Research Article
Comparison of Antibacterial Activity of (–) Thioridazine and Racemic Thioridazine in Staphylococcus aureus
Marianne Ø. Poulsen, Janne K. Klitgaard, Jørn B. Christensen, Birgitte H. Kallipolitis, Glenn W. Kaatz, Per Plenge, Stephen J. Fey and Jette E. Kristiansen*
Marianne Ø. Poulsen1,2, Janne K. Klitgaard1,2, Jørn B. Christensen3, Birgitte H. Kallipolitis2, Glenn W. Kaatz4, Per Plenge5, Stephen J. Fey2,6 and Jette E. Kristiansen7*
1Research Unit of Clinical Microbiology, Institute of Clinical Research, University of Southern Denmark, Odense, Denmark
2Department of Biochemistry and Molecular Biology, University of Southern Denmark, Odense, Denmark
3Department of Chemistry, University of Copenhagen, Frederiksberg, Denmark
4Department of Internal Medicine, Division of Infectious Diseases, John D. Dingell VA Medical Center and Wayne State University School of Medicine, Detroit, Michigan, USA
5Department of Neuroscience and Pharmacology. Panum Institute, University of Copenhagen, Copenhagen. Denmark
6CelVivo IVS, Blommenslyst, Denmark
7Center for Biomembrane Physics (Memphys), University of Southern Denmark, Odense, Denmark
*Address for Correspondence: Jette E. Kristiansen, Center for Biomembrane Physics (Memphys), University of Southern Denmark, Odense, Denmark, Tel: +457-444-9979; E-mail: [email protected]
Dates: 05 March 2018; Approved: 10 April 2018; Published: 13 April 2018
Citation this article: Poulsen MØ, Klitgaard JK, Christensen JB, Kallipolitis BH, Kristiansen JE, et al. Comparison of Antibacterial Activity of (–) Thioridazine and Racemic Thioridazine in Staphylococcus aureus. Am J Bioavailab Bioequiv. 2018;1(1): 001-009.
Copyright: © 2018 Kristiansen JE, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Non-antibiotics; Antibiotic resistance; MRSA; Dicloxacillin; Thioridazine; Enantiomer; Cardiotoxicity; Neurotoxicity
Abstract
Antibiotic resistance is an increasing problem globally. Non-antibiotics are therapeutics that have antibacterial properties in addition to their original purposes. Thioridazine is a non-antibiotic that has been shown to sensitize Staphylococcus aureus to classical antibiotics. However, the drug has been withdrawn from the market due to cardiotoxicity. Recent work has shown that the cardiotoxic side-effects are linked to the (+) enantiomer of thioridazine but not to the (–) form. The aim of this work was thus to investigate the antimicrobial efficacy of the (–) enantiomer (–TZ) as compared to the racemic mixture of Thioridazine (TZR). Viability assays on methicillin-sensitive and methicillin-resistant Staphylococcus aureus (S. aureus) strains show that combinations of TZR and–TZ together with Dicloxacillin (DCX) are equally effective showing that selecting the –TZ enantiomer does not compromise its activity. Importantly, –TZ binds with lower affinity to the dopamine 2 receptor, indicating that this formulation might provide therapeutic benefit with reduced side effects. This strengthens the potential for future application of combined treatment using –TZ and classical antibiotics.
Introduction
Phenothiazines and numerous other chemically related compounds, which are normally used for psychopharmaceutic purposes, have been shown to have valuable antimicrobial activities [1-7]. They are also called non-antibiotics and work either alone or synergistically as adjuvants with existing antibiotics. They have gained considerable interest because of the need for new chemotherapeutics against increasingly drug resistant microbial infections and cancer [8-11]. For these reasons, we have focused our research on the antimicrobial activity of the phenothiazines, their structural relatives, the thioxanthenes and other Central Nervous System (CNS) -active compounds [10,12-16].
Thioridazine (TZR) was synthesized in 1951 and commercialised by Novartis in 1959 under the trade name of Mellaril™ (or Melleril™) where after it became a common, long-term treatment of schizophrenia [7].
Pioneering work carried out using the severe multi-resistant TB strains collected in New York in the late 1980s and the beginning of the 1990s by Amaral et al. [17] focussed interest on the antimicrobial activity of TZR. An early structure-activity relationship study, comparing 18 different phenothiazine derivatives, obtained especially promising antimicrobial results using TZR [18]. Together, these studies led to TZR being intensively investigated by many laboratories all over the world [19].
Radhakrishnan et al. [20] tested 316 strains belonging to both Gram positive and Gram negative bacteria and showed that in addition to TZR being bactericidal against S. aureus and bacteriostatic against Vibrio cholera and parahaemolyticus, other Gram negative organisms were also affected.
Indicating that several mechanisms may be involved, TZR has also been shown to be able to reverse resistance by facilitating the elimination of R plasmids from twelve multiple antibiotic-resistant bacteria including Escherichia coli (E. coli) and Shigella flexneri but not in S. aureus [20].
One of the shortcomings precluding the utilization of TZR is that it has been reputed to have cardiotoxic properties [21]. Even at low concentrations, TZR was found to have a concentration-dependent prolongation of the QT interval (an indication of serious cardiotoxicity) [22,23]. Therefore, in 2005, Novartis discontinued Mellaril™ (TZR) worldwide, stating that “the benefit/risk profile of Mellaril™ could no longer meet current clinical and regulatory expectations”. The manufacturer cited evidence of a connection between QT prolongations, cardiac arrhythmias and sudden death in patients with schizophrenia [24]. Furthermore, Novartis claimed that new and improved antipsychotic treatments had become available. The antimicrobial effects were ignored.
It is essential to analyse this information in detail because an unfavourable clinical effect would significantly diminish the justification for developing TZR further. Side effects can be detected and described based on the retrospective description of cohorts of psychiatric patients. Reilly et al. [21] identified 74 cases meeting the study criteria for probable sudden death and 27 cases meeting the criteria for confirmed sudden death. Out of the 74 probable cases, seventeen were younger than 65 years and only one patient was younger than 50 years. This study was based on a total catchment population of 1.23 million people. The results are in accordance with the observations published by Glassman & Bigger who stated that “Although sudden death occurs almost twice as often in populations treated with antipsychotics as in normal populations, there are still only 10 – 15 such events in 10,000 person-years of observation” [24]. In conclusion, the side effect profile of TZR includes an estimated risk of sudden death due to QT prolongation of approximately 5 – 8 events in 10,000 cases from the normal healthy population. The TZR data include the notable observation that only one patient was younger than 50. In relation to the potential use of TZR for treatment of bacterial infections, TZR would be administered for only a few weeks (instead of years for psychiatric patients), and this would be expected to considerably ameliorate the side effect profile.
This information represents the relevant background for the withdrawal of TZR in 2005 for schizophrenia. These facts gain a completely different significance in the situation where there are growing limitations to antibiotic treatment. In 2015, globally, 10.4 million people fell ill with TB and 1.8 million died from the disease. In the same year an estimated 480,000 people developed Multidrug-Resistant TB (MDR-TB) or Extensively Drug-Resistant TB (XDR-TB) and of them, 242,000 died [25]. Expenses for MRSA treatment cost billions of dollars alone in the USA [26-27] and the EU have estimated that 25,000 persons in the EU have died from antibiotic resistant bacteria in 2015 [28]. This is not a situation where an older drug can be substituted by a newer one with a better side-effect profile. We anticipate that a lack of options might designate TZR as a potential treatment in situations where the natural course of the disease comprises a significantly higher risk of death than that attributed to sudden death. In other words, based on current data, TZR possesses considerable potential for the treatment of antibiotic resistant (and sensitive) microorganisms like Staphylococcus aureus and Mycobacterium tuberculosis. Thus, the potential benefits of TZR outweigh the implied shortcomings, and TZR needs therefore to be investigated further.
