Case Report
Refractory Postoperative Hypoxaemia Secondary to Pulmonary Leukostasis Syndrome. Can we think about this?
Ana Belen Fernandez1* and Marina Sanchez2
1Department of Anesthesiology, Intensive Care and Pain Treatment, Nuestra Sra de Candelaria University Hospital, Carretera del Rosario n Tenerife, Canary Island, Spain
2Resident Anesthesiology 4th, Spain
*Address for Correspondence: Ana Belen Fernandez, Department of Anesthesiology, Intensive Care and Pain Treatment, Nuestra Sra de Candelaria University Hospital, Santa Cruz of Tenerife, Canary Island, Spain, Tel: 0034-922-019-154/655-612-534; E-mail: anabfp@gmail.com
Dates: Submitted: 25 September 2017; Approved: 18 October 2017; Published: 21 October 2017
Citation this article: Fernandez AB, Sanchez M. Refractory Postoperative Hypoxaemia Secondary to Pulmonary Leukostasis Syndrome. Can we think about this? Am J Anesth Clin Res. 2017;3(2): 031-033.
Copyright: © 2017 Fernandez AB, er al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Pulmonary Leukostasis (PL); Acute Myeloid Leukaemia (AML); Respiratory Failure; Hypoxemia; Leukaemic Cells
Abstract
The incidence of Acute Myeloid Leukaemia is about 4 new cases/100,000 habitants per year, and only 10%-20% of patients present with hyperleukocytosis. 30%-40% of hyperleucocytic patients develop clinical signs of brain or pulmonary leukostasis, resulting from the obstruction of capillary vessels by leukaemic cells. Early recognition of leukostasis syndrome is of great importance since leucapheresis may improve short-term outcome. Our case is an AML debut that presents with hypoxemia and progressive respiratory failure, in early postsurgical period of corpo-caudal pancreatectomy, demonstrating the need to have this diagnosis in our minds to start treatment as soon as possible.
Introduction
Pulmonary Leukostasis (PL) occurs in patients with acute myeloid leukaemia and rapidly increasing white blood cell count and is consistently present when the count exceeds 100,000/mL. About 30%-40% of hyperleukocytic patients develop clinical signs of brain or PL, resulting from the obstruction of capillary vessels by leukemic cells. Overall the presence of hyperleukocytosis carries a poor prognosis with early mortality rates reaching 20%-30% at day 28. In retrospective clinicopathological studies on the causes of death in patients with Acute Myeloid Leukaemia (AML) and myeloproliferative disease, PL was found in 40% of the patients [1].
In patients with myelomonocytic or monocytic Acute Leukaemia (AML), leukaemia related pulmonary involvement is frecuent and severe, that include pulmonary leukostasis, leukaemic pulmonary infiltrates and lysis pneumopathy [1].
The management of acute hyperleukocytosis and leukostasis involves supportive measures and reducing the number of circulating leukemic blast cells, with careful monitoring of fluid balance, control of uric acid production and control of urine pH to prevent tumour lysis syndrome [2-5]. The high number of leukocytes may cause 3 main complications: Disseminated Intravascular Coagulation (DIC), Tumour Lysis Syndrome (TLS) and leukostasis.
Description
We experienced a rare case of PL secondary to debut AML in a patient with chronic myelomonocytic leukaemia without any CNS symptoms, in early postsurgical period of corpo-caudal pancreatectomy plus splenectomy for pancreatic tumour.
A 75-year-old woman with a history of essential hypertension and moderate aortic stenosis with auricular fibrilation was admitted at our Postsurgical Intensive Care Unit (PICU) after open corporocaudal pancreatectomy plus splenectomy for pancreatic tumour, laparoscopic surgery could not be performed by progressive desaturation with increased pressure and difficult management to mechanical ventilation.
Usual medications: bisoprolol, ramipril and acenocoumarol warfarin. Non-smoker patient.
In the last six months she had developed recurrent respiratory infections and pneumonia treated with amoxicillin-clavulanic acid and levofloxacin respectively. A fiberoptic bronchoscopy was performed with normal oropharyngeal flora.
Pre-operative echocardiography was stable, chest X-ray and laboratory tests were normal. No lymphadenopathy nor organomegaly. Oxygen saturation in surgery room was 92%.
Surgery time were five hours and blood transfusion is not performed. The patient arrives at PICU in spontaneous ventilation but poor ventilatory mechanics and accessory muscle use. Pulse oximetry reading was 91% despite oxygen 10L/min by facemask. Non-invasive ventilation was started with aceptable clinical result, however two days later the patient´s condition deteriorated with respiratory distress and severe hypoxia (oxygen saturation 55% and reservoir mask) requiring urgent endotracheal intubation and mechanical ventilation. Chest X-ray demonstrated bilateral diffuse alveolo-interstitial pulmonary infiltrates (Figures 1,2). The patient develops shock requiring norepinephrine moderate doses. CT showed no bleeding. A bronchoalveolar lavage for microbiological diagnosis was performed and an empiric antibiotic- antifungal therapy were added (meropenem, levofloxacin, linezolid and anidulafungin).
