Cytotoxicity of Roots Methanolic Extract of Maytenus senegalensis
Fatoumata Bah1, Pascale Marie Aimée Dozolme2, Mathilde Cabral1, Aminata Touré1, Absa Lam1, Théophile Amondo Mobio2, Edmond Ekoue Creppy2, Seck Matar3, Mamadou Fall1* and Serge Maria Moukha2
1Laboratoire de Toxicologie et Hydrologie, Faculte de Medecine, de Pharmacie et Odontologie, Universite Cheikh Anta Diop de Dakar, Senegal, B.P. 5005 Dakar-Fann
2Laboratoire de Toxicologie-INRA, UFR des Sciences Pharmaceutiques-Universite de Bordeaux – INRA 146, rue Leo Saignat - case 8833076 Bordeaux Cedex
3Laboratoire de Chimie Organique, Faculte de Medecine, de Pharmacie et Odontologie, Universite Cheikh Anta Diop de Dakar, Senegal, B.P. 5005 Dakar-Fann
*Address for Correspondence: Mamadou Fall, Laboratoire de Toxicologie et Hydrologie, Faculte de Medecine, de Pharmacie et Odontologie, Universite Cheikh Anta Diop de Dakar, Senegal, B.P. 5005 Dakar-Fann, E-mail: mamadou3.fall@ucad.edu.sn
Submitted: 26 February 2020; Approved: 26 May 2020; Published: 28 May 2020
Citation this article: Bah F, Aimée Dozolme PM, Cabral M, Touré A, Lam A, et al. Cytotoxicity of Roots Methanolic Extract of Maytenus senegalensis. Adv J Toxicol Curr Res. 2020;4(1): 011-016.
Copyright: © 2020 Bah F, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
The aim of this study was to evaluate toxicity of a methanolic root fraction of Maytenus senegalensis, a medicinal plant commonly used in senegalese pharmacopoeia. Toxicological tests were conducted by In vitro assay. Thus, cell viability was performed by MTT, Neutral Red and LDH activity assays on Caco-2 and HepG2 cell lines and IC50 were determined. Apoptotic activity was evaluated by electrophoretic migration of the DNA extract to determine a potential fragmentation. Results showed that the extract was cytotoxic ones whatever the cell type used, at IC50 < 40 µg/mL for MTT and LDH. With Neutral Red, IC50 was between 60-80 µg/mL DNA fragmentation has also been observed, indicating the involvement of apoptosis in the cell death mechanism. The date will be supplemented by an in vivo evaluation for a better appreciation of the safety of use of M. senegalensis.
Introduction
Medicinal plants have been used for centuries throughout the world. The fact is that plants being natural and people think that they no negative effect. They do not take into account the harmful consequences that could be linked to dosage or duration of therapy. Recently, study showed that methanolic extracts of M. senegalensis (Celastraceae) have In vitro anti-sickle-cell properties at 5 mg/mL [1].
Celastraceae is a family of plants including Maytenus senegalensis, widely distributed around the world and used in traditional medicine. It includes more than 90 genera with more than 1,200 recognized species [2]. Maytenus senegalensis is a plant species localized and widespread in Saharan and sub-Saharan Africa. Different parts of the plant are widely used in traditional medicine for the treatment of infectious and inflammatory disease [3,4]. It is a small thorny tree with several active substances like triterpenes, sterols, saponosids, alkaloids and tannins which have been identified, particularly in the roots [5,6]. Many of these substances have biological activities which were confirmed in pharmacology assays.
Extracts of M. senegalensis are said to have anti-inflammatory, antioxidant, anti-plasmodial, anti-leishmanial and anti-bacterial activities In vitro [7-10)]. Some composites are believed to have anti-carcinogenic properties but with high toxicity [11,12]. In Senegal, M. senegalensis is one of the most important medicinal plants; its roots especially, are purchased in the markets of traditional medicines to cure various diseases in the population [4,11]. The local population uses this plant against malaria, fever, chest pains, rheumatism, dysmenorrhea, diarrhea, dyspepsia eye infections, wounds, and snake bites. Its leaves are used for asthma, cough, and as remedy for sores on the tongue [4]. The same traditional uses of M. senegalensis are also reported from other African countries namely Benin, Ivory Coast, Kenya, Tanzania, Sudan, Zambia, and Zimbabwe [11].