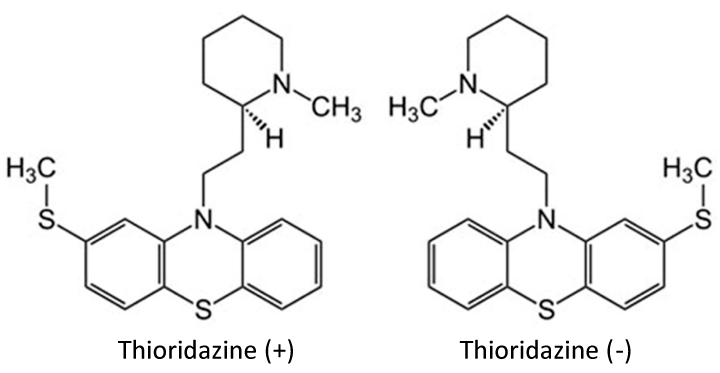
Like several other phenothiazines, thioridazine is a racemic mixture consisting of two enantiomers comprising a dextrorotary (+TZ) and a laevorotatory (–TZ) form (Figure1). The experiments showing synergistic recovery of antibiotic sensitivity were performed with the racemic mixture TZR [15,29-32]. In 2005, Hendricks et al. showed that TZR and –TZ (also known as “JEK47”) were able to reverse resistance in vancomycin-resistant enterococci and also in Methicillin-Resistant and –Sensitive Staphylococcus aureus strains (MRSA and MSSA respectively) [10, 15,29].
In respect of the cardiotoxicity question, a recent study indicated that –TZ has a negligible effect on the electrophysiological properties of cardiac muscle, while both +TZ and the racemate cause significant prolongation of the ventricular Action Potential Duration (APD) [16,33]. These prolonging effects appear to be caused by a significantly higher inhibition of the rapid component of the delayed rectifier potassium current, IKr, by the +TZ [34].
Concerning the neurological properties, –TZ also has a less challenging CNS pharmacodynamic activity (e.g. weaker blockade of dopamine D2-receptors) and is the enantiomer that is concentrated in human tissue at higher levels than +TZ [35-37].
Results, obtained using stereochemical analogues and new synthesized structures, have pointed to the possibility to separate their classical pharmacological activities from their antimicrobial effects. Micro-gravimetric titration studies of the molar ratios of phenothiazine hydrochloride derivatives with various lipids have suggested that their ionization potential is related to their sedative effects. In contrast, their differences in lipophilicity to different membranes in the cell (i.e. due to variations in phospholipids present in these membranes) may be related to non-neuronal physiological properties. The correlation between the molar ratio and the Fibonacci series suggests that similar spiral adducts are formed at water-phospholipid boundaries (e.g. membranes) irrespective of the actual analogue/phospholipid pair tested and that these adducts self-organize to minimize energy relationships [38]. This possibility to separate pharmacological and antimicrobial effects has thus started a search to find analogues with minimal cardiotoxic and neurological activities (peripheral and central nervous system) while further increasing their antimicrobial potency [16-39].
Additional studies comparing +TZ, –TZ and the racemate on 55 different bacteria (both Gram positive and Gram negative) showed that –TZ was superior in terms of both its in vitro and in vivo efficacies. Of relevance to the S. aureus studies presented here, –TZ was found to be effective on both PAN sensitive strains: ‘Oxford’ NCTC 6571 and NCTC 8530, the ATCC strain 25923; as well as the resistant strains ML 16, ML 152, ML 266, ML277, ML 329, ML 358, ML 422 [40].
Provided that –TZ has at least the same antibacterial activity as the racemate, these pharmacological attributes make it a better option to reverse resistance or as a “helper compound” in combination with antibiotics [8,30]. The purpose of this investigation was to extend the S. aureus studies, by comparing the antimicrobial activity of racemic Thioridazine (TZR) and the –TZ on selected S. aureus clones which are currently of serious concern in Denmark. We have used clones isolated from pigs (which are silent carriers) and humans. These studies are made more significant by the increasing number of MRSA CC398 cases detected. The use of +TZ thus has little interest from the clinical viewpoint and we therefore have focused on –TZ as a potential antibiotic helper compound. The aim of this work was thus to investigate the antimicrobial efficacy of –TZ as compared to the racemic mixture using clinically relevant strains and tests.
Materials and methods
Bacterial strains
The MSSA strains used included strain Newman (English hospital, 1952, [41]) and ATCC 25293 (US, 1945, Staphylococcus aureus subsp. aureus (ATCC® 25923™). The MRSA strains employed were COL (English hospital, 1966 [42]), 51203 and 51726 (Clinical isolates provided by Statens Serum Institute, Denmark (SSI)). The latter is a member of Clonal Complex (CC) 398 and is considered to have originated from pigs [43]. The strains were maintained as pure cultures in standard media throughout the entire project. Strain SA-1199B was used for efflux inhibition assays [44]. This strain has a norA promoter region mutation that results in high-level gene expression, producing a multidrug efflux-related resistance phenotype.
Antimicrobial agents and other chemical reagents
Dicloxacillin (DCX) was obtained from Bristol-Myers Squibb AB, and oxacillin was obtained from BioMerieux-Diagnostics. All other chemicals were purchased from Sigma Aldrich.
Media
Mueller-Hinton II 90 mm plates were purchased from SSI, Denmark and used for the E-test. E-test strips were obtained from Biomerieux. Brain Heart Infusion (BHI) broth was obtained from Oxoid, Mueller-Hinton (MH) broth from Merck, and granulated Difco agar (BD) were used for subculture and maintenance of all the bacterial strains investigated.
Isolation of –TZ
Clinically prescribed Thioridazine (TZR) hydrochloride is a racemic mixture of equal amounts of the (+) and (–) enantiomers with an asymmetric carbon at position 2 of the piperidyl ring (Figure 1). The enantiomers were separated by resolution according to the procedure described by Bourquin et al. [45]. Briefly, TZR was first converted to the free base. –TZ was obtained by fractional crystallisation out of acetone before being converted back into the hydrochloride.
Using optical rotation analysis only [46,47] we estimate that the purity of this batch to be approximately 80%. Robert has shown that the pure – enantiomer (free base) has a specific rotation of -37° in ethanol at 20°C while the + enantiomer has +33° [48]. Subsequent Enantiomeric Excess (EE) analysis was determined by using an Aurora Fusion A5/Agilent SFC system operating at 3 ml/min at 40°C and 150 bar backpressure. The column was a ChiralpakAD3 3m (150 x 4.6 mm). The eluent was CO2 (50%) and ethanol + 0.1 % diethylamine (50%). The – enantiomer purity was found to be 68%.
