Associated with the Time of Exposure of Peripheral Intravenous Devices Risk of Developing Phlebitis: A Prospective Cohort Study at a Latin American University Hospital?
Arenas Villamizar Angel Ricardo1*, Velasquez Serna Diana Lorena2 and Leon Giraldo Hoover3
1General Practitioner and Surgeon from Surcolombian University, Clinical Epidemiology obtained at University of the Border, Chile
2Professional Nurse Graduated from Andean Area University Foundation, Specialist in Health Management, Graduated from Javeriana University
3Statistician, Department of Self-Assessment and Academic Quality - DACA Academic Vice-Rector’s Office University of the Valley
*Address for Correspondence: Angel Ricardo Arenas Villamizar, Clinical Epidemiology, Medical Critical Patients Unit, Clinica Indisa, Santiago de Chile, Tel: +56987988420; E-mail: arenasvangelr@gmail.com
Submitted: 11 September 2016; Approved: 16 October 2017; Published: 20 October 2017
Citation this article: Angel Ricardo AV, Diana Lorena VS, Hoover LG. Associated with the Time of Exposure of Peripheral Intravenous Devices Risk of Developing Phlebitis: A Prospective Cohort Study at a Latin American University Hospital. Adv J Vasc Med. 2017;2(1): 020-025.
Copyright: © 2017 Angel Ricardo AV, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Phlebitis; Catheterization; Vascular Access Devices; Polyurethanes; Device Medical; Bandages
Download Fulltext PDF
Introduction: The Center for Disease Control CDC recommends the replacement of peripheral intravenous catheter (IV) every 72 up to 96 hours. Routine replacement is thought to reduce the risk of phlebitis and bacteremia; however multiple studies have not shown a significant difference of such practice.
Objective: To evaluate the risk of phlebitis in patients with catheters change every 72 hours Vs changes under indications of phlebitis and determine the factors that contribute to the development of phlebitis.
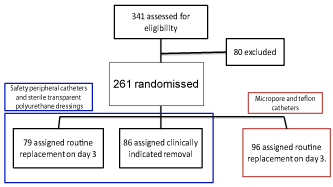
Methodology: A three-arm (n = 341) prospective cohorts study was proposed, in which patients to this study were randomized into three groups ,for the first group of patients the catheter was routinely taken off every 72 hours, in the second group the catheter was only removed in light of phlebitis signs. The third group kept the current regular conditions of routinely changes used in catheters and dressings.
Results: The study included a total of 261 patients that meet the inclusion criteria, from which 14% presented mechanical phlebitis (n = 28), 80% chemical phlebitis (n = 29). 67% of the cases of phlebitis appeared in the second arm of study (n = 24). Infective phlebitis was not documented in any of the three groups. The patients on whom the device was used for more than 72 hours have a 3 times higher probability to develop chemical phlebitis (RR = 2.92, CI: 1.35 - 6.33). The ratio catheter per patient was superior in the use of catheters made of teflon of 3.2 catheters per patient.
Conclusion: No cases of infectious phlebitis occurred in the study groups related this finding with adequate hand washing and strict supervision during the time of study techniques. The time of removal of catheters every 72 hours did not evidence a reduction of infectious phlebitis higher than expected by proper hand washing techniques.
Introduction
The Center for Disease Control CDC recommends the replacement of peripheral intravenous catheters (IV) every 72 up to 96 hours. The routinely replacement is thought to reduce the risk of phlebitis and bacteremia. The catheter insertion is an unpleasant experience for patients and the replacement of it might not be necessary if the catheter remains functional and if the patient does not show inflammation signs. The costs associated with the routinely replacement can be considerable [1]. This routinely replacement increases the costs of health care and the workload for the staff and it also requires that the patients would also undergo repeated invasive procedures. The effectiveness of such practice is not well established. Phlebitis occurred in 7% of the cases of patients when intravenous catheters were removed at the moment that was clinically advised and also when they were normally removed every 3 days. The absolute difference was small (0, 41%) and within the pre-established equivalence margin of 3%, 2 other studies have shown similar results [3-7].
