Levin RM, Monson FC, Longhurst PA, Wein AJ. Rabbit as a model of urinary bladder function. Neurourol Urodyn. 1994; 13: 119-135. https://goo.gl/YckjRz
- Yu HJ, Levin RM, Longhurst PA, Damaser MS. Effect of age and outlet resistance on rabbit urinary bladder emptying. J Urol. 1997. 158: 924-930. https://goo.gl/tvdn9h
- Buttyan R, chen MW, Levin RM. Animal models of bladder outlet obstruction and molecular insights into the basis for the development of bladder dysfunction. Eur Urol. 1997; 32: 32-39. https://goo.gl/B3hjir
- Levin R, Chichester P, Levin S, Buttyan R. Role of angiogenesis in bladder response to partial outlet obstruction. Scand J Urol Nephrol Suppl. 2004; 215: 37-47. https://goo.gl/Pebccr
- Stein R, Gong CL, Hutcheson J, Krasnopolsky L, Canning DA, Carr M, et al. The fate of urinary bladder smooth muscle after outlet obstruction--a role for the sarcoplasmic reticulum. Adv Exp Med Biol. 2003; 539: 773-90. https://goo.gl/ywJY9w
- Chapple CR, Smith D. The pathophysiological changes in the bladder obstructed by benign prostatic hyperplasia. Br J Urol. 1994; 73: 117-123. https://goo.gl/iPd7uW
- Lin VK, JD McConnell. Effects of obstruction on bladder contractile proteins. Prog Clin Biol Res. 1994; 386: 263-269. https://goo.gl/5dt9XW
- Lin WY, Mannikarottu A, Li S, Juan YS, Schuler C, Javed Z. Correlation of in vivo bladder blood flow measurements with tissue hypoxia. World J Urol. 2011; 29: 165-170. https://goo.gl/mFXZJz
- Wein AJ. Role of the urothelium in bladder function. J Urol. 2005; 173: 2199-2200. https://goo.gl/jPKkUU
- Birder L. Role of the urothelium in bladder function. Scand J Urol Nephrol Suppl. 2004: 48-53. https://goo.gl/ZPVLe3
- Lazzeri M. The physiological function of the urothelium--more than a simple barrier. Urol Int. 2006; 76: 289-295. https://goo.gl/truF49
- Birder L, Andersson KE. Urothelial signaling. Physiol Rev. 2013; 93: 653-680. https://goo.gl/7iTKhg
- Birder LA, Andersson KE, Kanai AJ, Hanna-Mitchell AT, Fry CH. Urothelial mucosal signaling and the overactive bladder-ICI-RS 2013. Neurourol Urodyn. 2014; 33: 597-601.
- Brading AF. The physiology of the mammalian urinary outflow tract. Exp Physiol. 1999; 84: 215-221. https://goo.gl/v7h2hP
- Zimmern PE, Lin VK, McConnell JD. Smooth-muscle physiology. Urol Clin North Am. 1996; 23: 211-219. https://goo.gl/EbFVfz
- Levin RM, Wein AJ, Buttyan R, Monson FC, Longhurst PA. Update on bladder smooth-muscle physiology. World J Urol. 1994; 12: 226-232. https://goo.gl/P5Mybv
- Kanai A, Fry C, Ikeda Y, Kullmann FA, Parsons B, Birder L. Implications for bidirectional signaling between afferent nerves and urothelial cells-ICI-RS 2014. Neurourol Urodyn. 2016; 35: 273-277. https://goo.gl/ZkSqwY
- Birder LA. Urothelial signaling. Handb Exp Pharmacol. 2011; 202: 207-231. https://goo.gl/rpTGGs
- Birder LA. Urothelial signaling. Auton Neurosci. 2010; 153: 33-40. https://goo.gl/7fDMQU
- Lounsbury KM, Hu Q, Ziegelstein RC. Calcium signaling and oxidant stress in the vasculature. Free Radic Biol Med. 2000; 28: 1362-1369. https://goo.gl/WT69ay
- Hashitani H, Takano H, Fujita K, Mitsui R, Suzuki H. Functional properties of suburothelial microvessels in the rat bladder. J Urol. 2011; 185: 2382-2391. https://goo.gl/xiYRpZ
- Mei Y, Thompson MD, Shiraishi Y, Cohen RA, Tong X. Sarcoplasmic/endoplasmic reticulum Ca2+ ATPase C674 promotes ischemia- and hypoxia-induced angiogenesis via coordinated endothelial cell and macrophage function. J Mol Cell Cardiol. 2014; 76: 275-282. https://goo.gl/bDViia
- Adachi T. Modulation of vascular sarco/endoplasmic reticulum calcium ATPase in cardiovascular pathophysiology. Adv Pharmacol. 2010; 59: 165-195. https://goo.gl/bWck1f
- Chichester P, Schroder A, Horan P, Levin RM. Vascular response of the rabbit bladder to chronic partial outlet obstruction. Mol Cell Biochem. 2001; 226: 1-8. https://goo.gl/kTgCov
- Pirzada AM, Ali HH, Naeem M, Latif M, Bukhari AH, Tanveer A. Cyperusrotundus L.: Traditional uses, phytochemistry, and pharmacological activities. J Ethno pharmacol. 201; 174: 540-560. https://goo.gl/ubNfCL
- Payne H, Adamson A, Bahl A, Borwell J, Dodds D, Heath C. Chemical- and radiation-induced haemorrhagic cystitis: current treatments and challenges. BJU Int. 2013; 112: 885-897. https://goo.gl/scC2Ek
- Guay DR. Cranberry and urinary tract infections. Drugs. 2009; 69: 775-807. https://goo.gl/Ju6AqQ
- Wein AJ. Chronic administration of anticholinergics in rats induces a shift from muscarinic to purinergic transmission in the bladder wall. J Urol. 2015. 193: 2149. https://goo.gl/xpyKk9
- Wein AJ. Re: Chronic administration of anticholinergics in rats induces a shift from muscarinic to purinergic transmission in the bladder wall. J Urol. 2014; 192: 616. https://goo.gl/vyos9j
- Uvin P, Boudes M, Menigoz A, Franken J, Pinto S, Gevaert T. Chronic administration of anticholinergics in rats induces a shift from muscarinic to purinergic transmission in the bladder wall. Eur Urol. 2013; 64: 502-510. https://goo.gl/ei2kwY
- Mumtaz FH, Lau DH, Siddiqui EJ, Morgan RJ, Thompson CS, Mikhailidis DP. Changes in cholinergic and purinergic neurotransmission in the diabetic rabbit bladder. In Vivo. 2006; 20: 1-4. https://goo.gl/ZcKbCR
- Yoshida M, Homma Y, Inadome A, Yono M, Seshita H, Miyamoto Y. Age-related changes in cholinergic and purinergic neurotransmission in human isolated bladder smooth muscles. Exp Gerontol. 2001; 36: 99-109. https://goo.gl/u23t62
Authors submit all Proposals and manuscripts via Electronic Form!