Minimal Inhibitory Concentration (MIC) determined on solid media by E-test
The minimal inhibitory concentration of DCX was determined by E-test according to the manufacturer’s instructions (Biomerieux). A colony was picked with a 1 µl white inoculation stick (McFarland 0.5) and suspended in 4.5 ml sterile 0.9% w/v sodium chloride and spread on a Mueller-Hinton II 90 mm plate (SSI) with a sterile swab and left to dry for approximately 20 min after which an E-test strip was placed on the plate. The plate was incubated for 20 hours at 35°C and the MIC was read the next day according to the manufacturer’s instructions.
MIC determined by the tube dilution technique
For all strains, MICs for TZR, +TZ, and –TZ were determined by the tube dilution technique [49]. A series of two-fold dilutions were prepared and the MIC was determined after incubation for 20 hours as the lowest concentration that inhibited growth (Table 1). The experiments were repeated twice.
| Table 1: Strains used in the present study, the MIC values determined and the concentrations of the drugs chosen for viability assays. | ||||||
| Minimal inhibitory concentration (mg/L) determined on solid media a) |
Sub-inhibitory concentrations (mg/L) used in viability assays b) |
|||||
| Strain | TZR | –TZ | DCX | TZR | –TZ | DCX |
| MSSA | ||||||
| Newman | 64 | 64 | 1 | 16 | 16 | 0.04 |
| ATCC25923 | 64 | 64 | 2 | 16 | 16 | 0.0625 |
| MRSA | ||||||
| COL | 64 | 64 | >256 | 16 | 16 | 16 |
| 51203 | 64 | 64 | >256 | 16 | 16 | 32 |
| 51726 | 64 | 64 | 16 | 16 | 16 | 0.125 |
|
||||||
Growth and viability of test bacteria in liquid medium
Growth and viability assays were performed as described by Poulsen et al. [28]. In order to perform viability assays, sub-inhibitory concentrations of the TZR forms and DCX were determined by growth experiments in liquid media. An overview of the sub-inhibitory concentrations of the TZR forms and DCX found by growth experiments are presented in table 1. For viability experiments, early exponential phase bacteria were exposed to the determined sub-inhibitory concentrations of thioridazine (TZR or –TZ) and DCX alone or in combination. The number of colony forming units was determined by 10 fold dilutions in saline buffer and plated on MH agar plates. The experiments were repeated twice.
Efflux inhibition assay
Ethidium Bromide (EtBr) is a substrate for many bacterial multidrug efflux pumps, including NorA. The efficiency of these EtBr pumps can be assessed fluorometrically by the loss of fluorescence over time from EtBr-loaded cells. SA-1199B was loaded with EtBr as previously described, and the effect of varying concentrations of TZR, +TZ, and –TZ on EtBr efflux was determined to generate a dose–response profile for each compound [44,50]. Results were expressed as percentage reduction of the total efflux observed for test strains in the absence of inhibitors. The 50% inhibitory concentration (IC50) for test compounds was determined by inspection of dose-response plots.
Dopamine receptor affinity assay
Human dopamine D2 receptors were expressed in COS7 cells. Membranes were prepared from COS7 cells after transient transfection with pcDNA2 plasmid expressing the human dopamine D2 receptor using the Lipo2000 transfection protocol (Invitrogen) as described previously [17]. The affinity of –TZ and +TZ to the D2 receptor was measured in buffer (25 mM HEPES, 120 mM NaCl, 5.4 mM KCl, 1.2 mM CaCl2, 1.2 mM MgSO4, pH 7.4) by competitive binding using [3H]-Raclopride. The measurements were performed in 96 well plates in a volume of 400 µL, at a concentration range from 0.1 – 2000 nM (10 determinations in triplicate). The binding mixture was incubated for 1 hour at room temperature and subsequently filtered through GF/B filters using a Tomtec cell harvester. After drying, the filters were saturated with Meltilex scintillator and counted in a Perkin Elmer Micro beta counter.
Results
Thioridazine has been shown to have non-antibiotic properties but its clinical introduction (for this indication) has been prohibited because of cardiotoxic side effects. Recent results have shown that the laevorotatory enantiomer (–TZ or JEK47) has been shown to be less toxic [33]. Therefore, the task remaining (and the goal of the experiments presented here) is to show that –TZ is as potent a non-antibiotic as the racemate TZR using clinically relevant MSSA and MRSA strains.
Towards this goal, we first determined the Minimal Inhibitory Concentration (MIC) values for TZR and –TZ for the MSSA Newman strain. These MIC values were found to be 64 mg/L for both drugs when determined by the tube dilution technique. The E-test MIC values of DCX were 1 mg/L for the Newman strain and 2 mg/L for S. aureus ATCC25923, and for S. aureus COL and strain 51203 were >256 mg/L and 16 mg/L for strain 51726 (Table 1).
Exponentially growing Newman strain bacteria (initially at an OD600 of 0.2) were exposed to concentrations of 8, 12, 16, 20, 24, 28, and 32 mg/L of each drug in order to compare the antimicrobial effect of –TZ and TZR. After 4 hours the optical densities were measured at 600 nm (OD600) and the data were used to construct dose-response curves for each compound. –TZ and TZR had IC50 values of 25.2 and 29.8 mg/L respectively. Thus these data revealed –TZ is a modestly more-potent antimicrobial agent than TZR (Figure 2).
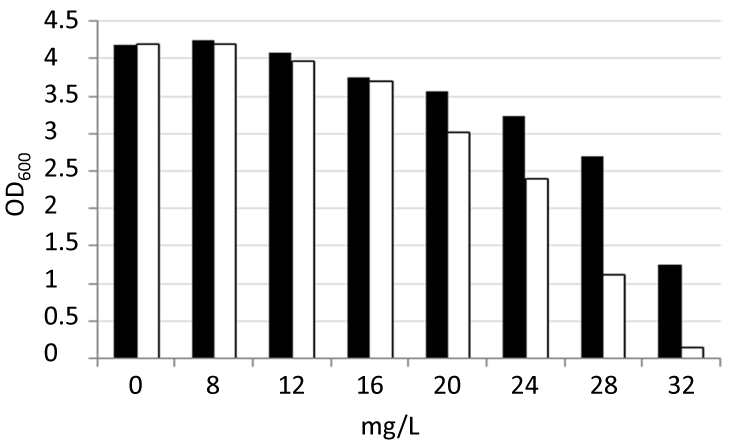 Figure 2: Dose-response curves of thioridazine racemic (TZR) and thioridazine (–) (–TZ (JEK47)). At OD600 0.2 a culture of MSSA strain Newman was exposed to various concentrations of TZR or –TZ (0 – 32 mg/L). The OD600 was measured after 3.7 hours of exposure is presented. Black bars; TZR, white bars; –TZ. The experiments were repeated twice and one set of data is presented. The raw data for figure 2 and data for additional time points (0.65, 1.1, 1.67, 2.7, 3.7, 4.65, 5.7 and 6.65 hours) can be found in the supporting information file S1_figure_2.pdf.
Figure 2: Dose-response curves of thioridazine racemic (TZR) and thioridazine (–) (–TZ (JEK47)). At OD600 0.2 a culture of MSSA strain Newman was exposed to various concentrations of TZR or –TZ (0 – 32 mg/L). The OD600 was measured after 3.7 hours of exposure is presented. Black bars; TZR, white bars; –TZ. The experiments were repeated twice and one set of data is presented. The raw data for figure 2 and data for additional time points (0.65, 1.1, 1.67, 2.7, 3.7, 4.65, 5.7 and 6.65 hours) can be found in the supporting information file S1_figure_2.pdf.