The use of transparent dressings in polyurethane that replace the traditional dressing gauzes, have avoided the interruption of intravenous treatments and the lack of differences in phlebitis or infiltration rates in both types of bandages, transparent dressings constitute the preferred solution instead of gauze dressings in spots of insertion of peripheral venous catheters insertion [8-9].
Even if studies about the use of transparent dressings in central catheters has not shown a significant reduction in phlebitis and bacteremia cases and it is established that additional studies are required [10], what is clear is that there is a significant reduction of the costs associated to the routinely replacement every 72 hours [11].
Materials and Methods
A prospective cohorts study was carried out from the 15th of July up to the 25th of February in a University Hospital of Cali Colombia. Three arms of study were formed, in the first two arms of the study safety polyurethane catheters OcrilonR and transparent sterile dressings in polyurethane TegadermR, (Figure 1 and 2) were used, by following the institutional protocols of peripheral vein catheterization, for the infusion of intravenous liquids and/or medications.
Every patient that entered into the study was admitted through the emergency room service and they were directed to a same hospital service in which the entire staff was previously trained and evaluated in asepsis and antiseptic techniques, such as hand wash, protocol in peripheral catheterization by using safety peripheral catheters OcrilonR and sterile transparent dressings in polyurethane TegadermR, in order to avoid biases due to the inadequate manipulation of the supplies used. Patients were catheterized with catheters of 16, 18, 20 or 22 by a same group of medical assistants for 24 hours per day and they were also previously trained.
Inclusion Criteria
The patients that require a peripheral intravenous of intravenous liquids and/or medication. Patients with hospitalization criteria in the services of Internal Medicine and Surgery.
Exclusion Criteria
1. The patients that enter with adverse events caused in other medical institutions.
2. The patients that belong to different services.
3. Patients that re-enter within a period of 8 days and for the same cause.
5. Patients who suffer from conditions that may render difficult the access to their veins (patients suffering from edema, patients that are undergoing chemotherapy, or patients who suffer from acute malnutrition) or also the patients whom may need more than one catheterization shot.
6. The patients whom may not authorize their participation in the study.
A simple randomization was performed reason why the group the people in charge of receiving patients did not know which arm they were going to catheterize in patients. Patients signed an informed consent which was included in the investigation protocol and approved by the Ethics and Investigation Committee from the University Clinic Rafael Uribe Uribe in Cali Colombia.
A code going from 001 up 399 was assigned to every catheterization kit, where two groups’ were established pair group number and an odd number group. In the group of pair numbers codes there were the patients in which Arm I: patients that were exposed (Routine replacement every 72h, ocrilonR – TegadermR). Arm II: (Clinically indicated removal, ocrilonR – TegadermR) Patients in which catheters were marked with an odd number code were the patients that were NOT exposed and starting from the code 242 the data of Arm III (Routine replacement every 72h, device Conventional, Teflon) was entered (Figure 3).Up to the time of completion of the three arms of study each arm was constituted by 100 patients.
Patient follow up was executed by an independent group of observers different than main investigators in order to avoid measurement biases and it was also executed by applying the follow up protocol that was previously established.
A number of 295 patients a size sample was calculated for an average of 1250 monthly hospital discharges, with a trust level of 95% and margin of error inferior to 5%.
The ones that were considered as exposed patients were those to whom the catheter was replaced every 72 hours as institutional protocol.
The patients that were considered as non-exposed were those patients to whom the peripheral intravenous catheters were removed only if there was a clinical evidence of phlebitis or 10 days after in accordance with technical recommendation of the manufacturer.
The follow up was made for a period of time of seven months with a sample of 341 patients in which 80 patients were excluded.