With regard to toxicity, only a few studies have been described in the literature [11,13,14] despite the widespread use of this plant for many diseases. The present study was undertaken to provide additional data on cytotoxicity and apoptotic activities of crude methanolic extract of M. senegalensis by MTT and Neutral red tests, LDH activity but also to study DNA-fragmentation.
Materials and Methods
Chemicals and reagents
MTT reagent (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide); Neutral Red (NR), sterile cell culture media: Dulbecco’s Modified Eagle’s Medium (DMEM) and Minimum Essential Medium (MEM); Dimethyl sulfoxid (DMSO), Fetal Bovine Serum (FBS) were purchased from Sigma-Aldrich (USA). The Lactate Dehydrogenase (LDH) Cytotoxicity Assay Kit was obtained from Promega (USA). Methanol and other chemicals used in this study were analytical grade and were purchased from VWR Prolabo Chemicals (UK).
Cells lines and culture conditions
Cells were obtained from the American Type Culture Collection (ATCC). Caco-2 was cultured in DMEM and HepG2 in MEM. Mediums were supplemented of 10% fetal bovine serum (Sigma, France), 2% L-glutamine (8 mM) (Sigma, France), 1% penicillin-streptomycin stock solution (10,000 units of penicillin and 10mg of streptomycin in 0.9% NaCl) (Sigma, France) and 1% Neomycin (250 μg/ml) (Sigma, France), 1% Non-Essential Amino Acid (NEAA) for MEM. All cell lines were cultured at 37°C in a humidified incubator with 5% CO2 atmosphere. The experiments were carried out during the exponential phase of cell growth.
Plant and preparation of extract
Roots of Maytenus senegalensis were collected from a natural habitat in Sangalkam, Dakar, Senegal. The plants were identified at the Plant Biology Laboratory of the Faculty of Medicine and Pharmacy of Cheikh Anta Diop University in Dakar, Senegal. Roots were washed and shade- dried for five (5) days and pulverized using a plant mill. A double extraction by maceration. For this, the roots powder weighing 200 g was dissolved in 1.5 liter of methanol in a 3-liter stoppered container, for a period of 72 hours with agitation at room temperature. The resulting mixtures were filtered using cotton wool, then Whatman filter paper (Size No1). The extraction and filtration procedures were repeated with further 750 ml of methanol. The resulting methanol filtrate was concentrated to dryness in-vacuo using an evaporator and the resulting powder was kept in an air–tight container and refrigerated.
In vitro assays procedure
The cytotoxicity was determined by measurement of cell viability employing an MTT, NR assay and LDH activity determination. Apoptotic activity was evaluated by the DNA-fragmentation study on agarose gel.
Preparation of extract stock solution: Stock solution of 1 mg/ml was prepared by dissolving extract in 5% of methanol in free medium which served as the vehicle. From this stock solution, series of dilutions in free medium of 5, 10, 20, 40, 80 and 160 µg/ml for MTT and NR assays; 5, 10, 20, 40 and 100 µg/mL for LDH activity were prepared.
Evaluation of cell viability:
MTT assay: MTT test was performed using MTT assay as described as previously by Mosmann [15]. As described, about 1×106 ml-1 cells (Caco-2 and HepG2) in their exponential growth phase were seeded in a flat-bottomed 96-well plate at a concentration of 2-3×104 cells/well in 100 μl culture medium and were incubated for 24 h at 37°C in 5% CO2 humidified incubator. After incubation, the medium was replaced by extract at desired concentrations for 48h. The supernatant was removed, the cells were washed once using PBS, and an MTT solution of 100 µl of MTT at a final concentration 1mg/ml was added to each well. The plates were incubated for 45 min in order to form formazan crystals which are then re-dissolved in 200 µl of DMSO. After 5-10 min of incubation at 37°C, cell viability was detected by measuring the Optical Density (OD) at the absorbance of 545 nm using LT-4000 Labtech Microplate Readers.