Thioridazine and DCX have been previously suggested to exhibit synergistic anti-bacterial effects [51,52]. The next step of our analysis was to investigate whether the –TZ and TZR also exhibit different synergistic effects when combined with the antibiotic DCX. Bacterial strains were grown in the presence of either single drug or in drug combinations and compared to untreated controls grown in media alone. Sub-inhibitory concentrations (i.e. the highest concentration which did not significantly affect the growth of S. aureus in liquid media) were determined for the two compounds (TZR and –TZ) by viability assays. This was determined to be 16 mg/L for all strains (Table 1). Similarly, the sub-inhibitory concentrations of DCX were determined to be 0.04 mg/L for strain Newman, 0.0625 mg/L for S. aureus ATCC25923, 16 mg/L for COL, 32 mg/L for strain 51203 and 0.125 mg/L for strain 51726. The use of sub-inhibitory concentrations facilitates the identification of synergistic effects.
The results obtained showed that the presence of neither thioridazine derivative alone at their sub-inhibitory concentrations affected the bacterial growth as determined by CFU/ml (Figure 3: racemic Thioridazine, TZR ⧠ or (–)-thioridazine, –TZ ∆). When grown in DCX alone, all 5 test strains were slightly inhibited after 4 hours by 1 log or less (Figure 3: ⨯). However, when grown in the presence of DCX combined with either of the thioridazine formulations, CFU/mL was reduced compared to the control by respectively at least 1, 3.5, 3, and 2.5 log10 CFU/ml for S. aureus strains: Newman, ATCC25923, COL and 51203. In the 51726 CC398 strain, the reduction in CFU/mL was as high as 4.5 log10 CFU/mL. This showed a clear synergistic action of the combination of DCX and thioridazine. Similar bactericidal results were obtained when either TZR or –TZ were used showing that there were no significant differences between them.
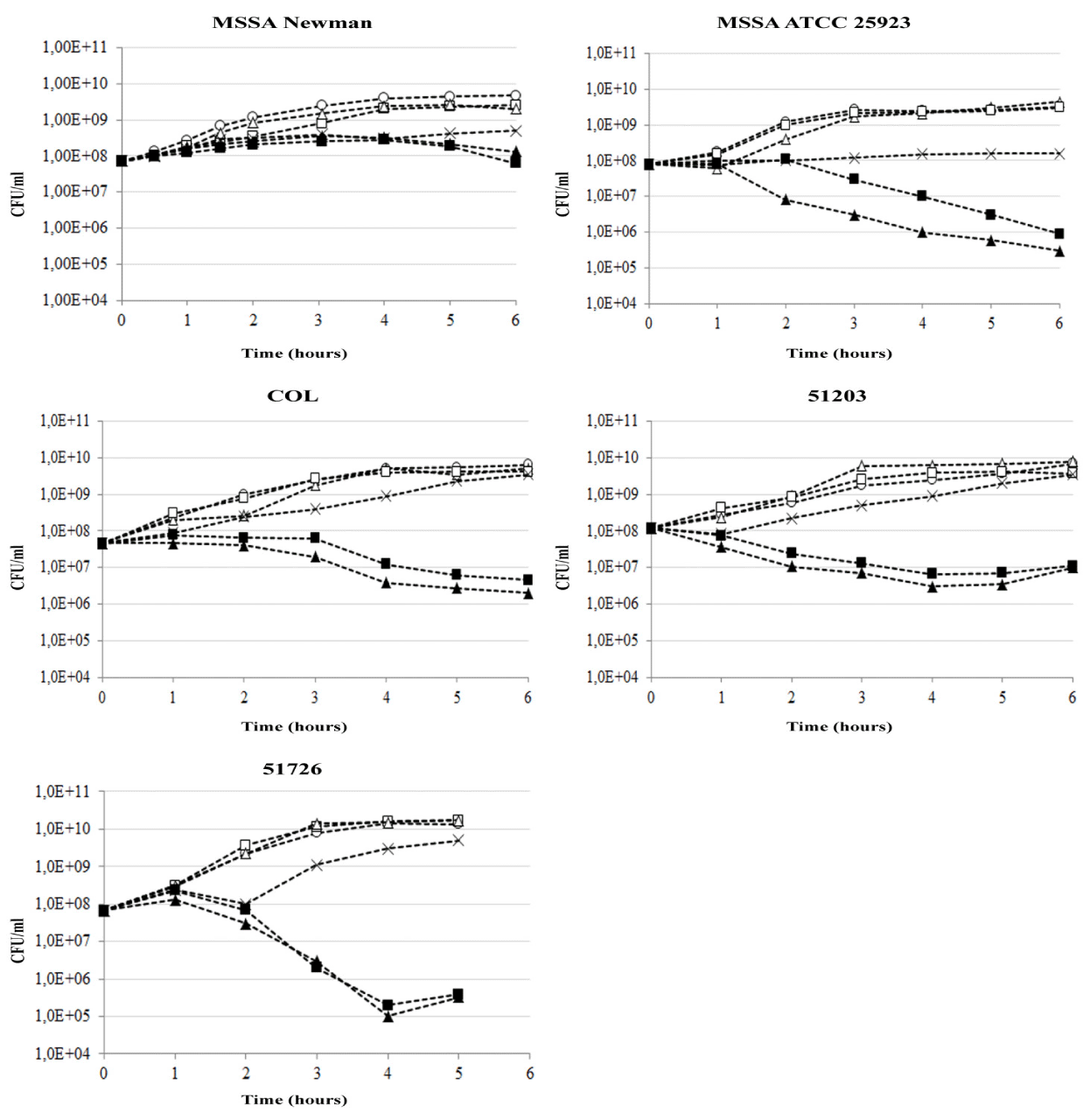 Figure 3: Viability assays of five Staphylococcal strains. Two MSSA strains (Newman and ATCC25293) and three MRSA strains (COL, 51203 and 51726). Bacterial cultures grown to OD600 0.2 was exposed to drugs either alone or in combination. As a control, bacteria were grown in media alone. The experiments were repeated twice and one set of data is presented. Control, ; thioridazine racemic (TZR), ; (–)-thioridazine (–TZ), ∆; dicloxacillin (DCX), x; thioridazine racemic (TZR) and DCX ; (–)-thioridazine (–TZ) and DCX, . The raw data for figure 3 can be found in the supporting information file S2_figure_3.pdf.
Figure 3: Viability assays of five Staphylococcal strains. Two MSSA strains (Newman and ATCC25293) and three MRSA strains (COL, 51203 and 51726). Bacterial cultures grown to OD600 0.2 was exposed to drugs either alone or in combination. As a control, bacteria were grown in media alone. The experiments were repeated twice and one set of data is presented. Control, ; thioridazine racemic (TZR), ; (–)-thioridazine (–TZ), ∆; dicloxacillin (DCX), x; thioridazine racemic (TZR) and DCX ; (–)-thioridazine (–TZ) and DCX, . The raw data for figure 3 can be found in the supporting information file S2_figure_3.pdf.
Strains Newman, ATCC 25923 and COL re-initiated growth within 9-22 hours (data not shown) while strains 51203 and 51726 re-initiated growth after 4-5 hours. We suggest that this is caused either by the instability of thioridazine, which is light-sensitive [53] or heterogeneity of the bacterial isolates. The more heterogenic, the more likely that a few bacteria will continue growth, unaffected by the single dose treatment because they are more drug-resistant. When treating patients for bacterial infections, drugs are administered repeatedly and thus growth re-initiation is not expected to be an issue.