The data collection was made through a check list used for phlebitis detection, by evaluating the following parameters:
1. Does not show any phlebitis sign.
2. Pain without inflammation erythema, induration or palpable venous cord.
3. Pain with erythema and inflammation without induration and no palpable venous cord.
4. Pain with erythema and inflammation, induration, palpable venous cord inferior to 3 cm.
5. Pain with erythema and inflammation, induration, palpable venous cord greater than 3 cm.
6. Full blown Stata version 11.0 (College Station, Texas, venous thrombosis and all the other signs are present.
Patients that belonged to the III Arm were catheterized with Teflon catheters as it is made in routinely conditions and by fixing the catheter with Micropore tape (Figure 4, 5).
Data was analyzed statistically in the software USA. Initially a descriptive analysis of the studied cohort was made, in which the quantitative variables are expressed through averages and standard deviation, in addition to the median and the range in accordance to it frequency distribution. Categorical variables were presented in proportions.
The different comparisons that were made through the Chi square test or through Fisher test as the case may be, for the case of categorical variables, in the case of quantitative variables the test of t.
Student was used, when the normality assumption the analysis was made through the Mann Whitney test, comparisons among experimental and the conventional groups, were made through variance analysis – ANOVA altogether with a post- hoc anova Bonferroni test. Associations were made through out the estimation of Relative Risk (RR) with its corresponding trust interval of 95%, significant differences were considered with a value of p < 0.05.
Results
A total follow up of 319 patients was made, out of which 261 patients were included in the study. The causes of exclusion of patients for the analysis were the loss in the follow up or their transfer to intensive or middle care units, or the beginning of intake of medications that cause chemical phlebitis.
The findings in the distribution per gender were that 55.6% were of male gender and 44.4% were of female gender. The average age was of 62 years old (DE ± 19) and the median age was of 67 years old in a range of 15 to 105 years old. A little more than half of the patients (56%) are of male gender; in average patients were followed up for 5,7 days (DE ± 5). 81.6% of patients were captured through access by the emergency service (Table 3).
Woman had an average age of 66 years old, while the average age in men was of 59 years old, this difference was statistically significant (p = 0.0044). (Table3)
In table 3 we can see that 67% of the patients that presented phlebitis belonged to the second group of study. Out of these 83% (n=20) corresponded to chemical phlebitis.
89.2% of patients did not have phlebitis, on the contrary in the patients in those that presented phlebitis there were 23 patients that presented one case of phlebitis (8.8%), 4 patients that had two cases of phlebitis and only one patient that had 5 cases of phlebitis during the period of study, there were no significant differences per gender.
In relation with the quantity of catheters used per each patient, approximatively 43% used one catheter, four patients used more than 10 catheters in the study (11 and 13 catheters), this distribution was similar per gender, on the contrary it was observed that the patients that had phlebitis had in average more catheters (3,5) by comparing them with the patients that did not have phlebitis (2,2), the said difference was statistically significant (p = 0.0015).
It was observed that the total number of patients that suffer from phlebitis (n = 23) 60.87% of the patients who had permanently the dressing and the safety catheter, while l 39,13% of the patients to whom the replacement of the catheter was made every 72 hours developed phlebitis.
The RR estimated showed an association between developing phlebitis that is related with the time of use and replacement of the security device and the transparent dressing, in this association it is established that the patients to whom the device is leaved on more than 72 horas have a 3 higher probability of developing phlebitis in comparison with those patients to whom the device is replaced every 72 hours (RR = 2.92), this association was statistically significant (1.35–6.33).
By taking into account the attributable fraction related to the exposure, it is observed that when by avoiding to leave the devices on, for more than 72 hours, 65.7 % is the maximum risk reduction to develop chemical phlebitis in the exposure groups that were related with the time of placement and not with the use of devices.