 Figure 1: The radiograph (A) on 2th day of admission to the PICU, showed diffuse bilateral alveolo-interstitial infiltrate.
Figure 1: The radiograph (A) on 2th day of admission to the PICU, showed diffuse bilateral alveolo-interstitial infiltrate.
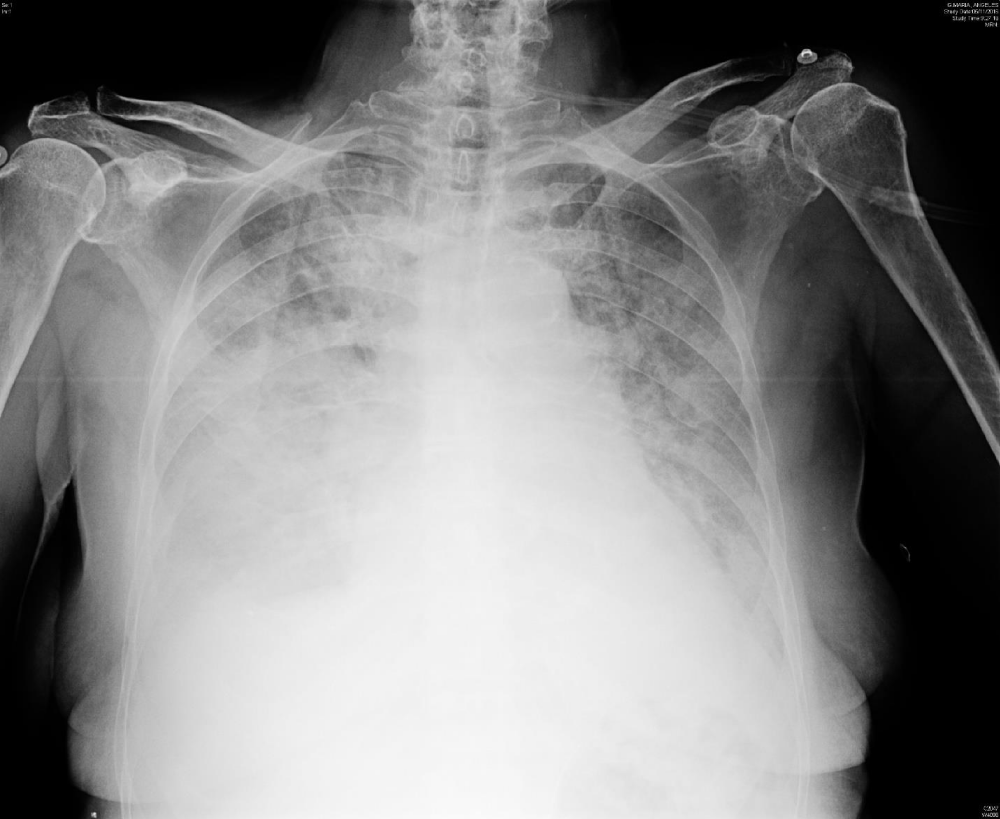 Figure 2: The radiograph (B) on 5th day of admission to the PICU, showed clear deterioration with respect to the A image after 4 days of mechanical ventilation: right pleural effusion and diffuse bilateral interstitial and airspace opacities.
Figure 2: The radiograph (B) on 5th day of admission to the PICU, showed clear deterioration with respect to the A image after 4 days of mechanical ventilation: right pleural effusion and diffuse bilateral interstitial and airspace opacities.
Laboratory tests showed an increasing leucocytosis (WCC 87000/mL), anaemia (haemoglobin 7,6 gr/dL), thrombocytopaenia (55,000 /microL), lactate dehydrogenase 1081 U/L (normal 135-214 U/L), creatinine 1,50 mg/dL (normal 0,60-1,00 mg/dL) and urea 120 mg/dL (normal 10-50 mg/dL). Microbiological studies were negative for pulmonary infection. She received two units of blood.
Hematology diagnosed AML secondary to probable asymptomatic myelomonocytic leukemia (monocytes 52390 /mL) with poor evolution after splenectomy.
In our case, the patient does not received polychemotherapy or leukapheresis due to poor vital prognosis, so we started with hydroxycarbamide, despite the high risk of tumour lysis syndrome.
Through low doses of sedation and an early -effective respiratory physiotherapy, she was extubated on the 5th day of her admission, but the patient´s condition deteriorated progressively in the next following hours developed irreversible hypoxaemia with a serious hemodynamic failure. Severe leukocytosis 97240/mL with monocytes 55400 /mL. The patient died on the 6th day of admission to the PICU.