One of the most common strategies that cells use when they develop multidrug resistance is to reduce drug accumulation by increasing the effectiveness of their efflux pumps. Thioridazine has been shown to inhibit the expression and activity of these efflux pumps [54-56]. Our next step was therefore to determine whether TZR, +TZ, and –TZ differed in their effectivity for inhibiting these pumps.
The efflux inhibition assay test strain SA-1199B showed MICs of 50 mg/L for all test compounds. Efflux inhibition assays revealed IC50 values of 9, 9, and 5 µM against SA-1199B for TZR, +TZ, and –TZ, respectively (data not shown). These values correspond to concentrations of 3.7, 3.7, and 2.0 mg/L, respectively, and revealed that NorA efflux pump inhibition occurs at concentrations of less than 10% of the respective MICs as reflected by IC50/MIC ratios of 0.07, 0.07, and 0.04, respectively. –TZ is slightly more potent than both TZR and +TZ in inhibiting the NorA efflux pump.
Since all antipsychotic drugs bind to the dopamine receptor D2, the final question we addressed was whether –TZ and +TZ bound equally to this receptor. Membranes were prepared from COS7 cells expressing Dopamine Receptor 2 (D2R) and their binding affinity measured by competitive binding with [3H]-Raclopride.
The affinity of –TZ was measured to 38.8 nM (SE ± 6.3) and that of +TZ to 11.3 nM (SE ± 3.7) to the human D2 receptor. Thus –TZ has a 3-fold lower affinity for the D2 receptor than +TZ (P = 0.0015). –TZ would therefore be expected to have a weaker antipsychotic side-effect.
Discussion
Multiple drug resistance is a serious and growing clinical problem. Non-antibiotics like Thioridazine (TZR) have the potential to sensitize antibiotic resistant bacteria and ‘re-activate’ existing antibiotics. Despite this, they are not currently widely used.
TZR was withdrawn from the market because of an estimated risk of sudden death due to QT prolongation of approximately 5-8 events in 10,000 cases (a doubling of the occurrence of this in the healthy population). In contrast, there are several reports indicating the value of TZR for treating Tuberculosis (TB) [57-60]. The potential benefit of using TZR as a treatment for TB may be as high as curing 11 out of 17 patients [61].
Considering that: 1.8 million died of TB and a further 242,000 died of multidrug-resistant or extensively drug-resistant TB [25] globally in 2015 and that MRSA treatment cost billions of dollars alone in the USA [26,27], there is a pressing need to elucidate the clinical utility of TZR.
The experiments presented in this manuscript show that combinations of TZR and–TZ together with DCX are equally effective. In other words, selecting the –TZ enantiomer does not compromise its activity. In addition we demonstrate that –TZ binds with lower affinity to the dopamine 2 receptor, indicating that this formulation might provide therapeutic benefit with reduced side effects. This strengthens the potential for future application of combined treatment using –TZ and classical antibiotics. These are important arguments needed to make an informed decision as to whether to use –TZ as a non-antibiotic against MRSA (and other microorganisms) in the clinic.
In the following discussion we therefore consider three different aspects of TZR and –TZ which are unclear from the literature. These are: 1) the apparent discrepancies relating to TZR; 2) our current knowledge of the mode of action of TZR and –TZ; and 3) pharmacokinetic aspects of TZR and –TZ.
Discrepancies relating to TZR concentrations
Our group has previously shown that TZR increases the antibiotic susceptibility of MRSA and MSSA strains [15,31,32]. The estimated MICs in the paper presented by Radhakrishnan et al. [20] are much higher (up to 800 mg/L) for some of the S. aureus strains and in contrast to data presented here (MIC of 64 mg/L). The explanation for this might lie in a technical issue. In the Radhakrishnan study, TZR was mixed with molten agar (50ᴼC) which may have caused its chemical breakdown because phenothiazines are thermolabile and can only withstand temperatures up to 40ᴼC.
We have previously investigated the in vitro antimicrobial activity of racemic TZR in both sensitive and resistant staphylococci [15,31,32,62,63]. In the current study, we have confirmed that –TZ has the same potent antimicrobial activity as the racemate on a spectrum of S. aureus strains exhibiting very different levels of drug resistance (Table 1). The dose-response curves in the viability assays showed that –TZ had a slightly better antimicrobial effect than TZR at concentrations greater than 20 mg/L, (Figure 3). Unexpectedly, S. aureus strain 51726 was the most sensitive in the synergy/reversal of resistance assay. This strain was isolated from a clinical case of an infection in man from the pig clone CC398. The significance of this is unknown, but could be due to genetic differences between this and other CC clones, as seen previously [32]. With a view to reducing nosocomial bloodstream infections, catheters were loaded with DCX alone or in combination with TZR. While pigs demonstrated severe toxicity towards the normal doses of DCX / TZR combination (possibly due to drug-drug interactions), reduced doses resulted in a significant decrease in the frequency of MSSA infections [64].
Mode of action of TZR
TZR was claimed to potentiate the effect of DCX in all test isolates comprising both sensitive and resistant staphylococci [32,52], indicating that the mechanism is independent of the resistance phenotype or expression of virulence genes [62]. Recent data suggests that TZR exerts its effect by weakening the cell wall. TZR has been shown to cause major changes in expression of many genes involved in peptidoglycan biosynthesis [63]. Muropeptide analysis of the MRSA strain USA300 showed that the peptidoglycan was depleted for glycine indicating that TZR could interfere with the formation of the pentaglycine branches and lead to a dysfunctional cell wall. In addition, insertion of phenothiazines into the interfacial region of membranes leads to significant membrane expansion [65]. The induced splaying of the diacyl chains of phospholipids would result in a change in its physical properties, possibly interfering with efflux pump activity [38].
Efflux pump inhibition data revealed that all compounds tested (TZR, +TZ, and –TZ) were relatively similar in their ability to interfere with NorA function. The fact that 50% pump inhibition occurred at concentrations well below the MIC (IC50/MIC ratios of less than 0.1) suggests that this activity is not directly related to antibacterial effects. Exposure of SA-1199B to a 30 µM concentration of the related compound prochlorperazine resulted in a modest depolarization of the cell membrane [9]. However, the IC50 for prochlorperazine against SA-1199B was only 9 µM. While these data suggest that direct pump inhibition is probable, it remains possible that indirect effects such as membrane depolarization or other changes in membrane functionality may contribute to the inhibition of pump activity.
Pharmacokinetic aspects of TZR and –TZ
The achievable plasma concentration of TZR in humans is only 0.5 mg/L: hence its potency as an adjuvant drug against MRSA has been questioned [66]. However, it has been demonstrated that human macrophages concentrate the drug up to 100 times thus providing a possibility for its clinical utility [57,58,67-69,70]. Considerably higher up-concentrations may occur in the cell membranes due to the differential lipophilicity of TZR in different phospholipids [38]. Therefore, TZR may reach the concentrations within phagocytic cells that are required for it to function as an adjuvant drug to DCX. This has already been seen with XMDR TB [71]. Macrophages have not shown any toxic responses. Liver toxicity and other pharmacokinetic parameters could be determined in vitro using human in vivo mimetic 3D hepatocyte cultures [72].