Neutral red assay: Neutral Red test was performed using assay as described as previously [16]. Caco2 and HepG2 cells were seeded in a 96 well plate, at a rate of 1.5-2×104 cells per well. The cells were then incubated for 24 hours at 37°C in 5% CO2 humidified incubator. The spent media was removed and the cells were washed with PBS then exposed at different concentrations of extract. The plates were then incubated for 48 h at 37°C in a humidified incubator with a 5% CO2. After the time of incubation, the supernatant was removed, the cells were washed with PBS then 100µl of 1% neutral red at 3.3 g/L in free medium were added to each well and the plates were further incubated for 4 hours. Liquid solution was removed from the wells by flipping the plates, and 150 μl of elution medium (EtOH 50% diluted in water/Ac-COOH; 99/1) into each well followed by gentle shaking for 10 min to obtain complete dissolution. Absorbance was read at 545 nm using a LT-4000 Labtech Microplate.
LDH activity assay: LDH activity assay PROMEGA- kit. Released LDH in culture supernatants is measured with a 30-min coupled enzymatic assay, which results in conversion of a tetrazolium salt into a red formazan product. The amount of colored product formed is proportional to the number of lysed cells in culture supernatants. This assay measures the activity release by cytoplasmic Lactate Dehydrogenase (LDH) from damaged cells. The cells were prepared, as described previously, and then incubated in various concentrations of extract for 48 hours. After time of exposure, the plates were centrifuged 5 min at room temperature using a Thermo Scientific Sorvall ST-16R centrifuge. The supernatant was collected then incubated with reaction mixture to measure the released LDH. Test was performed according to the recommendations of the manufacturer and quantification was performed using a LT-4000 Labtech Microplate at 450 nm.
Assessment of DNA damage (DNA fragmentation) in agarose gel: 3-5x105 cells per well were incubated in 6 flat-wells plate. After 24 hours incubation, the culture medium was removed from each well and plant extracts at concentrations of 10; 40; and 100 µg/ml were added in duplicated well. Control has been carried out with culture medium containing 5% methanol. After 24h incubation, the liquid was removed, and cells were washed with 5ml PBS. After addition of 3 ml SDS lysis buffer (in 100 mM Tris-HCl pH 8.0; 50 mM EDTA; 1% SDS) (plate placed on ice), 3 μl RNase (> 6 µg/mL final concentration) was added and mixed by pipetting up and down. After incubation time of 10 min at RT, 30 μl of proteinase K (10mg/ml) were added then the supernatant was collected in 1.5 ml Eppendorf tube, incubated 1h at 37°C, and carefully shaked each 10 min.
After centrifugation at 25,000 g for 10 min, at 20°C, DNA was extracted with phenol/chloroform/isoamyl alcohol (25/24/1). DNA was then centrifuged at > 6000 g for 5 mn at 20°C, before being precipitated in two equivalent volumes of isopropyl alcohol. After 1h at -20°C, DNA was pelleted (> 6000 g, 45mn, 4°C), then washed with ethanol 70°C, dried and resuspended in 50-200 µl of deionized water depending on DNA concentration. Purity of DNA was measured by absorbance read at 260nm and 280nm (1U of OD260 = 50 µg/ml of double-stranded DNA). DNA fragments were electrophoretically separated on a 2% (w/v) agarose gel containing 1μg/ml ethidium bromide and visualize by ultraviolet transillumination.
Statistical analysis
All experiments were performed three times in triplicate (except as mentioned in the text specifically) and data was statistically analyzed with GraphPad prism 6 software. The Mean Standard Errors (SEM) and Standard Deviation (SD) of the samples were calculated. Analysis of Variance (one-way ANOVA) followed by multiple comparison assays (two-sided Dunnett test) was performed to determine significant differences between exposed or non-exposed cells (using a p ≤ 0.05). The IC50 values were then calculated for each experiment.
Each result represents the mean viability + Standard Deviation (SD) as described in M&M. Cell viability was calculated as a percentage of the untreated viable cells.