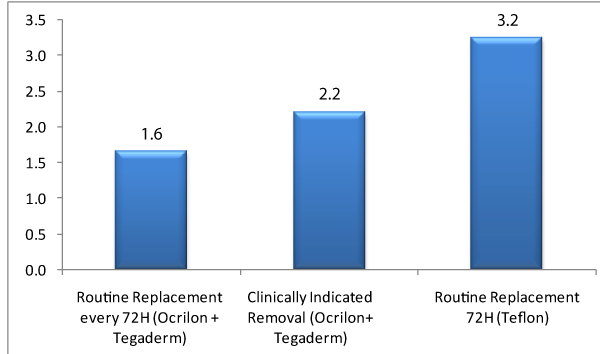
The average of catheters used was notoriously greater with the use of routine replacement 72H (Teflon) in comparison with routine replacement every 72H (Ocrilon + Tegaderm) in the other exposure groups, using an average of 3 conventional catheters in (Teflon) per patient in comparison to 2,2 and 1,6 catheters per patient in groups in which catheters were only clinically indicated removal and day sand routine replacement every 72H (Table 7 and Graph 2).
Variance analysis between averages of catheters according to the group assigned
By taking into account every patient that was included initially in the study, differences between the three groups were observed in relation with the average number of catheters (P < 0.000) (Table 8).
Bartlett’s test for equal variances: chi2 (2) = 118.0706 Prob > chi2 = 0.000 A post –anova analysis was applied in order to verify in which groups the said differences appeared; according to the results the conventional group had an average difference of 1.6 catheters relation with the 72 hour group (p < 0.000) and in relation to the clinically indicated removal group there was an average difference of 1.1 catheter. (P < 0.000).
As for the intervention costs, the average cost per patient and considering replacements every 72 hours with polyurethane catheters OcrilonR and sterile transparent dressings TegadermR which were used in the group of study, the average cost is of $3,01 American dollars per patient in comparison with $2,88 dollars with conventional catheters which allows to highlight the result of an inferior number of catheters per patient, and also an inferior number of punctures by using these devices, better follow up conditions by using transparent dressings and also an inferior risk of infection.
The projection of conventional catheters which would be used when taking them off within 10 days would be of 10 catheters per patient by doubling care costs.
In the Arms follow up 1 and 2 in which safety polyurethane catheters OcrilonR biological accidents did not appear during the follow up months, which strikes us besides the cost antiretroviral treatments, are the medical, social and the life style implications of officers that suffer a biological accident due to neddlesticks injuries or venipuncture devices.
Discussion
The campaigns of previous hand wash at the begging of the study, showed a maximum effectiveness within the three groups of study a part from the devices used in the procedure of peripheral catheterization, at the time of not showing any infectious phlebitis during the follow up period.
In relation with the catheter time of exposure, we found that the patients to which the catheter is left on for more than 72 hours have a 3 higher probability of developing Chemical phlebitis in comparison with the patients to whom the catheter is replaced every 72 hours (RR = 2.92), This association was statistically significant (1.35 – 6.33), more related with the time of exposure to the medication instead than to the materials used. In our case the exposure time was associated with an increased incidence of chemical and non-infectious phlebitis, which does not rule out the benefit of a less invasive to the patient to whom the change is made only with clinical evidence of phlebitis [12-14].
There was a greater evidence of effectiveness regarding the use of safety intravenous catheters OcrilonR and the peripheral transparent dressing TegadermR in comparison to the conventional catheter Teflon, as far as the relation per patient was of 3.2 catheters /patient in comparison even with the patients which had a greater time of exposure of up to 10 days this relation was of 2.2 catheters/patients, what reduces the intervention costs, as less invasive processes in the patient.
Conclusions
The time of removal of catheters every 72 hours did not evidence a reduction of infectious phlebitis higher than expected by proper hand washing techniques. The use of appropriate hand washing technique and venipuncture reduces the incidence of infectious phlebitis, so the withdrawal of venipuncture devices only with clinical evidence of phlebitis is recommended but emphasizing adequate dilution and infusion of routine antibiotics, since their mismanagement increase the likelihood of chemical phlebitis. Transparent dressings allow better supervision of patients. Evidenced greater efficiency in the use of catheters ocrilon Vs Teflon.
The investment in catheters in polyurethane and sterile transparent dressings is justified in terms of costs, as there is an inferior invasiveness on the patient, and better supervision and follow up conditions, as there are less infection risks fact that leads to the reduction of costs per stay days and also to the reduction of secondary complications.