The usual dosage of TZR used to treat schizophrenia was 10 – 75 mg per day for mild cases, and up to 600 – 800 mg per day for severely disturbed patients. Several studies have demonstrated the in vivo potential of TZR and –TZ for treating not only MRSA but also S. typhimurium and TB. They illustrate that a clinically achievable dose can result in a significant improved survival rate [32,69,73].
A murine model of latent TB has been used to demonstrate that thioridazine treatment, in combination with isoniazid, can achieve complete clearance of the bacillus as early as 24 weeks [74].
Abbate et al. [61] performed a clinical intervention study on TB patients based on the off-label use of TZR in combination with linezolid and/or moxifloxacin. In that study, TZR was initially administered at a daily dose of 25 mg; thereafter the dose was increased by 25 mg weekly until it reached 200 mg/day. The drug was only used on inpatients, initially under strict cardiac monitoring in order to safeguard against eventual cardiac adverse events. After the end of the study 11 out of 17 patients met the cure criteria.
We demonstrate here that –TZ (JEK47) has a significant antimicrobial activity. This is similar to the racemic TZR and in some situations more potent. Taken together with the diminished or excluded side effects (reduced cardiotoxic effect and lower dopamine 2 receptor affinity) and the preferential concentration of the –TZ enantiomer in human tissue, this strengthens the case for implementing –TZ/antibiotic combined treatment strategies to combat antibiotic resistance. The microorganisms targeted need not only be MRSA but should also include S. typhimurium and TB [30,40,51,75,76]. It thus appears that, as with ‘methylene blue’ (one of the first phenothiazines identified) [61,77], the story of these derivatives has turned full circle and we are again standing in the clinic with a potentially valuable antimicrobial agent [12,78].
Author disclosure statement
The authors JEK, OH, and JBC are inventors on the patent on –TZ (known as JEK47) (WO 2005/046694 - Thioridazine and derivatives thereof for reversing anti-microbial drug-resistance).
Acknowledgements
The authors wish to thank Cristian Pablo Pennisi, Johannes Jan Struijk, Oliver Hendricks and Hans Jørn Kolmos for help with solutions to practical and theoretical problems and for constructive comments on the manuscript. We would also like to thank members of the COST action 1407 “Challenging organic syntheses inspired by nature - from natural products chemistry to drug discovery” for fruitful discussions.
References
- Himmelweit F. The collected papers of paul ehrlich: In four volumes including a complete bibliography. Elsevier Science: 1956.
- Kristiansen JE. Antimicrobial activity of non-antibiotics. ASM News. 1991; 57: 135-139.
- Stenger M, Hendel K, Bollen P, Licht PB, Kolmos HJ, Klitgaard JK. Assessments of thioridazine as a helper compound to dicloxacillin against methicillin-resistant Staphylococcus aureus: In vivo trials in a mouse peritonitis model. PLoS One. 2015; 10: e0135571. https://goo.gl/n5cCVb
- Zimmermann P, Curtis N. Antimicrobial effects of antipyretics. Antimicrob Agents Chemother. 2017; 61: e02268-2316. https://goo.gl/SWLmqp
- Bueno J. Antimicrobial adjuvants drug discovery, the challenge of avoid the resistance and recover the susceptibility of multidrug-resistant strains. J Microb Biochem Technol. 2016; 8: 169-176. https://goo.gl/YHfyj5
- Macedo D, Filho AJMC, Soares de Sousa CN, Quevedo J, Barichello T, Junior HVN, et al. Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J Affect Disord. 2017; 208: 22-32. https://goo.gl/fR54rZ
- Musuka S1, Srivastava S, Siyambalapitiyage Dona CW, Meek C, Leff R, Pasipanodya J, et al. Thioridazine pharmacokinetic-pharmacodynamic parameters "wobble" during treatment of tuberculosis: a theoretical basis for shorter-duration curative monotherapy with congeners. Antimicrob Agents Chemother. 2013; 57: 5870-5877. https://goo.gl/qgupu4
- Kristiansen JEH, Fey SJ. The accepted “clinical interaction model”: a special case of reality. Journal of Bioequivalence & Bioavailability. 2017; 9: 418-423. https://goo.gl/4Fcfo4
- Kaatz GW, Moudgal VV, Seo SM, Kristiansen JE. Phenothiazines and thioxanthenes inhibit multidrug efflux pump activity in staphylococcus aureus. Antimicrob Agents Chemother. 2003; 47: 719-726. https://goo.gl/BMLKy3
- Kristiansen JE, Hendricks O, Delvin T, Butterworth TS, Aagaard L, Christensen JB, et al. Reversal of resistance in microorganisms by help of non-antibiotics. J Antimicrob Chemother. 2007; 59: 1271-1279. https://goo.gl/iGzZxH
- Nigam A, Gupta D, Sharma A. Treatment of infectious disease: beyond antibiotics. Microbiol Res. 2014; 169: 643-651. https://goo.gl/2ddBhD
- Kristiansen JE. Dyes, antipsychotic drugs, and antimicrobial activity. Fragments of a development, with special reference to the influence of paul ehrlich. Dan Med Bull. 1989; 36: 178-185. https://goo.gl/hpYhtK
- Kristiansen JE. The antimicrobial activity of psychotherapeutic drugs and stereo-isomeric analogues. Dan Med Bull. 1990; 37: 165-182. https://goo.gl/pGKqZ8
- Bender AB, Kristiansen JE. Antimicrobial effects of anesthetics and analgesics. Ugeskr Laeger. 1999; 161: 5814-5817. https://goo.gl/vRYJRS
- Hendricks O. Antimicrobial effects of selected non-antibiotics on sensitivity and invasion of gram-positive bacteria. Faculty of Health Sciences. University of Southern Denmark. 2006. https://goo.gl/EM13fi
- Jensen AS. Cardiac action potential prolongation induced by isolated thioridazine enantiomers. Aalborg University. Denmark. 2014.
- Amaral L, Kristiansen JE, Abebe LS, Millett W. Inhibition of the respiration of multi-drug resistant clinical isolates of mycobacterium tuberculosis by thioridazine: Potential use for initial therapy of freshly diagnosed tuberculosis. J Antimicrob Chemother. 1996; 38: 1049-1053. https://goo.gl/ujWY5Z
- Bourlioux P, Moreaux JM, Su WJ, Boureau H. In vitro antimicrobial activity of 18 phenothiazine derivatives: Structure-activity relationship. APMIS Suppl. 1992; 30: 40-43. https://goo.gl/XeT1bA
- Dutta NK, Pinn ML, Karakousis PC. Reduced emergence of isoniazid resistance with concurrent use of thioridazine against acute murine tuberculosis. Antimicrob Agents Chemother. 2014, 58, 4048-4053. https://goo.gl/p2CKwg
- Radhakrishnan V, Ganguly K, Ganguly M, Dastidar SG, Chakrabarty AN. Potentiality of tricyclic compound thioridazine as an effective antibacterial and antiplasmid agent. Indian J Exp Biol. 1999; 37: 671-675. https://goo.gl/adzYWc
- Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SH. Thioridazine and sudden unexplained death in psychiatric in-patients. Br J Psychiatry. 2002; 180: 515-522. https://goo.gl/c9qoeD
- Thanacoody HK. Thioridazine: resurrection as an antimicrobial agent? Br J Clin Pharmacol. 2007; 64: 566-574. https://goo.gl/wCEy4M
- Thanacoody HK. Thioridazine: The good and the bad. Recent patents on anti-infective drug discovery.2011; 6: 92-98.