Results
Cell viability
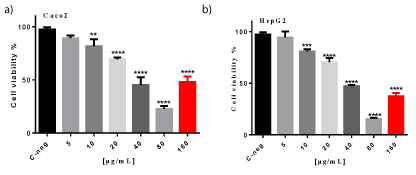
The results of cell viability (48h) are shown in figure 1 (a) (b); figure 2 (a) (b) and figure 3 (a) (b) respectively, for MTT assay, Neutral Red assay, and LDH activity on Caco2 (a) and HepG2 (b) cell lines.
MTT assay: Results of MTT method, show a significant reduction in cell viability depending on concentration up to 80 µg/mL .Similar results were found with Caco2 and HepG-2 cell line (Figure 1a and figure 1b).Therefore, results found with the MTT assay demonstrate that the crude extract from M. senegalensis roots is toxic from 10 µg/mL for both cell line viability with an IC50 of 29.07 µg/mL and 39.40 µg/mL for Caco2 and HepG2, respectively. Although there is a slight difference for IC50, but is not statistically significant. Thus, cytotoxicity of extract showed no cell type selectivity.
Neutral red assay: Figure 2a and 2b show the effects of M. senegalensis extract on the cell viability, as measured by Neutral red test. Cells were affected by the extract from 80µg/mL and the IC50 was estimated at 60.95 µg/mL and 75.24 µg/mL for Caco2 and HepG2 respectively. Overall cell viability decreased with increasing concentrations up to 80µg/mL, as previously observed. In contrast, Neutral Red assay gives higher IC50 with the cell viability that can be observed at a concentration up to 75 µg/mL.
LDH assay: LDH activity determination in cells (Caco-2 and HepG2) treated by extract for 48 hours showed similar results in both cell lines with a toxicity from 10 µg/mL The IC50 are 25.38 µg/mL (Figure 3b) and 24.40 µg/mL (Figure 3b) for Caco-2 and HepG2, respectively. The cytotoxicity was concentration dependent and cell type independent. This result is similar to those shown in MTT test.
The table 1 shown IC50 obtained with MTT, Neutral Red and LDH assays.
DNA damage
DNA fragmentation assay shows typical pattern of apoptosis DNA fragmentation (Figure 4). Control cell DNA appeared unfragmented, whereas DNA fragments size from 200 kb could be seen with incrementation of 200 bp, in both cell lines Caco-2 and HepG2, exposed with 10 µg/mL and 100 µg/mL methanolic extracts, respectively.
Discussion
In a previous work [10], Maytenus senegalensis roots was successively extracted with methanol, n-hexane, and ethyl acetate. The corresponding extracts were further screened to reduce In vitro formation of sickle-shaped red blood cells and the methanolic extract was shown to be the most effective.
In this study, following incubation with methanolic root extract, the cytotoxicity and apoptotic activity on the HepG2 and Caco-2 cells lines have been evaluated using cell proliferation, cell membrane integrity, and DNA fragmentation assays.
Cells lines were exposed for 48 hours to increasing concentrations of extract in a range from [1µg mL-1 to 1000 mg mL-1], but for convenience we reported results ranging from [5 µg. mL-1 to 100 or 160 mg mL-1].
At each series of concentrations, there were significant cytotoxicity effects is produced starting from 10µg/mL The cell viability results indicate that M. senegalensis roots are toxic to all of the cells lines tested and globally is not cell line dependent as it can be found in literature.
Results of cytotoxic cell assay, based with different methods, shows very small variation among MTT, and LDH assays. The IC50 found were similar with lower values for HepG2 cells. HepG2 cell appear to be relatively more sensitive than Caco-2 cell line. In contrast, Neutral Red assay results were different, Caco-2 cells appear relatively more sensitive and the IC50 higher than tests mentioned above. In comparison to MTT and LDH methods, these results seem to suggest that the Neutral Red method would be much less sensitive. It may be that the dosing principles are different.
The results clearly point at mitochondria as a target of the M. senegalensis extract on the two cell lines HepG2 and Caco-2 in approximately the same range (HepG2 IC50 =29.07 µg/mL and Caco-2 IC50 = 39.40 µg/mL).