Acknowledgments
Cobo & Asociados S.A.S. Importer for Colombia safety catheters Jelco Protectiv Plus de Smiths medical Intl. 3M Colombia S.A Lina del Mar Zea Candelo. Professional nurse. Cheif of Ob-Gyn service at Clínica Universitaria Rafael Uribe Uribe Jose Alberto Vásquez. Nursery Auxiliary. Emergency Room Service. Clínica Universitaria Rafael Uribe Uribe. For the Princess at all times motivated my inspiration.
- Webster J, Osborne S, Rickard C, Hall J. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2010; 17: 7798. https://goo.gl/QtihMK
- Rickard CM, Webster J, Wallis MC, Marsh N, McGrail MR, French V, et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012; 22: 380: 1066-1074. https://goo.gl/2ieWN5
- Van Donk P, Rickard CM, McGrail MR, Doolan G. Routine replacement versus clinical monitoring of peripheral intravenous catheters in a regional hospital in the home program: A randomized controlled trial. Infect Control Hosp Epidemiol. 2009; 30: 915-917. https://goo.gl/J2Pqkb
- Oishi LA. The necessity of routinely replacing peripheral intravenous catheters in hospitalized children. A review of the literature. J Intraven Nurs. 2001; 24: 174-179. https://goo.gl/rRjmD6
- Rickard CM, McCann D, Munnings J, McGrail MR. Routine resite of peripheral intravenous devices every 3 days did not reduce complications compared with clinically indicated resite: a randomized controlled trial. BMC Med. 2010; 8: 53. https://goo.gl/1p6UWp
- Webster J, Clarke S, Paterson D, Hutton A, van Dyk S, Gale C, et al. Routine care of peripheral intravenous catheters versus clinically indicated replacement: randomised controlled trial. BMJ. 2008; 337: 339. https://goo.gl/zaGUB3
- Gillies D, O'Riordan L, Wallen M, Rankin K, Morrison A, Nagy S. Timing of intravenous administration set changes: a systematic review. Infect Control Hosp Epidemiol. 2004; 25: 240-250. https://goo.gl/ufe9xc
- Tripepi-Bova KA, Woods KD, Loach MC. A comparison of transparent polyurethane and dry gauze dressings for peripheral i.v. catheter sites: rates of phlebitis, infiltration, and dislodgment by patients. Am J Crit Care. 1997; 6: 377-381. https://goo.gl/4BJ4tB
- Maki DG, Ringer M. Evaluation of dressing regimens for prevention of infection with peripheral intravenous catheters. Gauze, a transparent polyurethane dressing, and an iodophor-transparent dressing. JAMA. 1987; 258: 2396-2403. https://goo.gl/NdHBjR
- Gillies D, O'Riordan E, Carr D, O'Brien I, Frost J, Gunning R. Central venous catheter dressings: a systematic review. J Adv Nurs. 2003; 44: 623-632. https://goo.gl/S49jt3
- Tuffaha HW, Rickard CM, Webster J, Marsh N, Gordon L, Wallis M, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014; 12: 51-58. https://goo.gl/2MYVdp
- Claire M Rickard, Joan Webster, Marianne C Wallis, Nicole Marsh, Matthew R McGrail, Venessa French et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012; 380: 1066-1074. https://goo.gl/2Xaax8
- Webster J, Lloyd S, Hopkins T, Osborne S, Yaxley M. Developing a Research base for Intravenous Peripheral cannula re-sites (DRIP trial). A randomised controlled trial of hospital in-patients. Int J Nurs Stud. 2007; 44: 664–671. https://goo.gl/biVnwt
- C Ferrete-Morales, M A Vazquez Perez, M Sanchez Berna, I Gilabert Cerro, J E Corzo-Delgado, J A Pineda-Vergara et al. Incidence of secondary phlebitis by peripheral venous catheter and impact of a management protocol. Enferm Clin. 2010; 20: 3–9. https://goo.gl/mrg34y



Sign up for Article Alerts