- Glassman AH, Bigger JT Jr. Antipsychotic drugs: prolonged qTc interval, torsade de pointes, and sudden death. Am J Psychiatry. 2001; 158: 1774-1782. https://goo.gl/3697Xs
- Anderson L, Dias HM, Falzon D, Floyd K, Baena IG, Gilpin C, et al. Global tuberculosis report2016. WHO Press: New York. 2016.
- Gould IM. Costs of hospital-acquired Methicillin-Resistant Staphylococcus aureus (MRSA) and its control. Int J Antimicrob Agents. 2006; 28: 379-384. https://goo.gl/2BnD2P
- Suaya JA, Mera RM, Cassidy A, O'Hara P, Amrine-Madsen H, Burstin S, et al. Incidence and cost of hospitalizations associated with Staphylococcus aureus skin and soft tissue infections in the united states from 2001 through 2009. BMC Infect Dis. 2014; 14: 296. https://goo.gl/Yqrybx
- Beloeil PA, Guerra B, Stoicescu AV, Mulligan K, Riolo F, Nagy K, et al. The european union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2015. EFSA Journal. 2017; 15: 1-212. https://goo.gl/WJTnxp
- Hendricks O, Molnar A, Butterworth TS, Butaye P, Kolmos HJ, Christensen JB, et al. In vitro activity of phenothiazine derivatives in Enterococcus faecalis and Enterococcus faecium. Basic Clin Pharmacol Toxicol. 2005; 96: 33-36. https://goo.gl/fK1wvK
- Hendricks O, Poulsen MØ, Christensen JB, Kristiansen JE. Antibacterial synergy between jek 47 and oxacillin in a murine model of mrsa. SEEC, Slovenia. 2014. https://goo.gl/TtjrGF
- Klitgaard JK, Skov MN, Kallipolitis BH, Kolmos HJ. Reversal of methicillin resistance in Staphylococcus aureus by thioridazine. J Antimicrob Chemother.2008; 62: 1215-1221. https://goo.gl/bLExdG
- Poulsen MØ, Jacobsen K, Thorsing M, Kristensen NR, Clasen J, Lillebæk EM, et al. Thioridazine potentiates the effect of a beta-lactam antibiotic against Staphylococcus aureus independently of meca expression. Res Microbiol. 2013; 164: 181-188. https://goo.gl/LyDxKE
- Jensen AS, Pennisi CP, Sevcencu C, Christensen JB, Kristiansen JE, Struijk JJ. Differential effects of thioridazine enantiomers on action potential duration in rabbit papillary muscle. Eur J Pharmacol. 2015; 747: 7-12. https://goo.gl/th1H1S
- Jensen AS, Pennisi CP, Sevcencu C, Christensen JB, Kristiansen JE, Struijk JJ. In Model-based analysis of the effects of thioridazine enantiomers on the rabbit papillary action potential. 2015 Computing in Cardiology Conference (CinC). 2015; 1089-1092.
- Jortani SA, Valentour JC, Poklis A. Thioridazine enantiomers in human tissues. Forensic Sci Int. 1994; 64: 165-170. https://goo.gl/YR9pww
- Svendsen CN, Froimowitz M, Hrbek C, Campbell A, Kula N, Baldessarini RJ, et al. Receptor affinity, neurochemistry and behavioral characteristics of the enantiomers of thioridazine: Evidence for different stereoselectivities at d1 and d2 receptors in rat brain. Neuropharmacology. 1988; 27: 1117-1124. https://goo.gl/1Ly5Xq
- Getinet A. First generation antipsychotics: Pharmacokinetics, pharmacodynamics, therapeutic effects and side effects: a review. Research & Reviews: Journal of Chemistry. 2016; 5: 53-63. https://goo.gl/9MHwyr
- Keyzer H, Fey SJ, Thornton B, Kristiansen JE. Molar ratios of therapeutic water-soluble phenothiazine center dot water-insoluble phospholipid adducts reveal a fibonacci correlation and a putative link for structure-activity relationships. Rsc Advances 2015; 5: 20865-20877. https://goo.gl/Km5DjH
- Plenge P, Shi L, Beuming T, Te J, Newman AH, Weinstein H, et al. Steric hindrance mutagenesis in the conserved extracellular vestibule impedes allosteric binding of antidepressants to the serotonin transporter. J Biol Chem 2012; 287: 39316-39326. https://goo.gl/Yw7Ufi
- Christensen JB, Hendricks O, Chaki S, Mukherjee S, Das A, Pal TK, et al. A comparative analysis of In vitro and In vivo efficacies of the enantiomers of thioridazine and its racemate. PLoS One. 2013; 8: e57493. https://goo.gl/ovQ8VE
- Duthie ES, Lorenz LL. Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol. 1952; 6: 95-107. https://goo.gl/VUr4YK
- de Lencastre H, Tomasz A. Reassessment of the number of auxiliary genes essential for expression of high-level methicillin resistance in staphylococcus aureus. Antimicrob Agents Chemother. 1994; 38: 2590-2598. https://goo.gl/5rh7oZ
- Ruhlmann CH, Kolmos HJ, Kristiansen JE, Skov R. Pigs as an infection source for methicillin resistant Staphylococcus aureus infections in humans. Ugeskr Laeger. 2008; 170: 3436.
- Kaatz GW, Seo SM, Ruble CA. Efflux-mediated fluoroquinolone resistance in Staphylococcus-aureus Antimicrob Agents Chemother. 1993; 37: 1086-1094. https://goo.gl/JUho3P
- Bourquin JP, Schwarb G, Gamboni G, Fischer R, Ruesch L, Guldimann S, Theus V, Schenker E, Renz J, Synthesen auf dem phenothiazin-gebiet .2. N-substituierte mercaptophenothiazin-derivate. Helvetica Chimica Acta.1958; 41: 1072-1108.