Neutral Red is a dye that stains lysosomes in viable cells that can take up neutral red via active transport and incorporate the dye into their lysosomes. Neutral Red assay point at mechanisms that perturb the accumulation of the dye in lysosomal compartment, because non-viable cells cannot absorb this chromophore [16,17]. As for MTT, both cell lines are equally affected and in the same range by the methanolic extract (IC50 = 75.24 µg/mL and IC50 = 60.95 µg/mL respectively). In our experiments, the active processes for the trafficking uptake and accumulation of the dye seem to be stopped in most of the cells. This is partially occurring by cell lysis (necroptosis) mechanism as seen by LDH release.
The IC50 which represents the concentration of xenobiotics responsible for the 50% decrease in cell viability is a parameter conventionally used in cellular toxicology. The results presented in these studies did reveal 50% decrease in the viability (IC50) from 24 µg/mL to 40µg/mL for MTT and LDH test, respectively and 60 µg/mL to 76 µg/ml for RN. Crude methanolic roots extract of M. senegalensis which was tested showed IC50 more or less high, suggesting moderate cytotoxicity. These results are consistent with previous investigations performed with work of Nabende [18] which showed that the methanol extract from the stem bark of M. senegalensis had moderately high toxicity against breast cancer (4T1) cell lines. Investigation in Kenya has further revealed that, some isolated compounds of Maytenus extract showed cytotoxicity on Vero E6 cells at IC50 = 37.5 ng/mL a for others at IC50 > 39.52 μg/mL [8]. However, an earlier study in Tanzania found that extract of root of M. senegalensis have high cytotoxic activity [14]. Furthmore, maytansine compound isolated from the Maytenus. genus is known for its marked cytotoxic effect [11].
The pathway of cell death by apoptosis was also determined by DNA fragmentation assay which is employed to identify a possible apoptotic effect at different concentrations for 24 hours. The results showed clearly an apoptotic effect at 10 and 100 µg/mL, suggesting that extract induces apoptosis on Caco-2 and HepG2 cell lines. At low concentration of extract, apoptosis (Figure 4) seems to be abundant. Then with the different concentrations of M. senegalensis extract, it appears that apoptosis decreases drastically while the release of LDH, showing the necroptosis, is now very important. This suggests that with the concentration M. senegalensis extract the cell lysis increases (necroptosis and by consequence the release of pro-inflammatory molecules) and outweighs the apoptosis, this further strongly suggests that the extract preparation becomes much more toxic for cells with higher concentration.
The toxicity of roots of M. senegalensis although very used in traditional medicine, is not well reported in the literature. It is known that the roots are widely used in Senegal, but the scientific evidence is not clear enough to establish their safety. Furthermore, a recent study focused on the effect of the methanolic extract of M. senegalensis roots on the reversibility of sickling revealed that the best result (77% in 120 min) was found from 10 mg/ml [1]. This dose, supposed to be the pharmacological one, is well above the toxic doses for our cells. Hence in vivo studies must be conducted which could give us more information about the toxicity.
Conclusion
The main purpose of this study is to identify the potential toxicity of M. senegalensis root extract in an In vitro system, and to provide additional information for safe use. The study has shown concentration-dependent cytotoxicity up to 80µg/mL. Then, there is an increase in cell viability which can be explained by the fact that the substance is naturally colored or by possible compensatory cellular proliferation in response to cytotoxicity. In addition, the cytotoxicity was observed at doses below the pharmacological doses found. However, these results are not enough; they have to be complemented given the interesting pharmacological activities of this plant and his use by population. Although our studies provide information on the cytotoxicity of the extract and part of the mechanism of toxicity, more research is needed to reveal the toxicity in vivo, in order to predict and identify the adverse effect profiles of the substance.