Discussion
Although AML is the most frequent acute leukemia in adults, its incidence is about 4 new cases/ 100,000 inhabitants per year, and only 10%-20% of patients present with hyperleukocytosis [6].
In patients with AML, hyperleukocytosis is more common in patients with myelomonocytic leukaemia, monocytic leukaemia or the microgranular variant of acute promyelocytic leukaemia and should be as a medical emergency.
Leukaemic pulmonary infiltrates occur in patients with or without hyperleukocytosis [7]. The lung leukemic infiltration may cause hypoxaemia and clinical symptoms related to the vascular obstruction by leukemic cells and blast invasion of the interstitium and alveolar spaces, respectively [8], but their clinical and radiographic manifestations are difficult to distinguish from those of nonleukemic complications such as pneumonia or pulmonary edema, which may coexist. Pneumonia and acute pulmonar emboli may also contribute to acute respiratory failure, with infections accounting for up to one-third of early acute respiratory events in newly diagnosed AML patients [9]. In this situation, progression to acute respiratory failure requiring ventilatory support is a severe complication that is not only fatal [10], but also delays the administration of optimal chemotherapy.
Despite characteristic clinical presentations, the diagnosis of leukostasis is rarely made with high confidence. The main goal of the management of hyperleukocytosis and/or leukostasis in AML is to achieve effective cytoreduction as soon as possible with leukoapheresis and/or hydroxyurea [11].
Our patient debuted with AML in the perioperative period and after splenectomy, with no previous clinical data on leukemia, severe hypoxaemic respiratory failure, multiorgan failure and death in a few days. In our opinion, this is an important diagnosis that we must keep in mind, especially if it coexists with hyperleukocytosis, since it may be the first clinical manifestation of an acute leukemia.
References
- Kuo KH, Callum JL, Panzarella T, Jacks LM, Brandwein J, Crump M, et al. A retrospective observational study of leicoreductive strategies to manage patients with acute myeloid leukaemia presenting with hyperleukocytosis. Br J Haematol. 2015; 168: 384-394. https://goo.gl/vnSeHQ
- Porcu P, Cripe LD, Ng EW, Bhatia S, Danielson CM, Orazi A, et al. Hyperleukocytic leukemias and leukostasis: a review of pathophysiology, clinical presentation and management. Leuk Lymphoma. 2000; 39:1-18. https://goo.gl/d3P57x
- Awad C, Parikh R, Fardous Y. Pulmonary leucostasis in a patient with chronic lymphocytic leukaemia. BMJ Case Rep. 2015; 2015. https://goo.gl/3zQjT2
- Rico Rodriguez J, Villanueva Ortiz A, Santana Cabrera L, Rodriguez Perez H. Pulmonary leukostasis with severe respiratory impairment as a debut of acute myeloid leukemia. Int J Crit Illn Inj Sci. 2015; 5: 125-126. https://goo.gl/MeF74U
- Trof JR, Schaafsma R, Beishuizen B. Pulmonary Leucostasis Syndrome presented by unilateral pulmonary infiltrates. BMJ Case Rep. 2014; 2014. https://goo.gl/BWP5ir
- Albert-Coll M, Minguez Gallego C, Burgues Gasion O. Pulmonary leukostasis as a first manifestation of an acute myeloid leukemia without hyperleukocytosis. Med Clin (Barc). 2006; 127:437-8. https://goo.gl/ScseBd
- Rollig C, Ehninger G. How I treat hyperleukocytosis in acute myeloid leukemia. Blood. 2015; 125: 3246-52. https://goo.gl/mZ47B2
- Azoulay E, Canet E, Raffoux E, Lengline E, Lemiale V, Vincent F, et al. Dexamethasone in patients with acute lung injury from acute monocytic leukaemia. Eur Respir J. 2012; 39: 648-653.
- Chaoui D, Legrand O, Roche N, Cornet M, Lefebvre A, Peffault de Latour R, et al. Incidence and prognosis value of respiratory events in acute leukemia. Leukemia. 2004; 18: 670-675. https://goo.gl/cy199z
- Azoulay E, Thiery G, Chevret S, Moreau D, Darmon M, Bergeron A, et al. The prognosis of acute respiratory failure in critically ill cancer patients. Medicine (Baltimore). 2004; 83: 360-370. https://goo.gl/WDEMps
- Ganzel C, Becker J, Mintz PD, Lazarus HM, Rowe JM. Hyperleukocytosis, leukostasis and leukapheresis: practice management. Blood Rev. 2012; 26: 117-122. https://goo.gl/41wxEE
Authors submit all Proposals and manuscripts via Electronic Form!




