- Pascu ML, Smarandache A, Boni M, Kristiansen JE, Nastasa V, Andrei IR. Spectral properties of some molecular solutions. Romanian Reports in Physics. 2011; 63: 1267-1284. https://goo.gl/BfBUp7
- Smarandache A, Kristiansen J, Christensen JB, Pascu ML. Optical studies of the spectral properties of phenothiazines. Lett Drug Des Discov 2012; 9: 352-360. https://goo.gl/9grrsp
- Robert TE. Enantiomeric influences in neuroleptic binding to models of the dopamine receptor. 1979. https://goo.gl/mu3UG8
- Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (mic) of antimicrobial substances. Nat Protoc. 2008; 3: 163-175. https://goo.gl/Rmpqmh
- Kaatz GW, Seo SM, O Brien L, Wahiduzzaman M, Foster TJ. Evidence for the existence of a multidrug efflux transporter distinct from nora in Staphylococcus aureus. Antimicrob Agents Chemother. 2000; 44: 1404-1406. https://goo.gl/dG267g
- Poulsen MO, Scholer L, Nielsen A, Skov MN, Kolmos HJ, Kallipolitis BH, et al. Combination therapy with thioridazine and dicloxacillin combats meticillin-resistant Staphylococcus aureus infection in caenorhabditis elegans. J Med Microbiol. 2014; 63: 1174-1180. https://goo.gl/j8Tx81
- Rasmussen KS, Poulsen MO, Jacobsen K, Skov MN, Kolmos HJ, Kallipolitis BH, et al. Combination of thioridazine and dicloxacillin as a possible treatment strategy of staphylococci. New Microbiol. 2017; 40: 146-147. https://goo.gl/4XQBfv
- Eap CB, Souche A, Koeb L, Baumann P. Light-induced racemization: Artifacts in the analysis of the diastereoisomeric pairs of thioridazine 5-sulfoxide in the plasma and urine of patients treated with thioridazine. Ther Drug Monit. 1991; 13: 356-362. https://goo.gl/icBqSa
- Kristiansen MM, Leandro C, Ordway D, Martins M, Viveiros M, Pacheco T, et al. Thioridazine reduces resistance of methicillin-resistant staphylococcus aureus by inhibiting a reserpine-sensitive efflux pump. In vivo (Athens, Greece) 2006; 20: 361-366. https://goo.gl/bPF6qJ
- Amaral L, Viveiros M. Why thioridazine in combination with antibiotics cures extensively drug-resistant mycobacterium tuberculosis infections. Int J Antimicrob Agents. 2012; 39: 376-380. https://goo.gl/q2TCzh
- Song L, Wu X. Development of efflux pump inhibitors in antituberculosis therapy. Int J Antimicrob Agents. 2016; 47: 421-429. https://goo.gl/LMkUeu
- Kristiansen JE, Vergmann B. The antibacterial effect of selected phenothiazines and thioxanthenes on slow-growing mycobacteria. Acta Pathol Microbiol Immunol Scand B. 1986; 94: 393-398. https://goo.gl/y9isMc
- Crowle AJ, Douvas GS, May MH. Chlorpromazine: A drug potentially useful for treating mycobacterial infections. Chemotherapy. 1992; 38: 410-419. https://goo.gl/YHvskH
- Amaral L, Boeree MJ, Gillespie SH, Udwadia ZF, van Soolingen D. Thioridazine cures extensively drug-resistant tuberculosis (xdr-tb) and the need for global trials is now. Int J Antimicrob Agents. 2010; 35: 524-526. https://goo.gl/4EnwDN
- Hadji nejad S, Rahbar M, Mehrgan, H. Synergy between phenothiazines and oxacillin against clinical isolates of methicillin-resistant staphylococcus aureus. Trop J Pharm Res. 2010; 9: 243-249. https://goo.gl/rPJN3e
- Abbate E, Vescovo M, Natiello M, Cufré M, García A, Gonzalez Montaner P, et al. Successful alternative treatment of extensively drug-resistant tuberculosis in argentina with a combination of linezolid, moxifloxacin and thioridazine. J Antimicrob Chemother. 2012; 67: 473-477. https://goo.gl/NeNvSn
- Bonde M, Hojland DH, Kolmos HJ, Kallipolitis BH, Klitgaard JK. Thioridazine affects transcription of genes involved in cell wall biosynthesis in methicillin-resistant staphylococcus aureus. FEMS Microbiol Lett. 2011; 318: 168-176. https://goo.gl/nvvP48
- Thorsing M, Klitgaard JK, Atilano ML, Skov MN, Kolmos HJ, Filipe SR, et al. Thioridazine induces major changes in global gene expression and cell wall composition in methicillin-resistant Staphylococcus aureus USA 300. PLoS One. 2013; 8: 64518. https://goo.gl/noS18r
- Stenger M, Birkholm Gronnemose R, Klein K, Kolmos H J, Alm M, Andersen T E. A hydrogel interpenetrating polymer network in vascular catheters loaded with thioridazine and dicloxacillin facilitates slow surface release and inhibits staphylococcal biofilm formation In vitro and In vivo. https://goo.gl/7eEWCK
- Kopec W, Khandelia H. Reinforcing the membrane-mediated mechanism of action of the anti-tuberculosis candidate drug thioridazine with molecular simulations. J Comput Aided Mol Des. 2014; 28: 123-134. https://goo.gl/Ux7zRA
- Ordway D, Viveiros M, Leandro C, Arroz MJ, Amaral L. Intracellular activity of clinical concentrations of phenothiazines including thioridiazine against phagocytosed staphylococcus aureus. Int J Antimicrob Agents. 2002; 20: 34-43. https://goo.gl/9Soq8Z
- Forrest F M, Forrest I S, Roizin L. Clinical, biochemical and post mortem studies on a patient treated with chlorpromazine. Agressologie: revue internationale de physio-biologie et de pharmacologie appliquees aux effets de l'agression. 1963; 4: 259-265.
- Gardos G, Lassen U V, Pape L. Effect of antihistamines and chlorpromazine on the calcium-induced hyperpolarization of the amphiuma red cell membrane. Biochim Biophys Acta. 1976; 448: 599-606. https://goo.gl/z52kKb
- Martins M, Bleiss W, Marko A, Ordway D, Viveiros M, Leandro C, et al. Clinical concentrations of thioridazine enhance the killing of intracellular methicillin-resistant staphylococcus aureus: An In vivo, ex vivo and electron microscopy study. In vivo. 2004; 18: 787-794. https://goo.gl/AmwjoM
- Deshpande D, Srivastava S, Musuka S, Gumbo T. Thioridazine as chemotherapy for mycobacterium avium complex diseases. Antimicrob Agents Chemother. 2016; 60: 4652-4658. https://goo.gl/5ZBm5u
- Leonard Amaral, Joseph Molnar. Why and how the old neuroleptic thioridazine cures the xdr-tb patient. Pharmaceuticals (Basel). 2012; 5: 1021-1031. https://goo.gl/kyJyVZ
- Stephen J Fey, Krzysztof Wrzesinski. Determination of drug toxicity using 3D spheroids constructed from an immortal human hepatocyte cell line. Toxicol Sci. 2012; 127: 403-411. https://goo.gl/c4UEmv
- Dasgupta A, Mukherjee S, Chaki S, Dastidar SG, Hendricks O, Christensen JB, et al. Thioridazine protects the mouse from a virulent infection by salmonella enterica serovar typhimurium 74. Int J Antimicrob Agents. 2010; 35: 174-176. https://goo.gl/jKPVrL
- Singh, A.; Sharma, S. Chemotherapeutic efficacy of thioridazine as an adjunct drug in a murine model of latent tuberculosis. Tuberculosis (Edinb). 2014; 94: 695-700. https://goo.gl/uxyttY
- Kristiansen JE, Dastidar SG, Palchoudhuri S, Roy DS, Das S, Hendricks O, et al.Phenothiazines as a solution for multidrug resistant tuberculosis: From the origin to present. Int Microbiol. 2015; 18: 1-12. https://goo.gl/Za1Dzn
- Amaral L, Viveiros M. Thioridazine: A non-antibiotic drug highly effective, in combination with first line anti-tuberculosis drugs, against any form of antibiotic resistance of mycobacterium tuberculosis due to its multi-mechanisms of action. Antibiotics (Basel). 2017; 6. https://goo.gl/qpasws
- Schirmer RH, Adler H, Pickhardt M, Mandelkow E. Lest we forget you-methylene blue. Neurobiol Aging. 2011; 32: 2325. https://goo.gl/dZ6Qci
- Kristiansen J E. Are chlorpromazine and other phenothiazines also antibiotics. Ugeskr Laeger. 1981; 143: 1900-1904. https://goo.gl/qfxjbE
Authors submit all Proposals and manuscripts via Electronic Form!