- Sall C, Seck M, Faye B, Dioum MD, Seck I, Gueye PM, et al. Étude In vitro de l’effet antifalcémiant des globules rouges et de l’activité antioxydante d’extraits de la poudre de racines de Maytenus senegalensis Lam (Celestraceae). International Journal of Biological and Chemical Sciences. 2016; 10: 10-17. https://bit.ly/3d35Drr
- Simmons MP, Bacon CD, Cappa JJ, McKenna MJ. Phylogeny of Celastraceae subfamilies cassinoideae and tripterygioideae inferred from morphological characters and nuclear and plastid loci. Systematic Botany. 2012; 37: 456‑467. https://bit.ly/3d4y9sU
- Adam JG, Echard N, Lescot M. Plantes médicinales Hausa de l’Ader (République du Niger). Journal d’agriculture tropicale et de botanique appliquée. 1972; 19: 259‑399. https://bit.ly/3c56bvQ
- Kerharo J, Adam JG. Plantes médicinales et toxiques des Peul et des Toucouleur du Sénégal. 1964; 11: 384‑444. https://bit.ly/2XwdPtN
- da Silva G, Serrano R, Silva O. Maytenus heterophylla and maytenus senegalensis, two traditional herbal medicines. J Nat Sci Biol Med. 2011; 2: 59-65. PubMed: https://pubmed.ncbi.nlm.nih.gov/22470236/
- Leite JPV, Rastrelli L, Romussi G, Oliveira AB, Vilegas JHY, Vilegas W, et al. Isolation and HPLC quantitative analysis of flavonoid glycosides from brazilian beverages (Maytenus ilicifolia and M. aquifolium). J Agric Food Chem. 2001; 49: 3796‑3801. https://bit.ly/2yzeyCb
- Tahir AE, Ibrahim AM, Satti GMH, Theander TG, Kharazmi A, Khalid SA. The potential antileishmanial activity of some Sudanese medicinal plants. Phytotherapy Research. 1998; 12: 576‑579. https://bit.ly/2XpPypi
- Matu E.N. Medicinal properties and In vitro responses of Maytenus senegalensis (lam.) exell. University of Natal, Pietermaritzburg. Journal of Ethnopharmacology. 2003; 87: 35-41. https://bit.ly/2yvRRP4
- Haule EE, Moshi MJ, Nondo RSO, Mwangomo DT, Mahunnah RLA. A study of antimicrobial activity, acute toxicity and cytoprotective effect of a polyherbal extract in a rat ethanol-HCl gastric ulcer model. BMC Res Notes. 2012; 5: 546. PubMed: https://pubmed.ncbi.nlm.nih.gov/23031266/
- Muthaura C. Antiplasmodial, cytotoxicity and phytochemical constituents of four maytenus species used in traditional medicine in kenya. The Natural Products Journal. 2017; 7: 144‑152. https://bit.ly/3bYvGi9
- Mueller MS, Ernest M. Medicinal plants in tropical countries: Traditional Use - Experience - Facts. 1st edition. Stuttgart; New York: George Thieme Verlag. 2005; 101-106
- Kérharo J, Bouquet A, Debray M. Médecines et pharmacopées traditionnelles du Sénégal, du Congo et de Madagascar. Etudes médicales. 1975; 1: 92. https://bit.ly/3elw7F6
- Ahmed AS, McGaw LJ, Eloff JN. Evaluation of pharmacological activities, cytotoxicity and phenolic composition of four Maytenus species used in southern African traditional medicine to treat intestinal infections and diarrhoeal diseases. BMC Complement Altern Med. 2013; 13: 100. PubMed: https://pubmed.ncbi.nlm.nih.gov/23663902/
- Gessler MC, Tanner M, Chollet J, Nkunya MHH, Heinrich M. Tanzanian medicinal plants used traditionally for the treatment of malaria: in vivo antimalarial and In vitro cytotoxic activities. Phytotherapy Research. 1995; 504-508. https://bit.ly/2TFswtm
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983; 65: 55‑63. PubMed: https://pubmed.ncbi.nlm.nih.gov/6606682/
- Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nature Protocols. 2008; 3: 1125‑1131. PubMed: https://pubmed.ncbi.nlm.nih.gov/18600217/
- Barlovatz-Meimon G, Ronot X, Adolphe M, Demongeot J. Culture de cellules animales. Paris: Lavoisier-Tec & Doc, 3e édition, 2014. 667p.
- Nabende PN. Safety and anti-proliferative activity of Prunus africana, Warburgia stuhlmannii and Maytenus senegalensis extracts in breast and colon cancer cell lines. 2015; 86.



Sign up for Article Alerts